Assessing Controlled Traffic Farming as a Precision Agriculture Strategy for Minimising N2O Losses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Core Sampling
2.3. Soil Core Experiments
2.4. Gas (N2O) Sample Collection and Analysis
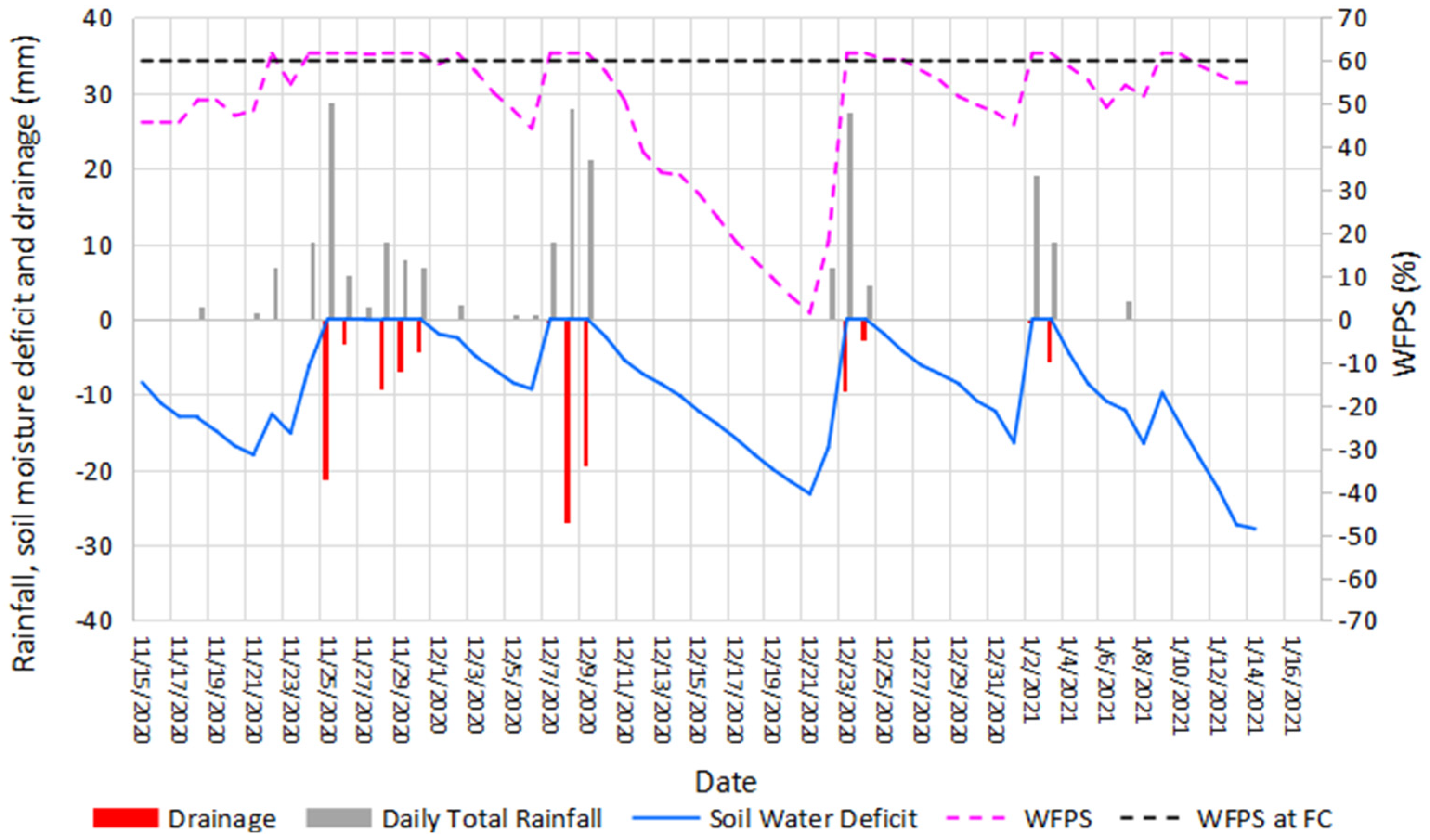
2.5. Weather Data
2.6. Grass Harvesting and Analysis for N Uptake
2.7. Soil Analysis
2.8. Statistical Analysis
3. Results
3.1. Climate
3.2. Initial and Final Soil Inorganic N Content
3.3. Traffic System–Fertiliser Nitrous Oxide Emission Profile
3.4. Grass N Uptake
4. Discussion
4.1. Effect of Traffic System on N2O Fluxes
4.2. Effect of WFPS on N2O Fluxes
4.3. Effect of N Fertiliser on N2O Fluxes
4.4. Nitrogen Uptake and N2O Emissions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTF | Controlled Traffic Farming |
| RTF | Random Traffic Farming |
| WFPS | Water-Filled Pore Space |
| N2O | Nitrous Oxide |
| N | Nitrogen |
References
- Statistics New Zealand. Land and Soil—Estimated Long—Term Soil Erosion. 2016. Available online: https://www.stats.govt.nz/topics/land/ (accessed on 19 April 2020).
- HortNZ. Annual Report 2020/21. Horticulture New Zealand. 2021. Available online: https://www.hortnz.co.nz (accessed on 15 August 2022).
- Ministry for Primary Industries (MPI). Fit for a Better World—Accelerating Our Economic Potential. New Zealand Government. 2020. Available online: https://www.mpi.govt.nz (accessed on 15 August 2022).
- Hu, W.; Drewry, J.; Beare, M.; Eger, A.; Müller, K. Compaction induced soil structural degradation affects productivity and environmental outcomes: A review and New Zealand case study. Geoderma 2021, 395, 115035. [Google Scholar] [CrossRef]
- Soane, B.D.; van Ouwerkerk, C. Implications of soil compaction in crop production for the quality of the environment. Soil Tillage Res. 1995, 35, 5–22. [Google Scholar] [CrossRef]
- Yamulki, S.; Jarvis, S.C. Short-term effects of tillage and compaction on nitrous oxide, nitric oxide, nitrogen dioxide, methane and carbon dioxide fluxes from grassland. Biol. Fertil. Soils 2002, 36, 224–231. [Google Scholar] [CrossRef]
- Saggar, S.; Tate, K.; Giltrap, D.; Singh, J. Soil-atmosphere exchange of nitrous oxide and methane in New Zealand terrestrial ecosystems and their mitigation options: A review. Plant Soil 2008, 309, 25–42. [Google Scholar] [CrossRef]
- Wuebbles, D.J. Nitrous oxide: No laughing matter. Science 2009, 326, 56–57. [Google Scholar] [CrossRef]
- Wu, X.; Du, J.; Gao, Y.; Wang, H.; Zhang, C.; Zhang, R.; He, H.; Lu, G.; Wu, Z. Progress and challenges in nitrous oxide decomposition and valorization. Chem. Soc. Rev. 2024, 53, 8379–8423. [Google Scholar] [CrossRef]
- Sitaula, B.K.; Hansen, S.; Sitaula, J.I.B.; Bakken, L.R. Effects of soil compaction on N2O emission in agricultural soil. Chemosphere—Glob. Change Sci. 2000, 2, 367–371. [Google Scholar] [CrossRef]
- Pulido-Moncada, M.; Petersen, S.O.; Munkholm, L.J. Soil compaction raises nitrous oxide emissions in managed agroecosystems: A review. Agron. Sustain. Dev. 2022, 42, 38. [Google Scholar] [CrossRef]
- Thomas, S.; Barlow, H.; Francis, G.; Hedderley, D. Emission of nitrous oxide from fertilised potatoes. In Proceedings of the SuperSoil 2004: The 3rd Australia New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004. [Google Scholar]
- Bochtis, D.D.; Vougioukas, S.G. Minimising the non-working distance travelled by machines operating in a headland field pattern. Biosyst. Eng. 2008, 101, 1–12. [Google Scholar] [CrossRef]
- Raper, R.L. Agricultural traffic impacts on soil. J. Terramech. 2005, 42, 259–280. [Google Scholar] [CrossRef]
- Tullberg, J.N.; Yule, D.F.; McGarry, D. Controlled traffic farming—from research to adoption in Australia. Soil Tillage Res. 2007, 97, 272–281. [Google Scholar] [CrossRef]
- Li, Y.X.; Tullberg, J.N.; Freebairn, D.M. Wheel traffic and tillage effects on runoff and crop yield. Soil Tillage Res. 2007, 97, 282–292. [Google Scholar] [CrossRef]
- Taylor, J.H. Reduction of traffic-induced soil compaction e special issue. Soil Tillage Res. 1992, 24, 301–302. [Google Scholar] [CrossRef]
- Tullberg, J.; Antille, D.L.; Bluett, C.; Eberhard, J.; Scheer, C. Controlled traffic farming effects on soil emissions of nitrous oxide and methane. Soil Tillage Res. 2018, 176, 18–25. [Google Scholar] [CrossRef]
- Vermeulen, G.; Mosquera, J. Soil, crop and emission responses to seasonal-controlled traffic in organic vegetable farming on loam soil. Soil Tillage Res. 2009, 102, 126–134. [Google Scholar] [CrossRef]
- Tullberg, J.; McHugh, A.D.; Khabbaz, B.G.; Scheer, C.; Grace, P. Controlled traffic/permanent bed farming reduces GHG emissions. In Proceedings of the 5th World Congress of Conservation Agriculture 2011: Resilient Food Systems for a Changing World, Canberra, Australia, 14 January 2011; Australian Centre for International Agricultural Research: Canberra, Australia, 2011. [Google Scholar]
- Gasso, V.; Oudshoorn, F.W.; Sørensen, C.A.G.; Pedersen, H.H. An environmental life cycle assessment of controlled traffic farming. J. Clean. Prod. 2014, 73, 175–182. [Google Scholar] [CrossRef]
- Tullberg, J.N. Developments in mechanization technology: Controlled traffic farming. In Advances in Agricultural Machinery and Technologies; Chen, G., Ed.; Taylor & Francis Group (CRC Press): London, UK, 2018; Chapter 2; pp. 27–47. [Google Scholar]
- Knowles, O.; Dawson, A. To grid or not to grid. A review of current soil sampling methods. In Farm Environmental Planning—Science, Policy and Practice; Currie, L.D., Christensen, C.L., Eds.; Massey University: Palmerston North, New Zealand, 2018; Available online: https://www.massey.ac.nz/~flrc/workshops/18/Manuscripts/Paper_Knowles_2018.pdf (accessed on 9 January 2022).
- Saggar, S.; Harvey, M.; Singh, J.; Giltrap, D.; Pattey, E.; Bromley, T.; McMillan, A. Chambers, micrometeorological measurements, and the New Zealand Denitrification–Decomposition model for nitrous oxide emission estimates from an irrigated dairy-grazed pasture. J. Integr. Environ. Sci. 2010, 7, 61–70. [Google Scholar] [CrossRef]
- NIWA. CliFlo: NIWA’s National Climate Database on the Web, NIWA. 2021. Available online: https://niwa.co.nz/ (accessed on 9 January 2022).
- McKenzie, H.; Wallace, H. The Kjeldahl determination of Nitrogen: A critical study of digestion conditions-Temperature, Catalyst, and Oxidizing agent. Aust. J. Chem. 1954, 7, 55–70. [Google Scholar] [CrossRef]
- Blakemore, L.; Searle, P.; Daly, B. Methods for Chemical Soil Analysis, New Zealand Soil Bureau Scientific Report 80; Department of Scientific and Industrial Research: Lower Hutt, New Zealand, 1987; pp. 72–103. [CrossRef]
- SAS Institute. Statistical Analysis Software for Windows, Version 9.4; SAS Institute: Cary, NC, USA, 2016.
- Gregorich, E.G.; Rochette, P.; VandenBygaart, A.J.; Angers, D.A. Greenhouse gas contributions of agricultural soils and potential mitigation practices in Eastern Canada. Soil Tillage Res. 2005, 83, 53–72. [Google Scholar] [CrossRef]
- Singurindy, O.; Molodovskaya, M.; Richards, B.K.; Steenhuis, T.S. Nitrous oxide emission at low temperatures from manure-amended soils under corn (Zea mays L.). Agric. Ecosyst. Environ. 2009, 132, 74–81. [Google Scholar] [CrossRef]
- Rousset, C.; Clough, T.J.; Grace, P.R.; Rowlings, D.W.; Scheer, C. Soil type, bulk density and drainage effects on relative gas diffusivity and N2O emissions. Soil Res. 2020, 58, 726–736. [Google Scholar] [CrossRef]
- Klefoth, R.R.; Clough, T.J.; Oenema, O.; van Groenigen, J.W. Soil bulk density and moisture content influence relative gas diffusivity and the reduction of nitrogen-15 nitrous oxide. Vadose Zone J. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Deepagoda, C.T.K.K.; Clough, T.J.; Thomas, S.M.; Balaine, N.; Elberling, B. Density effects on soil-water characteristics, soil-gas diffusivity, and emissions of N2O and N2 from a re-packed pasture soil. Soil Sci. Soc. Am. J. 2019, 83, 118–125. [Google Scholar] [CrossRef]
- Ali, A.; Bennett, J.M.; Roberton, S.; Krwanji, D.; Zhu, Y.; West, D. Selection of a stress-based soil compaction test to determine potential impact of machine wheel loads. Eur. J. Soil Sci. 2024, 75, e13501. [Google Scholar] [CrossRef]
- Ball, B.C.; Campbell, D.J.; Douglas, J.T.; Henshall, J.K.; O’sullivan, M.F. Soil structural quality, compaction and land management. Eur. J. Soil Sci. 1997, 48, 593–601. [Google Scholar] [CrossRef]
- Schmeer, M.; Loges, R.; Dittert, K.; Senbayram, M.; Horn, R.; Taube, F. Legume-based forage production systems reduce nitrous oxide emissions. Soil Tillage Res. 2014, 143, 17–25. [Google Scholar] [CrossRef]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- van der Weerden, T.J.; Kelliher, F.M.; de Klein, C.A.M. Influence of pore size distribution and soil water content on nitrous oxide emissions. Soil Res. 2012, 50, 125–135. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 69, 10–20. [Google Scholar] [CrossRef]
- Flechard, C.; Ambus, P.; Skiba, U.; Rees, R.; Hensen, A.; van Amstel, A.; Dasselaar, A.v.D.P.-V.; Soussana, J.-F.; Jones, M.; Clifton-Brown, J.; et al. Effects of climate and management intensity on nitrous oxide emissions in grassland systems across Europe. Agric. Ecosyst. Environ. 2007, 121, 135–152. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 20130122–20130197. [Google Scholar] [CrossRef]
- Bateman, E.; Baggs, E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Congreves, K.A.; Phan, T.; Farrell, R.E. Using 15N2O isotopomers to understand the relationship between soil moisture and N2O production pathways. Soil 2019, 5, 265–274. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Z.; Ju, X.; Song, X.; Zhang, J.; Li, S.; Zhu-Barker, X. Quantifying nitrous oxide production rates from nitrification and denitrification under various moisture conditions in agricultural soils. Front. Microbiol. 2022, 13, 1110151. [Google Scholar] [CrossRef]
- Schils, R.; Van Groenigen, J.; Velthof, G.; Kuikman, P. Nitrous oxide emissions from multiple combined applications of fertiliser and cattle slurry to grassland. Plant Soil 2008, 310, 89–101. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X. N2O emission from the semi-arid ecosystem under mineral fertilizer (urea and superphosphate) and increased precipitation in northern China. Atmos. Environ. 2008, 42, 291–302. [Google Scholar] [CrossRef]
- Kim, S.U.; Lee, H.H.; Moon, S.M.; Han, H.R.; Hong, C.O. Nitrous oxide emissions and maize yield as influenced by nitrogen fertilization and tillage operations in upland soil. Appl. Biol. Chem. 2021, 64, 18. [Google Scholar] [CrossRef]
- Carter, M.S. Contribution of nitrification and denitrification to N2O emissions from urine patches. Soil Biol. Biochem. 2007, 39, 2091–2102. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Mahama, G.; Prasad, P.; Roozeboom, K.; Nippert, J.; Rice, C. Reduction of nitrogen fertilizer requirements and nitrous oxide emissions using legume cover crops in a No-tillage sorghum production system. Sustainability 2020, 12, 4403. [Google Scholar] [CrossRef]
- Smith, L.C.; deKlein, C.A.M.; Catto, W.D. Effect of dicyandiamide applied in a granular form on nitrous oxide emissions from a grazed dairy pasture in Southland, New Zealand. N. Zeal. J. Agric. Res. 2008, 51, 387–396. [Google Scholar] [CrossRef]
- Ellis, S.; Yamulki, S.; Dixon, E.; Harrison, R.; Jarvis, S. Denitrification and N2O emissions from a UK pasture soil following the early spring application of cattle slurry and mineral fertiliser. Plant Soil 1998, 202, 15–25. [Google Scholar] [CrossRef]
- Freibauer, A.; Kaltschmitt, M. Controls and models for estimating direct nitrous oxide emissions from temperate and sub-boreal agricultural mineral soils in Europe. Biogeochemistry 2003, 63, 93–115. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]

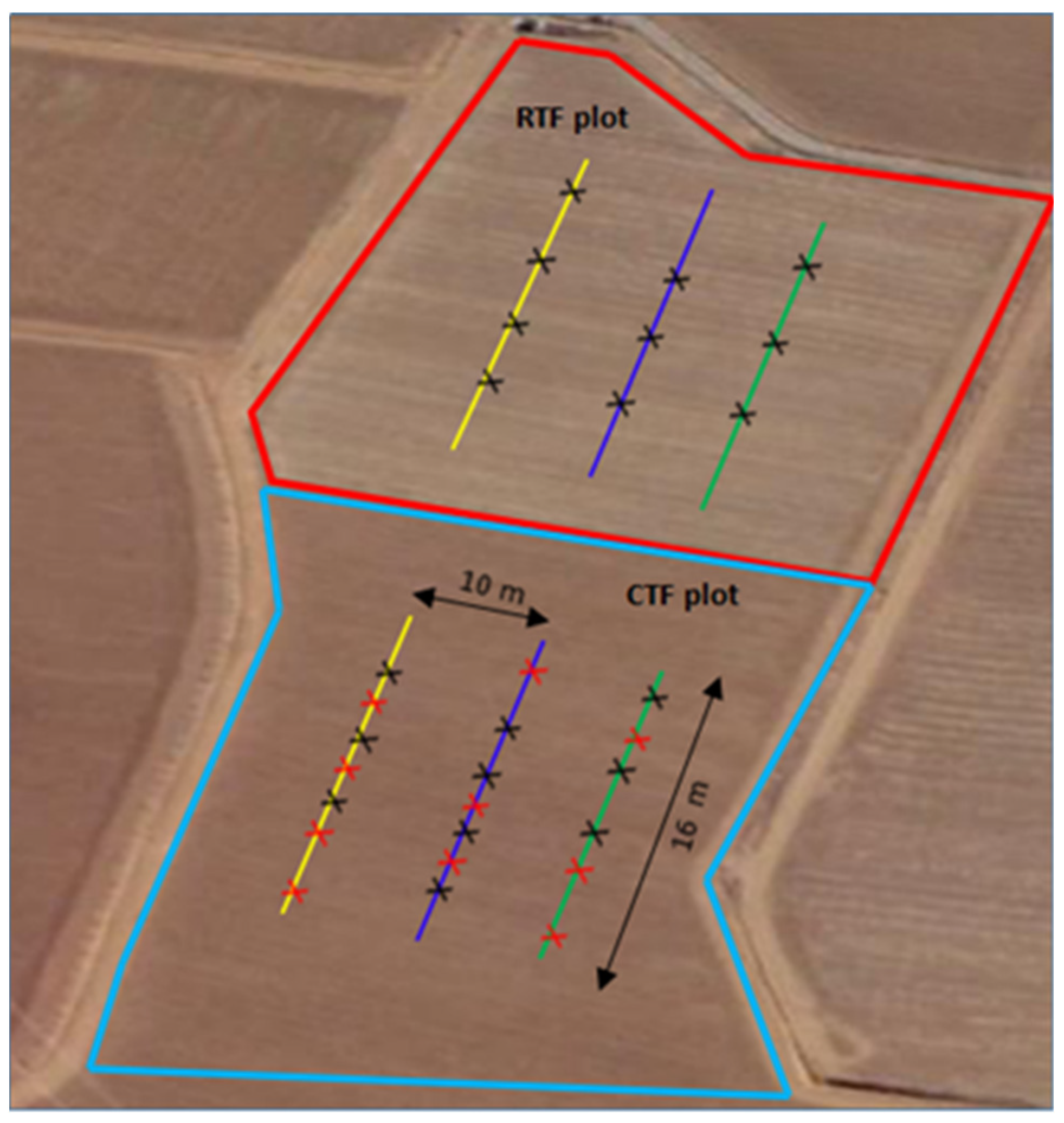
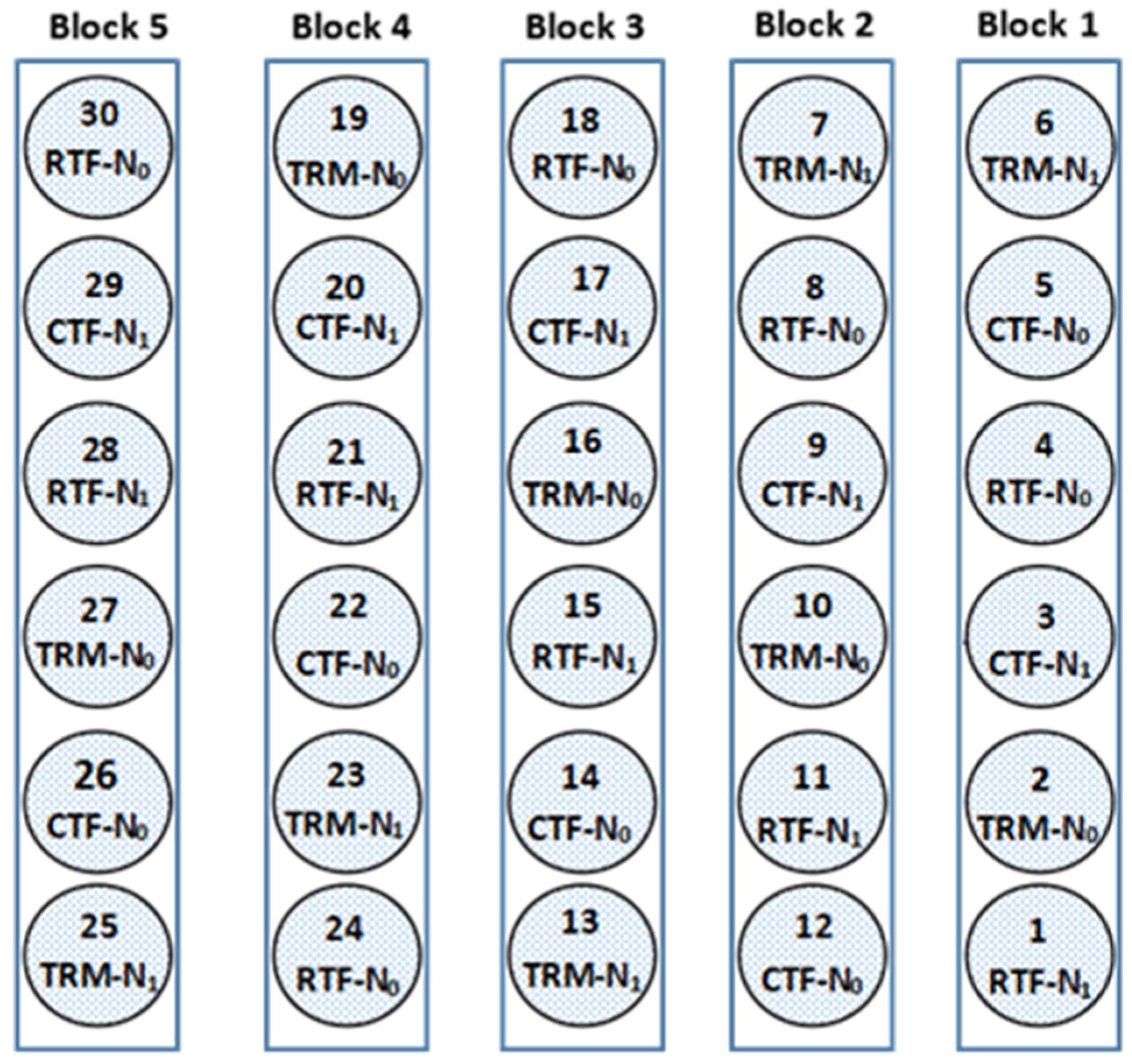

| Treatment | Description |
|---|---|
| CTF-N1 | Controlled Traffic Farming + 140 kg N/ha |
| RTF-N1 | Random Traffic Farming + 140 kg N/ha |
| TRM-N1 | Tramline + 140 kg N/ha |
| CTF-N0 | Controlled Traffic Farming + 0 kg N/ha |
| RTF-N0 | Random Traffic Farming + 0 kg N/ha |
| TRM-N0 | Tramline + 0 kg N/ha |
| Treatment | Mean Bulk Density (g/cm3) | Porosity |
|---|---|---|
| CTF-N1 | 0.81 | 0.69 |
| RTF-N1 | 0.83 | 0.69 |
| TRM-N1 | 0.87 | 0.66 |
| CTF-N0 | 0.77 | 0.71 |
| RTF-N0 | 0.76 | 0.67 |
| TRM-N0 | 0.87 | 0.67 |
| p value | ns | ns |
| Treatment | Initial Gravimetric Water Content (g/g soil) | Initial Residual Inorganic N (kg N/ha) | Final Residual Inorganic N (kg N/ha) |
|---|---|---|---|
| CTF-N1 | 0.25 ± 0.02 | 5.81 ± 2.05 | 34.04 ± 14.28 a |
| RTF-N1 | 0.23 ± 0.02 | 3.29 ± 0.10 | 43.52 ± 7.47 a |
| TRM-N1 | 0.22 ± 0.01 | 2.51 ± 0.40 | 56.03 ± 11.98 a |
| CTF-N0 | 0.23 ± 0.02 | 6.80 ± 4.30 | 1.92 ± 0.32 b |
| RTF-N0 | 0.25 ± 0.01 | 3.49 ± 0.30 | 1.86 ± 0.33 b |
| TRM-N0 | 0.2 ± 0.02 | 2.46 ± 0.35 | 1.71 ± 0.38 b |
| p value | ns | ns | <0.0001 |
| Days After Urea Application | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 2 | 6 | 9 | 14 | 17 | 24 | 27 | 34 | 41 | 49 | 56 | ||
| Treatments | g N2O/ha/day | Integrated flux (kg N/ha) | |||||||||||
| CTF-N1 | 9.08 a | 5.85 b | 17.07 a | 74.43 a | 109.88 a | 49.29 ab | 378.52 ab | 37.89 a | 9.56 ab | 25.33 ab | 29.36 a | 4.57 ab | 3.69 abc |
| RTF-N1 | 10.3 a | 26.3 a | 12.96 a | 59.61 a | 210.16 a | 68.69 a | 594.27 a | 30.6 ab | 16.46 a | 34.10 a | 43.33 a | 5.2 a | 5.40 a |
| TRM-N1 | 4.86 a | 3.12 b | 9.98 a | 86.88 a | 137.15 a | 32.40 ab | 414.39 ab | 30.2 ab | 5.44 ab | 14.85 b | 29.16 a | 2.82 ab | 3.99 ab |
| CTF-N0 | 8.25 a | 4.25 b | 8.92 a | 33.11 a | 125.2 a | 48.12 ab | 103.57 c | 15.6 ab | 8.09 ab | 7.13 c | 11.47 b | 3.87 ab | 1.41 c |
| RTF-N0 | 7.79 a | 7.92 b | 8.42 a | 71.74 a | 92.99 a | 16.63 b | 124.08 bc | 12.22 b | 8.27 ab | 5.36 c | 5.94 b | 3.64 ab | 1.53 c |
| TRM-N0 | 6.12 a | 5.67 b | 10.26 a | 47.93 a | 100.41 a | 33.29 ab | 303.35 ab | 20.49 ab | 4.65 b | 4.34 c | 7.64 b | 2.31 b | 2.49 bc |
| Treatments | 1st Cut | 2nd Cut | 3rd Cut | Total N Uptake |
|---|---|---|---|---|
| 14 dys after planting grass | 20 days after 1st cut | 41 days after 1st cut | ||
| CTF-N1 | 5.10 a | 19.08 a | 11.24 abc | 35.42 a |
| RTF-N1 | 5.84 a | 16.25 ab | 12.47 ab | 34.57 a |
| TRM-N1 | 3.54 a | 17.88 a | 15.99 a | 37.41 a |
| CTF-N0 | 2.48 a | 11.27 bc | 6.29 bcd | 20.06 b |
| RTF-N0 | 3.75 a | 7.20 cd | 3.08 cd | 14.04 bc |
| TRM-N0 | 4.43 a | 1.58 d | 0.59 d | 6.46 c |
| Treatments | Total Emissions from This Study (kg N2O/ha) | Reported Emissions from Other Studies (kg N2O/ha) |
|---|---|---|
| CTF-N1 | 3.69 | 0.45 [52] |
| RTF-N1 | 5.40 | 3.74 [42] |
| TRM-N1 | 3.99 | 2.40 [50] |
| CTF-N0 | 1.41 | 0.78 [18] |
| RTF-N0 | 1.53 | |
| TRM-N0 | 2.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveendrakumaran, B.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C. Assessing Controlled Traffic Farming as a Precision Agriculture Strategy for Minimising N2O Losses. Nitrogen 2025, 6, 63. https://doi.org/10.3390/nitrogen6030063
Raveendrakumaran B, Grafton M, Jeyakumar P, Bishop P, Davies C. Assessing Controlled Traffic Farming as a Precision Agriculture Strategy for Minimising N2O Losses. Nitrogen. 2025; 6(3):63. https://doi.org/10.3390/nitrogen6030063
Chicago/Turabian StyleRaveendrakumaran, Bawatharani, Miles Grafton, Paramsothy Jeyakumar, Peter Bishop, and Clive Davies. 2025. "Assessing Controlled Traffic Farming as a Precision Agriculture Strategy for Minimising N2O Losses" Nitrogen 6, no. 3: 63. https://doi.org/10.3390/nitrogen6030063
APA StyleRaveendrakumaran, B., Grafton, M., Jeyakumar, P., Bishop, P., & Davies, C. (2025). Assessing Controlled Traffic Farming as a Precision Agriculture Strategy for Minimising N2O Losses. Nitrogen, 6(3), 63. https://doi.org/10.3390/nitrogen6030063







