Reducing Nitrogen Input Increases the Efficacy of Soil Nitrogen Utilization by Regulating Cotton–Arbuscular Mycorrhizal Fungi–Soil Nitrogen Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Properties
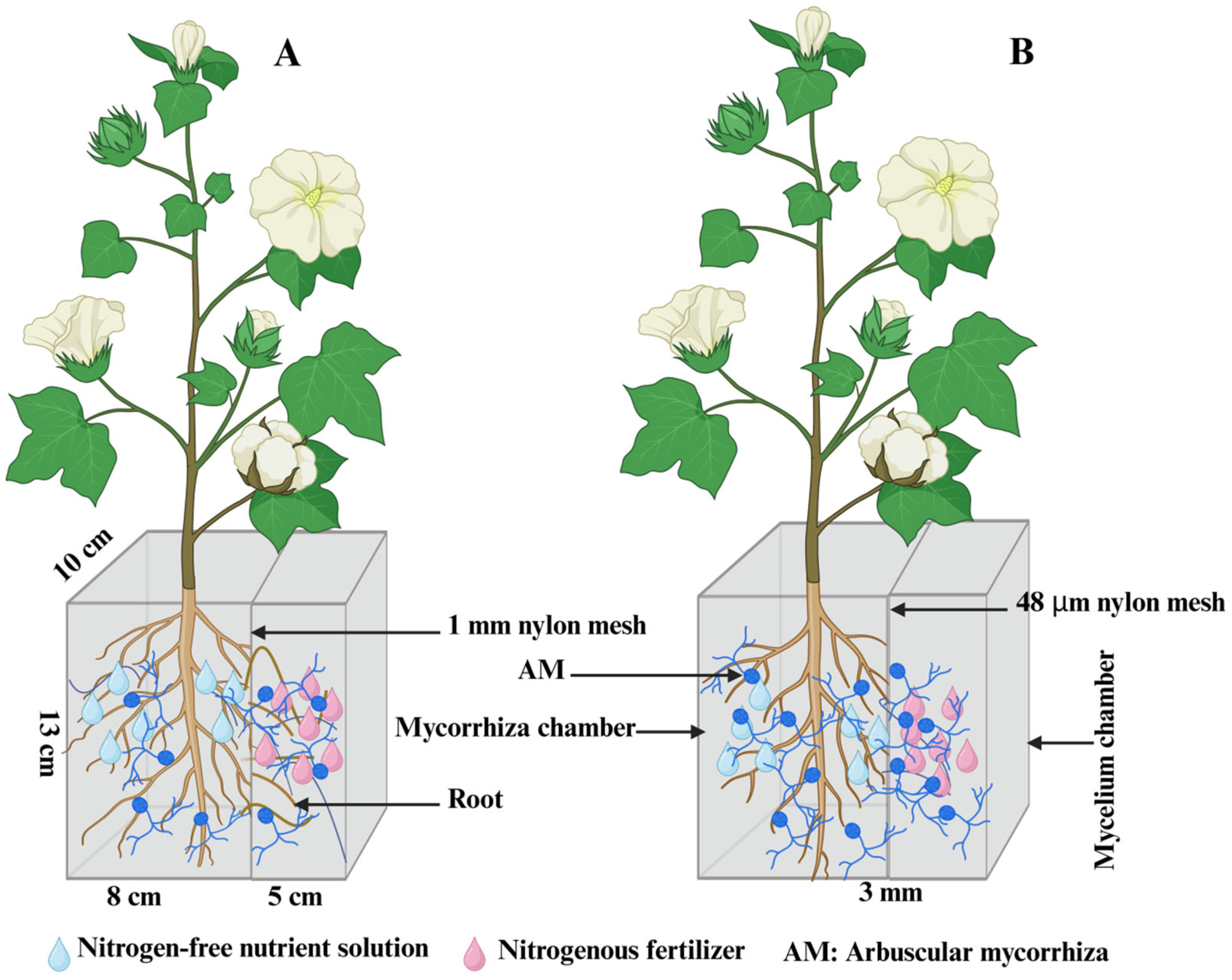
2.2. Experimental Design
2.3. Measurement of Plant- and Soil-Related Indicators
2.4. Statistical Analysis
3. Results
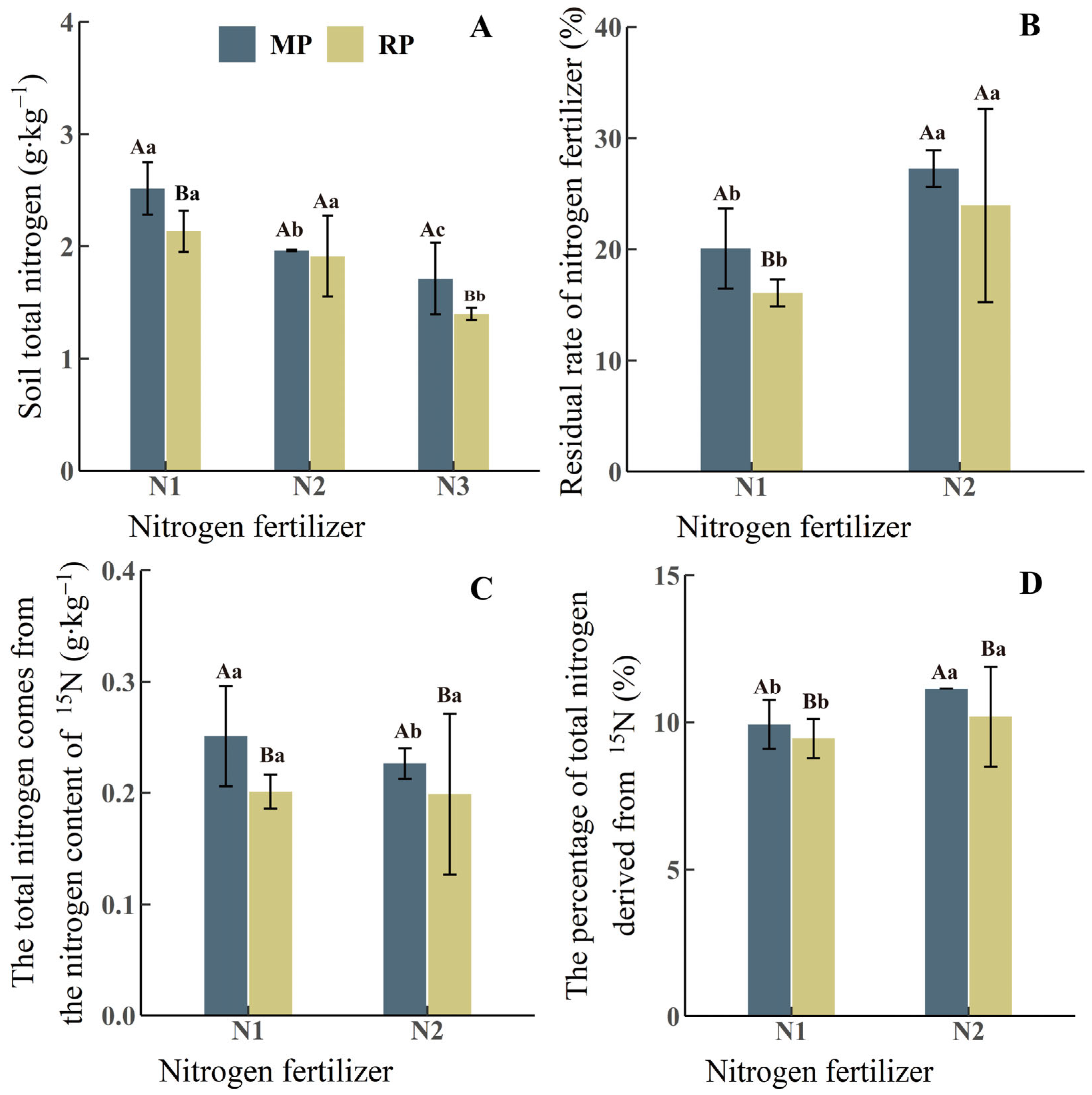
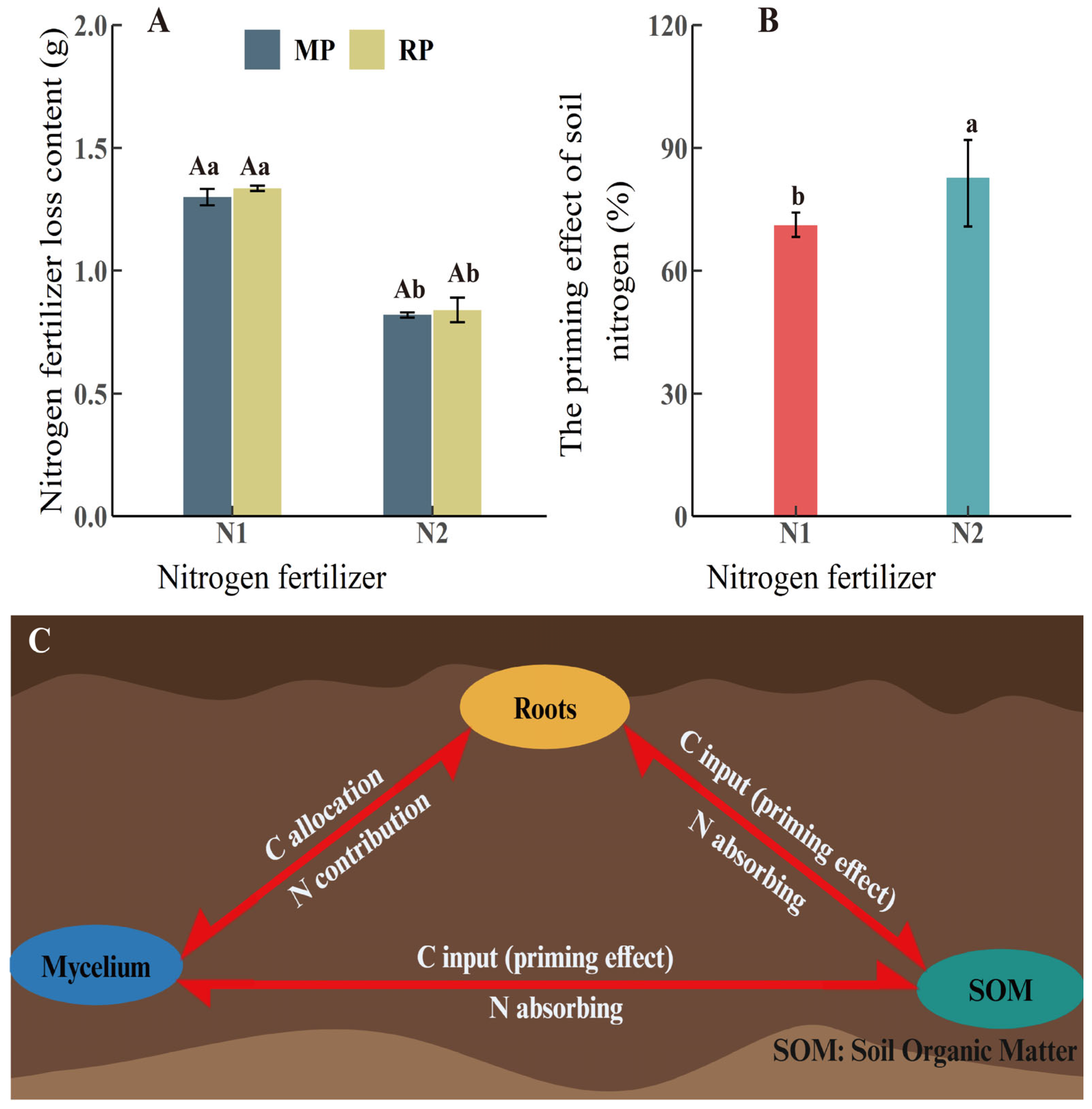
3.1. Impacts of Root and Mycelial Pathways on Soil N Content Under Different N Application Rates
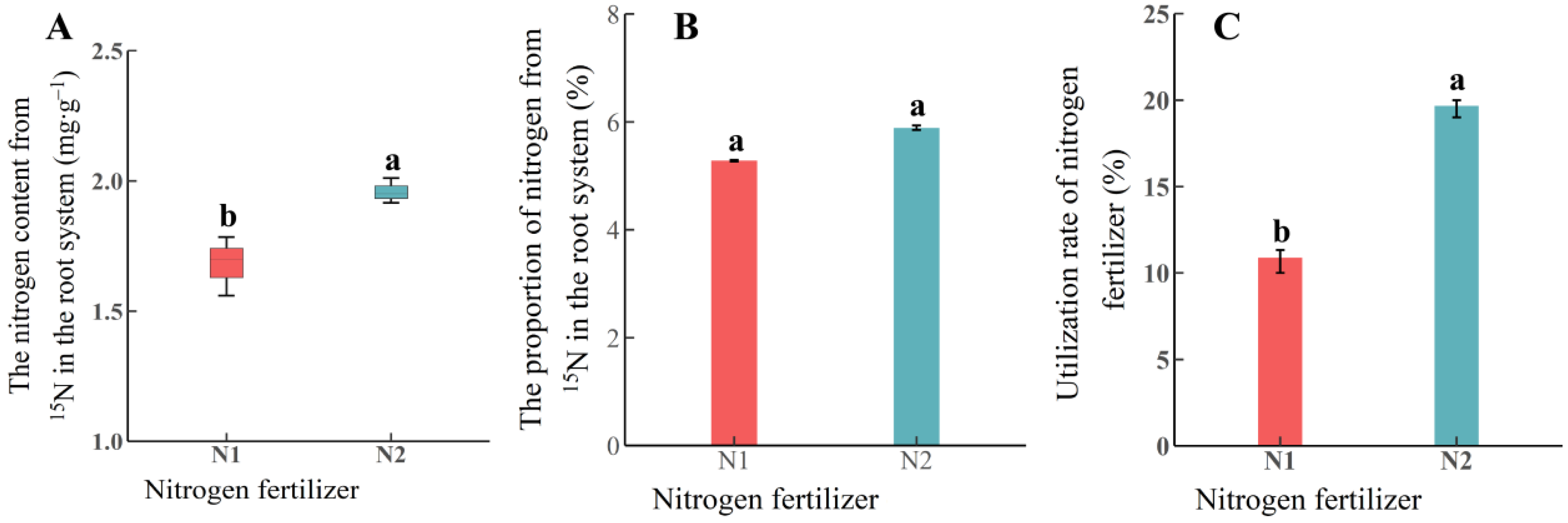
3.2. Absorption of Soil N by Roots Under Different N Application Rates
3.3. Changes in Cotton Growth and Plant Part N Accumulation Under Different N Application Rates
4. Discussion
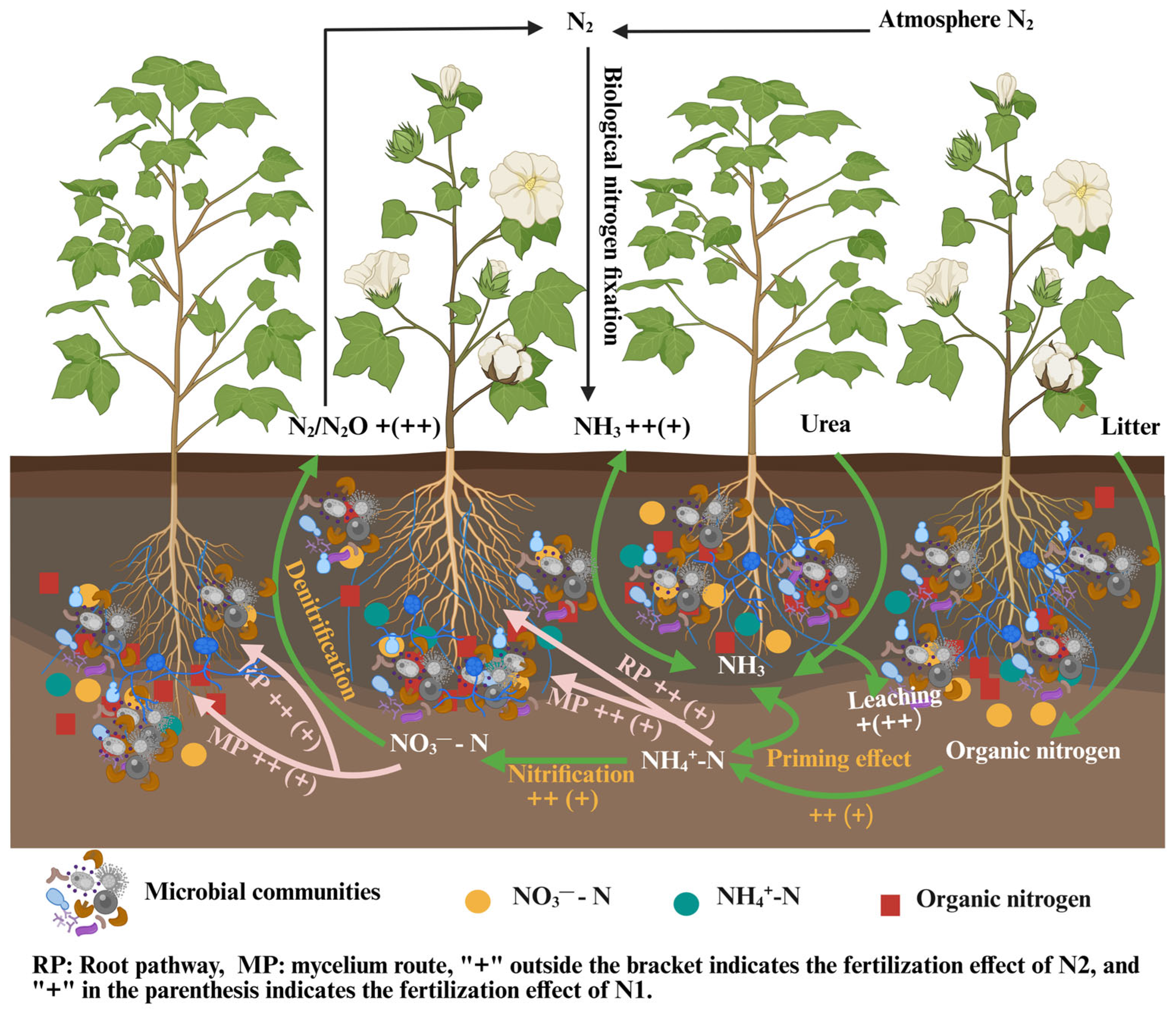
4.1. Regulation of the Symbiotic System of Cotton and AM Fungi by N Application
4.2. Effects of N Application and AM Fungi Interaction on Soil N
4.3. Effects of N Application and AM Fungi Interaction on N Distribution in Various Plant Parts of Cotton
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Arbuscular mycorrhizal |
| N | Nitrogen |
| C | Carbon |
| MP | Mycelium pathway |
| RP | Root pathway |
References
- Shi, H.L.; Yan, Q.Q.; Zhang, J.S.; Li, C.Y.; Dou, H.T. Compensation Effect of Nitrogen Fertilizer on Photosynthetic Characteristics and Yield during Cotton Flowering Boll-setting Stage under Non-sufficient Drip Irrigation. Acta Crops Sin. 2018, 44, 1196–1204. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H.; Song, W.; Liu, C.; Xu, J.; Zhao, W.; Zhou, Z. Effect of N fertilization rate on soil alkali-hydrolyzable N, subtending leaf N concentration, fiber yield, and quality of cotton. Crop J. 2016, 4, 323–330. [Google Scholar] [CrossRef]
- Hou, X.; Fan, J.; Hu, W.; Zhang, F.; Yan, F.; Xiao, C.; Li, Y.; Cheng, H. Optimal irrigation amount and nitrogen rate improved seed cotton yield while maintaining fiber quality of drip-fertigated cotton in northwest China. Ind. Crop Prod. 2021, 170, 113710. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Dhital, Y.; Ma, K.; Wen, Y.; Wang, Z. Increasing dissolved oxygen concentration of irrigation water is beneficial to nitrogen uptake of cotton under mulched drip irrigation. Field Crops Res. 2024, 316, 109494. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Z.; Li, H.; Wang, T.; Chen, R. Effects of nitrogen application and brackish water irrigation on yield and quality of cotton. Agr. Water Manag. 2022, 264, 107512. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, Y.; Wang, S.Z.; Wang, Z.Q.; Gu, J.C. Effects of elevated N availability on anatomy, morphology and mycorrhizal colonization of fine roots:A review. Chin. J. Appl. Ecol. 2016, 27, 1294–1302. [Google Scholar] [CrossRef]
- Hakulinen, J. Nitrogen-induced reduction in leaf phenolic level is not accompanied by increased rust frequency in a compatible willow (Salix myrsinifolia)—Melampsora rust interaction. Physiol. Plant. 1998, 102, 101–110. [Google Scholar] [CrossRef]
- Xie, H.; Xu, H.; Li, X.; Knapp, L.S.P.; Lu, D.; Jin, S. Does nitrogen fertilization alter the scaling relationships of multinutrients in tree organs? Evidence from Chinese hickory (Carya cathayensis) saplings. Plant Soil 2023, 484, 533–546. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lu, Y.; Zhang, H.; Ju, C.H.; Pei, W.M.; Hu, F.; Wan, F.X. Effects of exponential fertilization on growth and foliar nutrient status of pecan seedlings. Soil Fertil. Sci. China 2018, 6, 136–140. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.J.; Guo, T.T.; Cui, J.; Li, L.P.; Liang, D.F.; Li, Y.H.; Zhang, Z.H.; Zhu, X.X.; Ren, F. Effects of Multi-gradient Nitrogen and Phosphorus Additions on Biomass and Nitrogen and Phosphorus Content of Alpine Meadow Plant Community. Acta Agrestia Sin. 2023, 31, 751–759. [Google Scholar] [CrossRef]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.-N.; Fu, S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bi, Z.C.; Xiong, Z.Q. Dynamic responses of nitrous oxide emission and nitrogen use efficiency to nitrogen and biochar amendment in an intensified vegetable field in southeastern China. Gcb Bioenergy 2017, 9, 400–413. [Google Scholar] [CrossRef]
- Min, J.; Zhang, H.L.; Shi, W.M. Optimizing nitrogen input to reduce nitrate leaching loss in greenhouse vegetable production. Agr. Water Manag. 2012, 111, 53–59. [Google Scholar] [CrossRef]
- Huddell, A.M.; Galford, G.L.; Tully, K.L.; Crowley, C.; Palm, C.A.; Neill, C.; Hickman, J.E.; Menge, D.N.L. Meta-analysis on the potential for increasing nitrogen losses from intensifying tropical agriculture. Glob. Change Biol. 2020, 26, 1668–1680. [Google Scholar] [CrossRef]
- Wan, X.J.; Wu, W.; Liao, Y.C. Mitigating ammonia volatilization and increasing nitrogen use efficiency through appropriate nitrogen management under supplemental irrigation and rain-fed condition in winter wheat. Agr. Water Manag. 2021, 255, 107050. [Google Scholar] [CrossRef]
- Jalpa, L.; Mylavarapu, R.S.; Hochmuth, G.; Wright, A.; van Santen, E. Recovery efficiency of applied and residual nitrogen fertilizer in tomatoes grown on sandy soils using the 15N technique. Sci. Hortic. 2021, 278, 109861. [Google Scholar] [CrossRef]
- Wang, S.H.; Mao, L.L.; Shi, J.L.; Nie, J.J.; Song, X.L.; Sun, X.Z. Effects of plant density and nitrogen rate on cotton yield and nitrogen use in cotton stubble retaining fields. J. Integr. Agr. 2021, 20, 2090–2099. [Google Scholar] [CrossRef]
- Kong, X.W.; Ying, S.H.; Yang, L.C.; Xin, Y.C.; Cai, Z.; Zhu, S.M.; Liu, D.Z. Microbial and isotopomer analysis of N2O generation pathways in ammonia removal biofilters. Chemosphere 2020, 251, 126357. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Fang, J.Y. Review on characteristics and main hypotheses of plant ecological stoichiometry. Chin. J. Plant Ecol. 2021, 45, 682–713. [Google Scholar] [CrossRef]
- Estiarte, M.; Campioli, M.; Mayol, M.; Penuelas, J. Variability and limits of nitrogen and phosphorus resorption during foliar senescence. Plant Commun. 2023, 4, 100503. [Google Scholar] [CrossRef]
- Su, Y.; Ma, X.F.; Le, J.J.; Li, K.H.; Han, W.X.; Liu, X.J. Decoupling of nitrogen and phosphorus in dominant grass species in response to long-term nitrogen addition in an Alpine Grassland in Central Asia. Plant Ecol. 2021, 222, 261–274. [Google Scholar] [CrossRef]
- Liu, X.; Yin, C.M.; Xiang, L.; Jiang, W.T.; Xu, S.Z.; Mao, Z.Q. Transcription strategies related to photosynthesis and nitrogen metabolism of wheat in response to nitrogen deficiency. BMC Plant Biol. 2020, 20, 448. [Google Scholar] [CrossRef]
- Pan, X.Y.; Wang, P.F.; Wei, X.W.; Zhang, J.X.; Xu, B.C.; Chen, Y.L.; Wei, G.H.; Wang, Z. Exploring root system architecture and anatomical variability in alfalfa (Medicago sativa L.) seedlings. BMC Plant Biol. 2023, 23, 449. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.X.; Zhang, H.Y.; Wang, Q.M.; Xie, B.T.; Zhang, L.M. Regulation of root development in nitrogen-susceptible and nitrogen-tolerant sweet potato cultivars under different nitrogen and soil moisture conditions. BMC Plant Biol. 2023, 23, 454. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wang, P.; Xia, R.X. Effects of mycorrhizal fungi on phytate-phosphorus utilization in trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Acta Physiol. Plant 2014, 36, 1023–1032. [Google Scholar] [CrossRef]
- Zhang, C.F.; van der Heijden, M.G.A.; Dodds, B.K.; Nguyen, T.B.; Spooren, J.; Valzano-Held, A.; Cosme, M.; Berendsen, R.L. A tripartite bacterial-fungal-plant symbiosis in the mycorrhiza-shaped microbiome drives plant growth and mycorrhization. Microbiome 2024, 12, 13. [Google Scholar] [CrossRef]
- Basiru, S.; Mhand, K.A.S.; Hijri, M. Disentangling arbuscular mycorrhizal fungi and bacteria at the soil-root interface. Mycorrhiza 2023, 33, 119–137. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Jin, Y.; Gu, J.C. Effects of long-term nitrogen addition on soil fungal communities in two temperate plantations with different mycorrhizal associations. Appl. Soil Ecol. 2021, 168, 104111. [Google Scholar] [CrossRef]
- Duan, S.L.; Yan, W.H.; Feng, G.; Zhang, L. Carbon-phosphorus reciprocal mechanism for plants to acquire nutrients through the root/mycorrhizal pathway. J. Plant Nutr. Fertil. 2023, 29, 1160–1167. [Google Scholar] [CrossRef]
- Ge, S.; Jiang, X.; Wang, L.; Yu, J.; Zhou, Y. Recent advances in the role and mechanism of arbuscular mycorrhiza-induced improvement of abiotic stress tolerance in horticultural plants. Acta Hortic. Sin. 2020, 47, 1752–1776. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Wu, S.L.; Fu, W.; Rillig, M.C.; Chen, B.D.D.; Zhu, Y.G.; Huang, L.B. Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi—An updated conceptual framework. New Phytol. 2024, 242, 1417–1425. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Azcon, R.; Rodriguez, R.; Amora-Lazcano, E.; Ambrosano, E. Uptake and metabolism of nitrate in mycorrhizal plants as affected by water availability and N concentration in soil. Eur. J. Soil Sci. 2008, 59, 131–138. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, I.; Martinez-Garcia, L.B.; Barel, J.M.; Martens, H.; Snoek, L.B.; Hontoria, C.; De Deyn, G.B. Cover crop identity determines root fungal community and arbuscular mycorrhza colonization in following main crops. Eur. J. Soil Sci. 2023, 74, e13427. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, J.; Kou, L.; Ma, Z.; Lyu, S.; Wei, J.; Wang, H.; Wen, X. Phosphorus acquisition strategies of arbuscular mycorrhizal and ectomycorrhizal trees in subtropical plantations. Eur. J. Soil Sci. 2022, 73, e13303. [Google Scholar] [CrossRef]
- Khaekhum, S.; Ekprasert, J.; Suebrasri, T.; Seemakram, W.; Mongkolthanaruk, W.; Riddech, N.; Jogloy, S.; Boonlue, S. Co-Inoculation of an Endophytic and Arbuscular Mycorrhizal Fungus Improve Growth and Yield of Helianthus tuberosus L. under Field Condition. J. Fungi 2021, 7, 976. [Google Scholar] [CrossRef]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizas: Physiology and Function; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bender, S.F.; Conen, F.; Van der Heijden, M.G.A. Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol. Biochem. 2015, 80, 283–292. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Liu, R.J.; Shen, C.Y.; Li, H.F.; Qiu, W.F. Induction of pathogenesis-related proteins in cotton seedlings by vam fungi and verticillium dahliae. Acta Phytopathol. Sin. 1993, 2, 162. [Google Scholar] [CrossRef]
- Feng, G.; Bai, D.S.; Yang, M.Q.; Li, X.L.; Zhang, F.S.; Li, S.X. Effects of two Glomus mosseae isolates on cotton salinity tolerance. Acta Ecol. Sin. 2001, 2, 259–264. [Google Scholar]
- Wang, C.C.; Wang, Z.P.; Li, M.Y.; Wu, Y.H.; Qin, Z.Y.; Jin, H.R. Effects of different exogenous nitrogen on nitrogen and phosphorus transport in cotton infected by Rhizophagus irregularis. Ind. Microbiol. 2018, 48, 12–16. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Wang, X.Y.; Zhu, C.L.; Han, J.B.; Xu, Y.; Jin, H.R. Effects of Light Intensity and Nitrogen Forms on Carbon and Nitrogen Metabolism in Cotton Arbuscular Mycorrhizal Fungi Symbionts. J. Microbiol. 2024, 44, 32–39. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Q.; Liu, Z.; Wang, H.; Xiong, J.; Lei, Y.; Sun, Y. Diversity of arbuscular mycorrhizal fungi in cotton rhizosphere soil in Shihezi and surrounding areas, Xinjiang. Cotton Sci. 2022, 34, 69–78. [Google Scholar] [CrossRef]
- Wu, C.; Bi, Y.L.; Zhu, W.B. Is the amount of water transported by arbuscular mycorrhizal fungal hyphae negligible? Insights from a compartmentalized experimental study. Plant Soil 2024, 499, 537–552. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Xiao, J.; Yuan, Y.S.; Zhao, C.Z.; Liu, Q.; Yin, H.J. Mycelium- and root-derived C inputs differ in their impacts on soil organic C pools and decomposition in forests. Soil Biol. Biochem. 2018, 123, 257–265. [Google Scholar] [CrossRef]
- Totsche, K. 2—Quality Control and Quality Assurance in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995. [Google Scholar]
- Dickson, S.; Smith, S.E. Evaluation of Vesicular-Arbuscular Mycorrhizal Colonisation by Staining; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Chen, J.; Liu, Y.; Chu, C. Genetic improvement toward nitrogen-use efficiency in rice: Lessons and perspectives. Mol. Plant 2023, 16, 64–74. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, K.; Song, W.; Zhong, N.; Wu, Y.; Fu, X. Improving Crop Nitrogen Use Efficiency Toward Sustainable Green Revolution. Annu. Rev. Plant Biol. 2022, 73, 523–551. [Google Scholar] [CrossRef]
- Quan, Z.; Li, S.L.; Zhang, X.; Zhu, F.F.; Li, P.P.; Sheng, R.; Chen, X.; Zhang, L.M.; He, J.Z.; Wei, W.X.; et al. Fertilizer nitrogen use efficiency and fates in maize cropping systems across China: Field 15N tracer studies. Soil Till Res. 2020, 197, 104498. [Google Scholar] [CrossRef]
- Bender, S.F.; Plantenga, F.; Neftel, A.; Jocher, M.; Oberholzer, H.R.; Köhl, L.; Giles, M.; Daniell, T.J.; van der Heijden, M.G.A. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. ISME J. 2014, 8, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Zhou, J.; Rengel, Z.; George, T.S.; Feng, G. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: Processes and ecological functions. Plant Soil 2022, 481, 1–22. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Li, D.; Wang, G.; Bei, S.; Ju, X.; An, R.; Li, L.; Kuyper, T.W.; Christie, P.; et al. Mycorrhiza-mediated recruitment of complete denitrifying Pseudomonas reduces N2O emissions from soil. Microbiome 2023, 11, 45. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.R.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, aba3756. [Google Scholar] [CrossRef]
- Wang, R.Z.; Dijkstra, F.A.; Han, X.G.; Jiang, Y. Root nitrogen reallocation: What makes it matter? Trends Plant Sci. 2024, 29, 1077–1088. [Google Scholar] [CrossRef]
- Blanke, V.; Renker, C.; Wagner, M.; Füllner, K.; Held, M.; Kuhn, A.J.; Buscot, F. Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol. 2005, 166, 981–992. [Google Scholar] [CrossRef]
- Kemppainen, M.; Duplessis, S.; Martin, F.; Pardo, A.G. RNA silencing in the model mycorrhizal fungus Laccaria bicolor: Gene knock-down of nitrate reductase results in inhibition of symbiosis with Populus. Environ. Microbiol. 2009, 11, 1878–1896. [Google Scholar] [CrossRef]
- Eaton, G.K.; Ayres, M.P. Plasticity and constraint in growth and protein mineralization of ectomycorrhizal fungi under simulated nitrogen deposition. Mycologia 2002, 94, 921–932. [Google Scholar] [CrossRef][Green Version]
- Rineau, F.; Shah, F.; Smits, M.M.; Persson, P.; Johansson, T.; Carleer, R.; Troein, C.; Tunlid, A. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 2013, 7, 2010–2022. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J. Provenance differences in functional traits and N: P stoichiometry of the leaves and roots of Pinus tabulaeformis seedlings under N addition. Glob. Ecol. Conserv. 2020, 21, e00826. [Google Scholar] [CrossRef]
- Pu, Z.T.; Zhang, L.; Zhang, C.; Wang, H.; Wang, X.X. Research Progress of Arbuscular Mycorrhizal Fungi and Plant Symbiosis Affecting Plant Water Regime. Soil 2022, 54, 882–889. [Google Scholar] [CrossRef]
- Högberg, M.N.; Briones, M.J.I.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Thornton, B.; Hurry, V.; Linder, S.; Näsholm, T.; et al. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187, 485–493. [Google Scholar] [CrossRef]
- Xue, J.H.; Mo, J.M.; Li, J.; Fang, Y.T.; Li, D.J. Effects of nitrogen deposition on ectomycorrhizal fungi. Acta Ecol. Sin. 2004, 8, 1789–1796. [Google Scholar]
- Yin, L.M.; Xiao, W.; Dijkstra, F.A.; Zhu, B.; Wang, P.; Cheng, W.X. Linking absorptive roots and their functional traits with rhizosphere priming of tree species. Soil Biol. Biochem. 2020, 150, 107997. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Anckaert, A.; Declerck, S.; Poussart, L.A.; Lambert, S.; Helmus, C.; Boubsi, F.; Steels, S.; Argüelles-Arias, A.; Calonne-Salmon, M.; Ongena, M. The biology and chemistry of a mutualism between a soil bacterium and a mycorrhizal fungus. Curr. Biol. 2024, 34, 4934–4950. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Huo, J.; Wang, S.; Xu, L.; Chen, X.; Qiu, Y.; Liu, M. The balance between arbuscular mycorrhizal fungal diversity and plant growth benefits from optimizing nitrogen inputs in agroecosystems. Appl. Soil Ecol. 2023, 187, 104834. [Google Scholar] [CrossRef]
- Khanam, D.; Mridha, M.A.U.; Solaiman, A.R.M.; Hossain, T. Effect of edaphic factors on root colonization and spore population of arbuscular mycorrhizal fungi. Bull. Inst. Trop. Agric. Kyushu Univ. 2006, 29, 97–104. [Google Scholar] [CrossRef]
- Babalola, B.J.; Li, J.; Willing, C.E.; Zheng, Y.; Wang, Y.L.; Gan, H.Y.; Li, X.C.; Wang, C.; Adams, C.A.; Gao, C.; et al. Nitrogen fertilisation disrupts the temporal dynamics of arbuscular mycorrhizal fungal hyphae but not spore density and community composition in a wheat field. New Phytol. 2022, 234, 2057–2072. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Prieto, I.; Vargas, R.; Allen, M.F. Changes in soil hyphal abundance and viability can alter the patterns of hydraulic redistribution by plant roots. Plant Soil 2012, 355, 63–73. [Google Scholar] [CrossRef]
- Huang, S.; Liu, W.X.; Yang, S.; Yang, L.; Peng, Z.Y.; Deng, M.F.; Xu, S.; Zhang, B.B.; Ahirwal, J.; Liu, L.L. Plant carbon inputs through shoot, root, and mycorrhizal pathways affect soil organic carbon turnover differently. Soil Biol. Biochem. 2021, 160, 108322. [Google Scholar] [CrossRef]
- Nurmanov, Y.T.; Chernenok, V.G.; Kuzdanova, R.S. Potato in response to nitrogen nutrition regime and nitrogen fertilization. Field Crops Res. 2019, 231, 115–121. [Google Scholar] [CrossRef]
- Liu, Z.X.; Gao, F.; Yang, J.Q.; Zhen, X.Y.; Li, Y.; Zhao, J.H.; Li, J.R.; Qian, B.C.; Yang, D.Q.; Li, X.D. Photosynthetic Characteristics and Uptake and Translocation of Nitrogen in Peanut in a Wheat-Peanut Rotation System Under Different Fertilizer Management Regimes. Front. Plant Sci. 2019, 10, 86. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, S.; Maghraoui, T.; Hafidi, M.; Sanguin, H.; Oufdou, K.; Prin, Y.; Duponnois, R.; Galiana, A. Enhanced transfer of biologically fixed N from faba bean to intercropped wheat through mycorrhizal symbiosis. Appl. Soil Ecol. 2016, 107, 91–98. [Google Scholar] [CrossRef]
- Jarratt-Barnham, E.; Zarrabian, D.; Oldroyd, G.E.D. Symbiotic regulation: How plants seek salvation in starvation. Curr. Biol. 2022, 32, R46–R48. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Jiang, L.; Liu, W.; Wang, X.; Li, S.; Yang, S.; Wang, B. Ecosystem scale trade-off in nitrogen acquisition pathways. Nat. Ecol. Evol. 2018, 2, 1724–1734. [Google Scholar] [CrossRef]
- Ju, X.T.; Liu, X.J.; Zhang, F.S. Study on Effect of Nitrogen Fertilizer and Nitrogen Balance in Winter Wheat and Summer Maize Rotation System. Sci. Agric. Sin. 2002, 11, 1361–1368. [Google Scholar] [CrossRef]
- Xue, J.W.; Guo, L.L.; Li, L.L.; Zhang, Z.W.; Huang, M.; Cai, J.; Wang, X.; Zhong, Y.X.; Dai, T.B.; Jiang, D.; et al. Effects of arbuscular mycorrhizal fungi on uptake, partitioning and use efficiency of nitrogen in wheat. Field Crops Res. 2024, 306, 109244. [Google Scholar] [CrossRef]
- Dong, H.Z.; Kong, X.Q.; Li, W.J.; Tang, W.; Zhang, D.M. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crops Res. 2010, 119, 106–113. [Google Scholar] [CrossRef]
- Chen, L.Y.; Liu, L.; Mao, C.; Qin, S.Q.; Wang, J.; Liu, F.T.; Blagodatsky, S.; Yang, G.B.; Zhang, Q.W.; Zhang, D.Y.; et al. Nitrogen availability regulates topsoil carbon dynamics after permafrost thaw by altering microbial metabolic efficiency. Nat. Commun. 2018, 9, 3951. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Change Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Hurek, T.; Reinhold-Hurek, B. Effect of N-fertilization, plant genotype and environmental conditions on nifH gene pools in roots of rice. Environ. Microbiol. 2003, 5, 1009–1015. [Google Scholar] [CrossRef]
- Barron, A.R.; Wurzburger, N.; Bellenger, J.P.; Wright, S.J.; Kraepiel, A.M.L.; Hedin, L.O. Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nat. Geosci. 2009, 2, 42–45. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Song, W.F.; Wen, S.L.; Wang, B.R.; Zhu, C.Q.; Shen, R.F. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 2017, 113, 240–249. [Google Scholar] [CrossRef]
- Feng, M.M.; Adams, J.M.; Fan, K.K.; Shi, Y.; Sun, R.B.; Wang, D.Z.; Guo, X.S.; Chu, H.Y. Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol. Biochem. 2018, 126, 151–158. [Google Scholar] [CrossRef]
- Yue, G.H.; Huang, Y.C.; Gao, X.T.; Mei, X.; Liu, Y.A.; Pan, B.R. Effects of Different Sweet Maize/Vegetable Soybean Interplanting Systems on Rhizosphere Nitrogen-Fixing Bacteria and Arbuscular Mycorrhizal Fungi Communities. J. Maize Sci. 2024, 32, 67–77. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Enquist, B.J. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Zhao, C.T.; Lin, Q.H.; Tian, D.; Ji, C.J.; Shen, H.H.; Fan, D.Y.; Wang, X.P.; Fang, J.Y. Nitrogen addition promotes conservative resource-use strategies via aggravating phosphorus limitation of evergreen trees in subtropical forest. Sci. Total Environ. 2023, 889, 164047. [Google Scholar] [CrossRef]
- Zhao, D.L.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Fu, W.; Wu, H.; Zhao, A.H.; Hao, Z.P.; Chen, B.D. Ecological impacts of nitrogen deposition on terrestrial ecosystems: Research progresses and prospects. Chin. J. Plant Ecol. 2020, 44, 475–493. [Google Scholar] [CrossRef]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberte, E. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Robinson, D.; Davidson, H.; Trinder, C.; Brooker, R. Root-shoot growth responses during interspecific competition quantified using allometric modelling. Ann. Bot-Lond. 2010, 106, 921–926. [Google Scholar] [CrossRef]
- Cornejo, N.S.; Hertel, D.; Becker, J.N.; Hemp, A.; Leuschner, C. Biomass, Morphology, and Dynamics of the Fine Root System Across a 3000-M Elevation Gradient on Mt. Kilimanjaro. Front. Plant Sci. 2020, 11, 13. [Google Scholar] [CrossRef]
- Feng, W.N.; Sun, M.; Shao, J.J.; Pang, C.Y.; Zheng, C.S.; Dong, H.L.; Li, P.C. Optimizing nitrogen management to reconcile cotton yield and yield stability: A three-year field study. Ind. Crop Prod. 2024, 218, 118986. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, H.; Li, W.; Zhao, Q.; Dai, J.; Tian, L.; Dong, H. Effects of reduced nitrogen rate on cotton yield and nitrogen use efficiency as mediated by application mode or plant density. Field Crops Res. 2018, 218, 150–157. [Google Scholar] [CrossRef]
- Song, X.; Huang, Y.; Yuan, Y.; Shahbaz, A.T.; Biangkham, S.; Yang, G. Cotton N rate could be reduced further under the planting model of late sowing and high-density in the Yangtze River valley. J. Cotton Res. 2020, 3, 28. [Google Scholar] [CrossRef]
- Hou, J.W.; McCormack, M.L.; Reich, P.B.; Sun, T.; Phillips, R.P.; Lambers, H.; Chen, H.Y.H.; Ding, Y.Y.; Comas, L.H.; Valverde-Barrantes, O.J.; et al. Linking fine root lifespan to root chemical and morphological traits-A global analysis. Proc. Natl. Acad. Sci. USA 2024, 121, e2320623121. [Google Scholar] [CrossRef]
- Pierick, K.; Leuschner, C.; Homeier, J. Topography as a factor driving small-scale variation in tree fine root traits and root functional diversity in a species-rich tropical montane forest. New Phytol. 2021, 230, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Lin, G.G.; Liu, B.; Mao, R. Linking leaf nutrient resorption and litter decomposition to plant mycorrhizal associations in boreal peatlands. Plant Soil 2020, 448, 413–424. [Google Scholar] [CrossRef]
- Jiang, J.; Moore, J.A.M.; Priyadarshi, A.; Classen, A.T. Plant-mycorrhizal interactions mediate plant community coexistence by altering resource demand. Ecology 2017, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, aba1223. [Google Scholar] [CrossRef] [PubMed]
- Reckling, M.; Döring, T.F.; Bergkvist, G.; Stoddard, F.L.; Watson, C.A.; Seddig, S.; Chmielewski, F.M.; Bachinger, J. Grain legume yields are as stable as other spring crops in long-term experiments across northern Europe. Agron. Sustain. Dev. 2018, 38, 63. [Google Scholar] [CrossRef]
- Zhang, L.C.; Yuan, J.; Zhang, M.Q.; Zhang, Y.C.; Wang, L.M.; Li, J. Long effects of crop rotation and fertilization on crop yield stability in southeast China. Sci. Rep. 2022, 12, 14234. [Google Scholar] [CrossRef]
- Zhang, D.M.; Li, W.J.; Xin, C.S.; Tang, W.; Eneji, A.E.; Dong, H.Z. Lint yield and nitrogen use efficiency of field-grown cotton vary with soil salinity and nitrogen application rate. Field Crops Res. 2012, 138, 63–70. [Google Scholar] [CrossRef]
- Gabhane, V.V.; Ramteke, P.; Chary, G.R.; Patode, R.S.; Ganvir, M.M.; Chorey, A.; Tupe, A.R. Effects of long-term nutrient management in semi-arid Vertisols on soil quality and crop productivity in a cotton-greengram intercropping system. Field Crops Res. 2023, 303, 109115. [Google Scholar] [CrossRef]
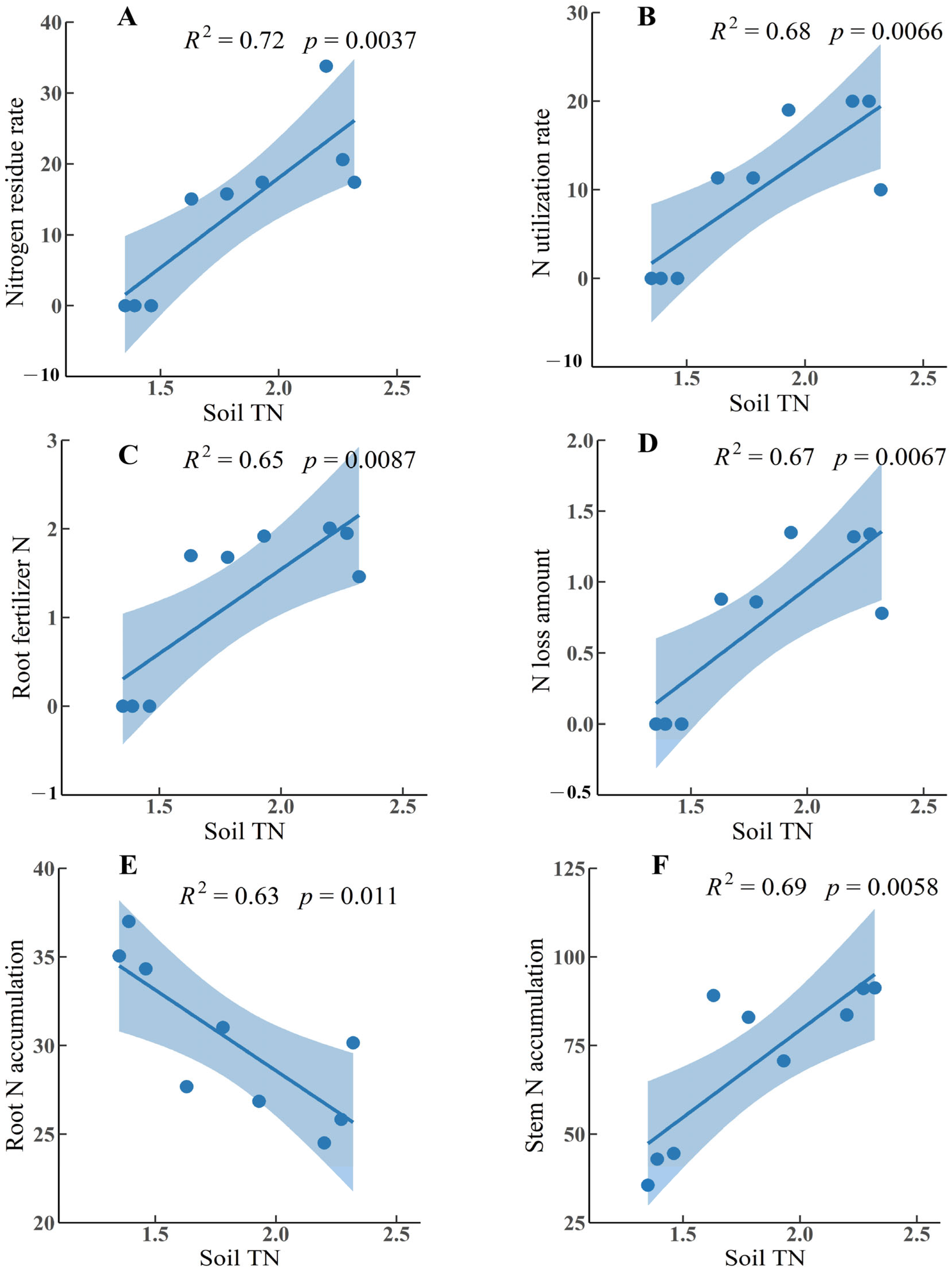
| Assessment Item | Association and Significance | Association Ranking |
|---|---|---|
| Stem N accumulation | 0.96 ** | 1 |
| Root fertilizer N | 0.94 ** | 2 |
| Root-to-shoot ratio | −0.90 ** | 3 |
| N application rates | 0.89 ** | 4 |
| N loss amount | 0.86 ** | 5 |
| N residual rate | 0.84 ** | 6 |
| N utilization rate | 0.82 ** | 7 |
| Root N accumulation | −0.80 ** | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; He, Y.; Shen, Z.; Liu, M.; Zhang, W.; Pu, X. Reducing Nitrogen Input Increases the Efficacy of Soil Nitrogen Utilization by Regulating Cotton–Arbuscular Mycorrhizal Fungi–Soil Nitrogen Interactions. Nitrogen 2025, 6, 55. https://doi.org/10.3390/nitrogen6030055
Wang H, He Y, Shen Z, Liu M, Zhang W, Pu X. Reducing Nitrogen Input Increases the Efficacy of Soil Nitrogen Utilization by Regulating Cotton–Arbuscular Mycorrhizal Fungi–Soil Nitrogen Interactions. Nitrogen. 2025; 6(3):55. https://doi.org/10.3390/nitrogen6030055
Chicago/Turabian StyleWang, Hushan, Yunzhu He, Zihui Shen, Mengjuan Liu, Wangfeng Zhang, and Xiaozhen Pu. 2025. "Reducing Nitrogen Input Increases the Efficacy of Soil Nitrogen Utilization by Regulating Cotton–Arbuscular Mycorrhizal Fungi–Soil Nitrogen Interactions" Nitrogen 6, no. 3: 55. https://doi.org/10.3390/nitrogen6030055
APA StyleWang, H., He, Y., Shen, Z., Liu, M., Zhang, W., & Pu, X. (2025). Reducing Nitrogen Input Increases the Efficacy of Soil Nitrogen Utilization by Regulating Cotton–Arbuscular Mycorrhizal Fungi–Soil Nitrogen Interactions. Nitrogen, 6(3), 55. https://doi.org/10.3390/nitrogen6030055






