Abstract
Alley cropping, an agroforestry system that integrates trees and arable crops, holds the potential to improve both crop yields and soil health. It has been found to be effective for upland crops in many countries of the world. However, the utilization of alley cropping to improve soil health in the terrace ecosystem of Bangladesh is poorly understood. Therefore, this study was undertaken to assess the changes in soil biochemical properties and quantify the cabbage yield under three alley widths of Leucaena leucocephala (3.0, 4.5, and 6.0 m size) and five nitrogen (N) levels [0, 40, 80, 120, and 160 kg N ha−1 (0, 25, 50, 75, and 100% of recommended N rates, respectively) with the addition of pruned materials of L. leucocephala (Ipil-ipil)]. The field experiment was conducted following a split-plot design, where alley width was considered as the main-plot factor and N rate as the sub-plot factor. Within each main plot, the five N rates were replicated thrice. Control plots with similar N doses were applied accordingly without addition of pruned materials to compare the results with alley cropping. Data were collected on the biochemical properties of the soil [soil pH, organic carbon (C), total N, available phosphorus (P), exchangeable potassium (K), microbial biomass C, and biomass N] and the yield of cabbage quantified [edible head weight (kg plant−1) and head yield (t ha−1)] under different alley widths and control. Findings revealed that organic C, total N, available P, exchangeable K, microbial biomass C, and biomass N in the topsoil exhibited maximum values in the L. leucocephala-based alley plot, which is proved to be a possible solution of restoration of degradable land. Additionally, L. leucocephala-based alley cropping improved the soil pH, indicating a potential avenue for more-sustainable land management practices. Results also showed that alley widths and N rates have significant effects on cabbage (Brassica oleracea L. var. capitata) yield. Alley width of 6.0 m along with 100% N provided the highest cabbage yield followed by 75% N in 6.0 m alley, and the control with 100%. The wider alley minimizes tree–crop competition, allowing for optimal cabbage production. These aforementioned results suggest that alley cropping with L. leucocephala is a promising approach to enhance soil fertility and crop productivity in the terrace ecosystem of Bangladesh.
1. Introduction
About 169.83 million people of Bangladesh rely on 8.09 million hectares (Mha) of cultivable land, which is inadequate to meet the food demand of this burgeoning population [1,2]. Moreover, agriculture faces challenges of climate change, soil health degradation, and environmental pollution [3,4]. Additionally, soil contains less than 1.5% organic matter (OM), while a productive soil should hold at least 2.5% OM [5]. Cereal-based intensive agriculture for achieving higher cropping intensity, accelerated decomposition of soil OM, sparse use of organic fertilizers, and inadequate green manure application has contributed to poor soil heath and low productivity. Furthermore, about 6 Mha of land area in Bangladesh is degraded and 0.69 Mha of agricultural land diminishes annually due to industrialization, urbanization, and other development activities [6,7]. Despite the difficulties, it is necessary to adopt sustainable agricultural technologies to reclaim the degraded lands and make them suitable for economic crop production.
There are a number of viable options to revitalize our degraded land, viz., organic farming, ecological farming, agroforestry systems, and regenerative agriculture [8,9]. In Bangladesh, the significance of sustainable crop production and enhancing soil fertility has greatly increased in recent years. Therefore, the transformation of degraded land to productive land needs sustainable farming practices like alley cropping. Alley cropping is an economically viable approach that involves the integration of hedges with leguminous species alongside the crop plants [10,11]. To reduce competition for light, water, and nutrients between trees and companion crops, these hedges periodically or continuously need to be pruned and added to soil. The decomposition of the pruned biomass improves the soil physicochemical and biological characteristics. According to Hombegowda et al. [12], alley cropping helps to regenerate degraded topsoil, leads to more stable aggregates, and provides a favorable soil health for crop cultivation. Moreover, alley cropping shrinks soil erosion, improves the water and nutrient holding capacity of soil, and minimizes nutrient leaching. Different tree and shrub species, viz., Faidherbia albida, Cajanus cajan, Senna siamea, Gliricidia sepium, Indigofera tysmanii, Leucaena leucocephala, Cassia siamea, Allanblackia floribunda, and Populus deltoides, etc., are used for the alley cropping system [13,14,15,16,17,18]. L. leucocephala is one of the most suitable species for alley cropping and has been extensively grown across different regions of the world [19,20].
L. leucocephala is considered a fast-growing species with multiple uses such as biomass production, source of nitrogen, being enriched in organic matter content, reducing soil erosion, improving water quality, and supplying high-quality fodder for livestock [21,22,23,24,25]. Notably, the leaves of L. leucocephala are rich in carbon and minerals containing 35.9–42.5% C, 3.8–4.2% N, 0.14–0.16% P, 0.8–2.36% Ca, and 0.19–0.4% Mg [26,27]. In addition, L. leucocephala as a N2-fixing tree species can fix 150–250 kg N ha−1 year−1 from the atmosphere; therefore, incorporating the biomass with soil can thus increase the availability of N to the crops [28,29]. Several studies reported that using Leucaena in the alley cropping improved the growth and yield of associated crops [30,31,32,33]. The grain yield, biological yield, and harvest index of wheat were increased by 29%, 12%, and 16%, respectively, when alley-cropped with Leucaena compared to the absence of alley biomass [9]. Moreover, soil physical and chemical properties were augmented under L. leucocephala−based alley cropping systems [3,13,34]. Fernández et al. [35] reported that organic matter, dehydrogenase activity, and soil nitrogen increased by 41%, 98%, and 35%, respectively, after 11 years of cultivation with Leucaena. Furthermore, it is an ideal fast-growing tree species that provides timber, shade, green manure, and erosion control [36,37]. The species also contributes to reduce nitrogen fertilization and greenhouse gas emissions, as well as to improve carbon sequestration [38,39]. Thus, L. leucocephala has appeared as a promising candidate for promoting healthy soil and sustainable agriculture, particularly through improving soil pH, organic carbon, soil primary nutrients (N, P, K), and microbial biomass. The incorporation of Leucaena-based alley cropping against conventional farming practices might enhance the fertility status of intensively cultivated soils, reduce the reliance on inorganic N fertilizer, and improve the overall crop productivity. Therefore, this study focuses on how the alley cropping system improves the low-fertility and degraded soil in the terrace ecosystem of Bangladesh and potentiates a prospect to minimize the usage of inorganic fertilizer to shift to more organic crop production.
In Bangladesh, winter is a suitable season for most of the vegetable production as 70% of the vegetable grow in this season [40]. Cultivation of different types of winter vegetables in alleys of L. leucocephala might be a better option to restore degraded terrace soils of the study area. Among the various winter leafy vegetables grown in Bangladesh, cabbage (Brassica oleracea var. capitata L.), which is rich in vitamins B and C, minerals (potassium and calcium), and dietary fibers holds noteworthy importance [41,42,43]. Therefore, the present study was conducted to examine the changes in soil properties in the alley cropping system and to evaluate the productivity of cabbage grown under different alley widths at varying N levels. The findings of the study might help improve soil health and ensure sustainable agriculture in the silty clay loam terrace soil of the Madhupur Tract of Bangladesh.
2. Materials and Methods
2.1. Description of the Experimental Site
The experiment was performed in the research field of the Department of Agroforestry and Environment, Gazipur Agricultural University, Gazipur 1706, Bangladesh. The soil of the study site was classified as shallow red-brown terrace soil under the order Inceptisols according to the United States Department of Agriculture (USDA) system [44]. Locally, the site belongs to the agroecological zone (AEZ) Madhupur Tract (AEZ 28). The overall soil fertility is low, with medium level of organic carbon (12.7–14.4 g kg−1), low level of total N (1.04–1.11 g kg−1), low to medium available P (9.66–11.99 mg kg−1), medium level of exchangeable K (0.26–0.30 c-mol (+) kg−1), and slightly acidic to acidic (pH 5.48–5.67) nature. During the experimental period, the monthly mean temperatures were recorded as 26.81 °C, 23.22 °C, 19.30 °C, 19.11 °C, and 21.08 °C from October to December 2018, and January to February 2019, respectively. Data on monthly rainfall, mean monthly air temperature, relative humidity, and actual evaporation were collected from the meteorological station of Gazipur Agricultural University and presented in Table 1.

Table 1.
Meteorological data during the experimental period from October 2018 to February 2019.
2.2. Incorporation of L. Leucocephala Biomass into Soil
Following an alley cropping design (3.0, 4.5, and 6.0 m alley), seedlings of L. leucocephala were planted in the experimental field on September 2005, maintaining a 50 cm distance from tree to tree. Therefore, a thirteen-year-old L. leucocephala alley field was used for the experiment. L. leucocephala trees were pruned twice during field preparation; the first pruning was performed during the 3rd week of September 2018, while the second pruning was performed during the 1st week of November 2018. Disc plowing and rotavator were used to mix fresh pruned materials (leaves and branches) into the soil. Consequently, the 3.0 m alley produced the highest amount of fresh pruned materials of 10.19 t ha−1, while the 4.5 and 6.0 m alleys produced 6.61 and 4.99 t ha−1 pruned material, respectively (Table 2). The pruned materials were collected and chemically analyzed to determine the concentrations of nitrogen, phosphorus, and potassium in the provided pruned materials, which were 2.31, 0.12, and 1.71%, respectively. From these concentrations, the amount of nutrients supplied to the soil was calculated and is presented in Table 2.

Table 2.
Fresh weight of pruned materials (leaves and branches) obtained from different alley widths of L. leucocephala and nutrients (N, P, and K) supplied to the soil from added pruned materials.
2.3. Experimental Design and Treatments
The experiment was undertaken according to a split-plot design: alley widths as the main-plot factor and N levels as the sub-plot factor. Alley widths were considered as the main plot treatment reflecting between-subject variability (variability among alley widths), and N levels were considered as sub-plot treatment representing within-subject variability (variability within each alley width due to N levels) to compare the interactions. Three alley widths (3.0, 4.5, and 6.0 m) were fixed in main plots and five different doses of N [N0 (0 kg N ha−1), N25 (40 kg N ha−1), N50 (80 kg N ha−1), N75 (120 kg N ha−1), and N100 (160 kg N ha−1)] were distributed to sub-plots within each main plot. Each N level was replicated thrice in 3.0, 4.5, and 6.0 alleys individually. The plot length for each N dose was 5.0 m; so, the total area of each N dose plot was 6.0 × 5.0 m, 4.5 × 5.0 m, and 3.0 × 5.0 m for 6.0, 4.5, and 3.0 m alley widths, respectively. The plots without trees (open field) were considered as the control, where five N doses were applied accordingly without pruned material. A promising commercial cabbage variety ATLAS 70 (2–3 kg/pieces) was employed as a test crop in the experiment.
2.4. Field Preparation, Transplanting, Fertilizer Application and Intercultural Operations
After completion of land preparation, 36-day-old cabbage seedlings were transplanted on 19 November 2018, maintaining a spacing of 60 × 60 cm between each plant. Fertilizers were applied according to the recommended doses where urea [CO(NH2)2; 46% N], triple superphosphate (TSP) [Ca(H2PO4)2.H2O; 20% P], and muriate of potash (MoP) [KCl; 50% K] were applied to provide N, P, and K nutrients at the rates of 160 kg N ha−1, 50 kg P ha−1, and 150 kg K ha−1, respectively [45]. Specifically, 160 kg N ha−1 was considered as 100% of recommended N dose, and from this rate, 25% (40 kg N ha−1), 50% (80 kg N ha−1), and 75% (120 kg N ha−1) of recommended N fertilizer were calculated and applied according to the treatments in all of the three alley widths and control. During the final land preparation, total TSP and half of the MoP were applied, and the remaining half of the MoP and total urea were applied in three equal installments, e.g., 10 days after transplanting (DAT), 25 DAT, and at 60 DAT. We provided four irrigations during the experiment (20, 45, 65, and 85 DAT) using a flexible hose pipe to facilitate the proper establishment of cabbage plants and to prevent any moisture-related stress. Hand weeding was performed on a regular basis to minimize the competition for resources between weeds and crops.
2.5. Soil Sampling, Preparation and Analyses
Both the initial and post-harvest soil samples were collected on 18 September 2018 and 28 February 2019, respectively, at depth of 0–15 cm. To comply with the specified number of doses for each unit of alley width, three soil samples were collected, dried in the air, pulverized, and sifted using a 10-mesh sieve. Eventually, the ground samples were blended together to create a single composite sample which was stored in the clean plastic bag for subsequent analyses. The soil pH, organic carbon, total nitrogen, available phosphorus, exchangeable potassium, biomass carbon, and biomass nitrogen were determined. The following protocols were used to conduct the aforementioned analyses.
2.5.1. Soil pH
Soil pH was measured by a glass electrode pH meter using a soil–water suspension ratio of 1:5 [46]. Ten grams of air-dried soil was taken in a conical flask; then, 50 mL water was added into it, and it was shaken for 1 min in a vortex mixture. Finally, soil pH was measured using the pH meter.
2.5.2. Organic C
Organic carbon was estimated by Walkley and Black’s wet oxidation method as described by FAO [47]. Briefly, 1 g of oven-dried soil was taken in a 500 mL Erlenmeyer flask; then, 10 mL 1N K2CR2O7 and 20 mL concentrated H2SO4 were added, and immediately, the flask was swirled to mix the soil and reagents. To avoid heat loss, the flask was kept in an insulated sheet for 30 min in a fume hood, and then, 200 mL water was added to the flask. After that, 3–4 drops of the o-phenanthroline indicator were added, and the solution was titrated against 0.5 M ammonium ferrous sulfate solution.
2.5.3. Total N
The total nitrogen concentration of soil was determined by the micro-Kjeldahl method [48]. A total of 1 g of air-dried soil sample was used following digestion of concentrated (98%) 10 mL H2SO4 using a mixture of 3 g CuSO4 and K2SO4 (1:9) as catalyst and steam distillation with 40% NaOH solution. The ammonia evolved was collected in a beaker containing 4% H3BO3 with an indicator composed of 95% alcohol, methyl red, and bromocresol green and finally titrated against 0.02 N H2SO4 to measure the amount of total N.
2.5.4. Available P
The authors used Bray and Kurtz [49] methodology to measure the available phosphorus concentration of soil. Firstly, 3.5 g air-dried soil was taken in a plastic pot, and 25 mL NH4F was added as an extracting solution, and this was shaken for 5 min in a rotary shaker. Filtering was performed in a conical flask, and 5 mL soil extract was taken in a 50 mL volumetric flask. Then, 10 mL ammonium molybdate solution was added and volumed up to the mark level. The absorbance reading was taken at 890 nm wavelength by using a spectrophotometer (PD-303UV, APEL Co. Ltd., Kawaguchi, Japan).
2.5.5. Exchangeable K
The exchangeable K concentration in soil was measured using an atomic absorption spectrophotometer (Hitachi 170-30 AAS, Tokyo, Japan) at 766.5 nm wavelength following standard laboratory protocol [50]. Briefly, 25 mL solution of 1M ammonium acetate was added into 2.5 g air-dried soil as an extracting solution, then shaken for 30 min and allowed to stay overnight. The supernatant was repeatedly collected to allow the exchangeable cations to be exchanged from the soil particles with NH4+ cations. The absorbance was measured at a wavelength of 766.5 nm.
2.5.6. Fumigation of Soil Samples for the Measurement of Biomass C and Biomass N
Soil fumigation was performed in a desiccator with alcohol-free chloroform (CHCl3). Generally, alcohol remains as an admixture with CHCl3. To obtain CHCl3 free from alcohol, 100 mL CHCl3 and 200 mL 5% H2SO4 (1:2) were taken in a separatory funnel and thoroughly shaken by hand for 20 times. CHCl3 free from alcohol was then collected in a beaker from the separatory funnel, and the process was repeated 5 times with 5% H2SO4 and 5 times with distilled water. After that, the desiccator was rinsed with boiled water; then, 200 mL of boiled water and a beaker containing 100 mL alcohol-free CHCl3 were placed at the bottom of the desiccator. Then, the conical flasks each containing 20 g of soil samples were placed inside the desiccator. After tightly closing the desiccator, it was evacuated with a mini vacuum pump (Hitachi−Minivac PD-52, Yamato Scientific Co. Ltd., Tokyo, Japan) for at least 10 min. The desiccator was then kept in the dark by wrapping it with a black polythene sheet for 24 h.
2.5.7. Measurement of Biomass C
Fumigated and non-fumigated soils were extracted with 0.5 M K2SO4 (soil:K2SO4 = 1:4) and shaken for 30 min and then filtered. From the extract, the amount of biomass carbon was determined according to the method described by Vance et al. [51].
2.5.8. Measurement of Biomass N
From the fumigated and non-fumigated soil extract, biomass nitrogen was determined according to the method described by Brookes et al. [52]. At first, 10 mL of extract was taken in a 100 mL Kjeldahl flask; then, 5 mL concentrated H2SO4, 0.5 mL CuSO4 (0.2 N), and 2–3 boiling stones were placed in it. After that, it was subjected to distillation with 10 N NaOH immediately after heating in a digestion chamber for 4 h. An amount of 5 mL of 2% boric acid was used to collect 50 mL distillate in it, and finally, it was titrated against 0.005 N H2SO4 to determine biomass N.
2.6. Yield-Contributing Characteristics of Cabbage
The cabbage was harvested at 92 DAT when it had reached the edible stage to estimate the edible head weight (kg plant−1) and edible head yield (t ha−1). To minimize the experimental error, 15 cabbages were harvested from each replicated N dose, and then averaged to be considered as one replication. A similar technique was employed to sample the cabbages from every N dose plot under 6.0 m, 4.5 m, 3.0 m, and control.
2.7. Statistical Analysis
In the experiment, split-plot design was used to investigate the effects of alley widths and N levels on cabbage yield and soil properties. The experimental data were statistically analyzed carrying out the two-way analysis of variance (ANOVA) using “Statistix 10” software to determine the significance of the variations in results attributed to the main-plot factor, sub-plot factor, or their interaction. In this study, alley widths and N levels were considered as main- and sub-plot factors, respectively. When the interaction of alley widths and N levels was found to be significant, we only focused on the interaction, and in case of insignificant interaction, the main effects of single factors were reported. Further, the least significant difference (LSD) test was applied to determine which specific treatment means significantly differed from each other at a 5% probability level. Boxplots were made using the “ggplot2” package, and principal component analysis (PCA) was performed using the “ggplot2”, “factoMineR,” and “factoextra” packages [53,54] in RStudio (R-4.4.3).
3. Results
3.1. Impacts of Alley Cropping on Soil Properties
The combined effects of alley width and N levels were significant on soil pH, organic carbon (OC), and exchangeable K in post-harvest soil. The 3.0 m × N0 combination showed a significant improvement in soil pH (5.87) compared to that of other combinations, while the lowest soil pH (5.48) was recorded in the control × N50 treatment combination (Table 3). It is worth mentioning that N0, N25, N50, N75, and N100 denote 0 kg N ha−1, 40 kg N ha−1, 80 kg N ha−1, 120 kg N ha−1, and 160 kg N ha−1, respectively. The highest soil OC (17.00 g kg−1) was found in the 3.0 m × N50 treatment combination followed by 4.5 m × N100 (16.57 g kg−1), 3.0 m × N75 (16.37 g kg−1), 4.5 m × N75 (16.07 g kg−1), 6.0 m × N75 (16.1 g kg−1), and 6.0 m × N100 (16.03 g kg−1) treatment combinations (Table 3). However, the control × N100 treatment combination had the lowest OC (12.1 g kg−1), which was considerably lower than all other treatment combinations of alley widths except control. In case of exchangeable K, the interaction of 4.5 m alley and N50 exhibited the significantly highest exchangeable K (0.41 c-mol (+) kg−1) compared to all other treatment combinations (Table 3). On the contrary, the lowest exchangeable K (0.26 c-mol (+) kg−1) was recorded in the control × N50 treatment combination. In post-harvest soil, soil pH and exchangeable K were increased in both the alley-cropped and control plots, where the rates of increment were found as 2.12%, 1.97%, 1.80%, and 2.37% for soil pH and 18%, 13%, 7%, and 4% for exchangeable K in 3.0 m, 4.5 m, 6.0 m alleys, and control, respectively, compared to the initial level (Table 4). Soil OC was increased by 7.64%, 7.75%, and 8.57% in 3.0 m, 4.5 m, and 6.0 m alleys, respectively, whereas it was reduced by 1.57% in the control treatment.

Table 3.
Interaction effect of alley widths and nitrogen levels on soil pH, organic C, total N, available P, and exchangeable K in soil after harvesting of cabbage.

Table 4.
Changes in soil biochemical properties after harvesting of cabbage.
The combined effects of alley width and N levels had no significant effects on total N and available P in soil, yet the highest total N (1.30 g kg−1) and available P (14.18 mg kg−1) were recorded in the 3.0 m × N100 treatment combination (Table 3). In response to the single effects, the total N and available P in soil were significantly varied due to the main effect of alley widths. The 3.0 m alley width provided the highest total N (1.23 g kg−1) as well as available P (13.27 mg kg−1) in soil (Figure 1A,B). In contrast, the lowest total N (1.01 g kg−1) and available P (10.14 mg kg−1) were recorded in the control plot. N contents in soils were increased by 11%, 6%, and 5% in 3.0 m, 4.5 m, and 6.0 m alleys, respectively, compared to the initial soil N, while, the post-harvest control soil resulted in a 3% reduction in total N (Table 4). In parallel, available P was increased by 5%, 11%, 15%, and 16% in the control, 3.0 m, 4.5 m, and 6.0 m alleys, respectively, compared to the pre-experimental soil P. The effect of N levels (sub-plot factor) in total N of soils was found to be statistically insignificant; however, the highest total N (1.20 g kg−1) was found in the N100 treatment (Figure 1C). On the other hand, the effect of N levels on the available P in soil was significant, where the highest available P (12.55 mg kg−1) was recorded in the N100 treatment followed by N50 (12.44 mg kg−1), N25 (12.12 mg kg−1), and N75 (11.99 mg kg−1) (Figure 1D). The lowest available P (11.55 mg kg−1) was recorded in the N0 treatment.
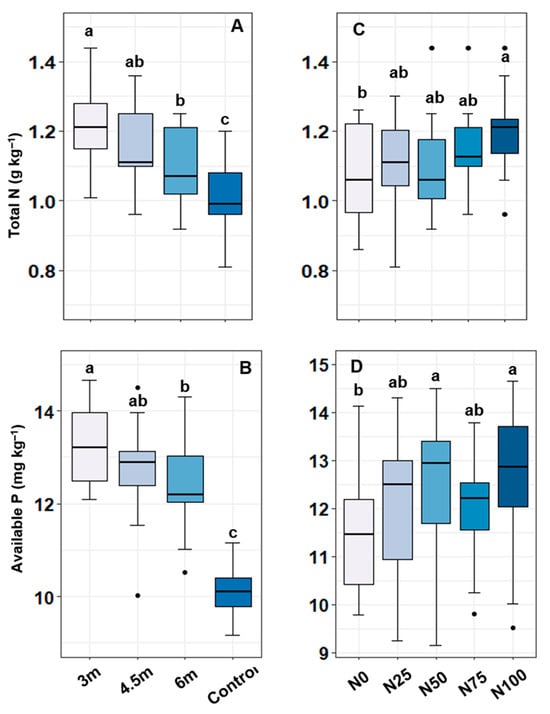
Figure 1.
Total N (A,C) and available P (B,D) in soil under different alley widths and varying nitrogen levels after harvesting of cabbage. Different alphabetical letters showed the significant differences among the treatments (n = 15 for each alley width and control; n = 12 for each N dose).
Alley widths and N levels jointly showed an insignificant effect on microbial biomass carbon (BC); however, it was statistically significant for biomass nitrogen (BN). In response to different treatment combinations, the highest BC (561 mg kg−1 soil) was recorded in the 3.0 m × N50 treatment combination followed by 3.0 m × N75, 4.5 m × N75 and 3.0 m × N100 treatment combinations (Table 5). The 3.0 m × N100 treatment combination had the significantly highest BN (114 mg kg−1 soil). In contrast, control × N0 treatment combination had the lowest BC (323.17 mg kg−1 soil) and BN (29.97 mg kg−1 soil) in post-harvest soil (Table 5). Biomass C/N ratios across the treatment combinations ranged from 4.71 to 13.45. The control × N50 treatment combination displayed the highest ratio of 13.45, while the lowest ratio of 4.71 was observed in the 3.0 m × N100 treatment combination (Table 5). Results also showed that contribution of BC to the soil OC was the highest in 3.0 m × N0 (4%) treatment combination and lowest in the control × N0 (3%). In terms of BN, the 3.0 m × N100 treatment combination demonstrated the highest contribution, accounting for 8.77% of the soil’s total nitrogen (Table 5).

Table 5.
Interaction effect of alley widths and nitrogen levels on microbial biomass carbon and biomass nitrogen in soil after harvesting of cabbage.
The interaction effect of alley widths and N levels was found insignificant on BC; however, their individual effect was significant for the content of soil microbial BC. The highest BC (531 mg kg−1 soil) was found in the 3.0 m alley which was statistically comparable with the 4.5 and 6.0 m alley width (Figure 2A), while the control soil had the lowest BC (387 mg kg−1 soil). The biomass C/N ratios ranged from 5.87 to 9.64 for the studied alley widths and control, where the maximum C/N ratio (9.64) was exhibited in the control and the minimum (5.87) in the 3.0 m alley width (Figure 2C). The contribution of BC to the soil OC varied from 3.09 to 3.43%; however, the 3.0 m alley width contributed the highest (Figure 2D). Similarly to alley width, N levels exhibited significant variations in soil microbial BC. Notably, the N50 treatment displayed the highest BC (505 mg kg−1 soil), which was statistically similar to N75 (494 mg kg−1 soil) and N100 (476 mg kg−1 soil) treatments, while the N0 treatment showed the lowest BC (444 mg kg−1 soil) in post-harvest soil (Figure 2B). The biomass C/N ratios ranged from 5.13 to 8.41 for the studied N levels, where the highest and lowest values were in the N0 and N100 treatments, respectively (Figure 2E). According to BC/OC, the utmost level was noticed in N50 treatment (3.37%) and lowest in N25 (3.14%) (Figure 2F). Post-harvest soil samples showed increments in BC (7.65%, 4.88%, and 4.47%) and BN (4.90%, 3.42%, and 2.24%) in the 3.0 m, 4.5 m, and 6.0 m alleys, respectively, compared to their initial level, while a 3% reduction in both BC and BN was estimated in post-harvest control soil (Table 4).
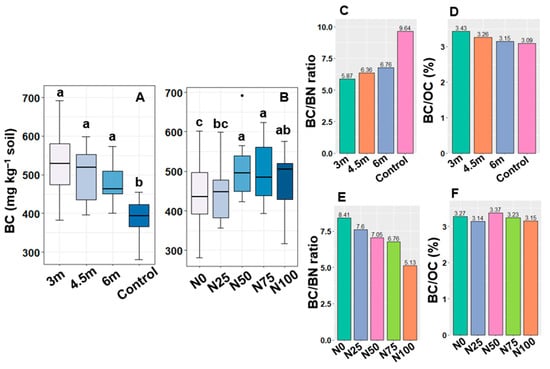
Figure 2.
Microbial biomass C (A,B) and its ratio with biomass N (C,E) and contribution of biomass C to the organic C (D,F) in soil under different alley widths and varying nitrogen levels after harvesting of cabbage. Different alphabetical letters show the significant differences among the treatments (n = 15 for each alley width and control; n = 12 for each N dose).
3.2. Productivity of Cabbage
The combined effect of alley widths and N levels on edible head weights and head yields were found to be insignificant, indicating that the main-plot and sub-plot factors worked independently. However, the 6.0 m × N100 combination achieved the highest head weight (3.08 kg plant−1) and head yield (85.65 t ha−1) of cabbage followed by control × N100 and 6.0 m × N75 treatment combinations (Table 6). There was a sharp increase in cabbage yields along with the increase in N levels irrespective of alley widths and control, and it was observed that the yields steadily declined with the decrease in alley width. The edible head weight and head yield of cabbage varied significantly among different alley widths. The significantly highest edible head weights were found in the 6.0 m alley (2.22 kg plant−1) and in control (2.06 kg plant−1) (Figure 3A). The significantly lowest edible head weight (1.42 kg plant−1) was recorded in the 3.0 m alley. Similar trends in terms of head yields were observed where the 6.0 m alley provided the highest edible head yield of 61.67 t ha−1, while the lowest edible head yield of 39.43 t ha−1 was found in the 3.0 m alley (Figure 3B). The head weight and head yield were significantly augmented with the increase in N levels. The significantly highest head weight (2.64 kg plant−1) and head yield (73.26 t ha−1) were recorded in N100 treatment, while N0 showed a considerable reduction in edible head weight (0.77 kg plant−1) and head yield (21.38 t ha−1) (Figure 3C,D).

Table 6.
Interaction effect of alley widths and nitrogen levels on edible head weight and edible head yield of cabbage.
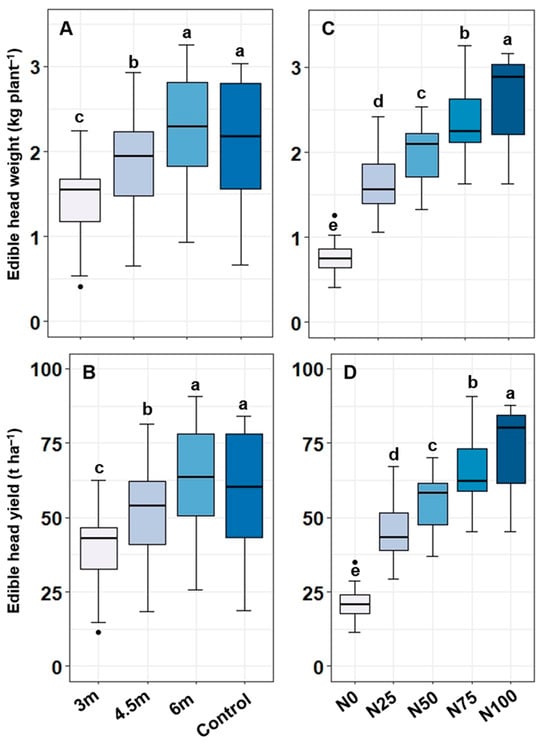
Figure 3.
Edible head weight (A,C) and edible head yield (B,D) of cabbage grown under different alley widths and varying nitrogen levels. Different alphabetical letters showed the significant differences among the treatments (n = 15 for each alley width and control; n = 12 for each N dose).
3.3. Correlation of Treatments (Main-Plot × Sub-Plot) with Soil Biochemical Properties and Cabbage Yield Using Principal Component Analysis (PCA)
The PC1 (56.4%) and PC2 (23.4%) accounted for the majority of the variability and collectively explained 79.8% of the variability (Figure 4). Furthermore, PCA showed that the 3.0 m × N25, 3.0 m × N50, 3.0 m × N75, 3.0 m × N100, 4.5 m × N25, 4.5 m × N50, 4.5 m × N75, 4.5 m × N100, 6.0 m × N50 and 6.0 m × N100 treatments exhibited a stronger correlation with both of the yield and soil properties, whereas control × N0, control × N25, control × N50, control × N75, control × N100, 3.0 m × N0, 4.5 m × N0, and 6.0 m × N25 showed negative correlations with most of the attributes studied.
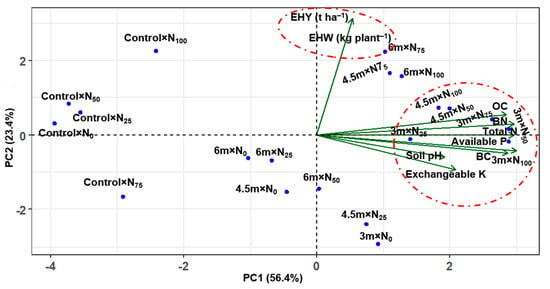
Figure 4.
Principal component analysis (PCA) represents the relationship among the different treatments and parameters. The biplot was created with the two components (PC1 and PC2) that collectively explain 79.8% of the variability among the datasets. The vector lines of the biplot display positive or negative associations of different biochemical properties of soil and the productivity of cabbage with different treatments. The angle value between the parameter and treatment specifies the intensity of association between treatment and subsequent parameters, where a small angle indicates a weak association and a large angle indicates a strong association. EHW (kg plant–1) stands for edible head weight and EHY (t ha–1) means edible head yield.
4. Discussion
Shrinkage and degradation of the cultivable land cause food insecurity for a vast majority of the global population including Bangladesh. Crop production through intensive farming using synthetic fertilizers results in soil health degradation and unsustainable agriculture. Therefore, to keep soil alive and healthy, a sustainable farming practice comprising organic and inorganic fertilizers, crop rotation with legumes, minimum tillage, agroforestry systems, etc., might be advisable. To date, agroforestry ensures maximum land utilization, reduces the risk of total crop failure, confirms maximum output from the same piece of land, increases nutrient retention, reduces soil erosion, and improves soil health and environment [7,55,56,57,58]. The alley cropping system is an inherent part of agroforestry which has already acknowledged several possibilities [59,60]. In Bangladesh, the agroforestry system has enormous opportunities to manifest the alley cropping technology which are yet to be explored. Findings of the present study of alley cropping may contribute in improving soil health, reducing the use of inorganic fertilizers, and improving crop productivity.
In the present study, the alley cropping showed encouraging results on soil pH, OC, available P, exchangeable K, BC, and BN (Table 3, Table 4 and Table 5; Figure 1 and Figure 2). The highest soil pH was found in the 3.0 m alley at 0% N level (Table 3). Addition and decomposition of maximum pruned materials in the 3.0 m alley might have reduced the soil acidification which ultimately improved soil pH. Fascinatingly, soil pH was slightly increased in both the control and alley-cropped plots, where the rates of increment were 2.37%, 2.12%, 1.97%, and 1.80% in the control, 3.0 m, 4.5 m, and 6.0 m alleys, respectively, compared to the initial soil pH (Table 4). The findings of the present study are supported by several researchers across the world [18,61,62], who have reported that organic fertilizer and litter production by trees can reduce soil acidity by enhancing soil pH. Moreover, Wang et al. [63] reported that soil pH has a strong correlation with ash alkalinity. Legume biomass has higher ash alkalinity which contributes to the amelioration of soil acidity. Soil pH was higher under different treatment combinations of alley widths and N levels compared to the control. Thus, our experiment demonstrated that while L. leucocephala has a beneficial effect on improving soil pH, application of synthetic N fertilizer increases soil acidity.
Compared to the control, i.e., treatment plots without the L. leucocephala trees, soil OC and other nutrients increased in the alley system with the decrease in alley widths (Table 3; Figure 1A,B); nonetheless, these corresponded irregularly with N levels. Sirohi et al. [17] reported that OC, total N, available P, and exchangeable K were decreased with the advancement in the row–row distance of poplar trees. Supplied pruned biomass from L. leucocephala (Table 2) might be responsible for the augmentation of OC and other soil nutrients in this study. The findings are corroborated by Sirohi et al. [17] and Fernández et al. [35]. In the present investigation, the highest OC was found in the 3.0 m × N50 treatment combination (Table 3) and might be attributed to a higher amount of biomass applied from L. leucocephala compared to the other combinations of the 4.5 m and 6.0 m alleys. It is evident that the incorporation of pruned materials enhanced the carbon contents in the soils of the alleys. Compared with the initial soil C, it increased by 7.64%, 7.75%, and 8.57% in the 3.0 m, 4.5 m, and 6.0 m alleys, respectively, whereas OC was reduced by 1.57% in the control treatment (Table 4). Similar results were also described with other legume species [13,14,64]. However, we found that exchangeable K exhibited higher values in the 4.5 m alley with 50% N dose (Table 3). The exerted nutrients from L. leucocephala biomass, mineralization of organic compounds, biological N fixation, recycling of P and K through litter decomposition (Figure 5), coupled with the application of organic and inorganic fertilizer may all have contributed to the increase in N, P, and K in post-harvest soil.
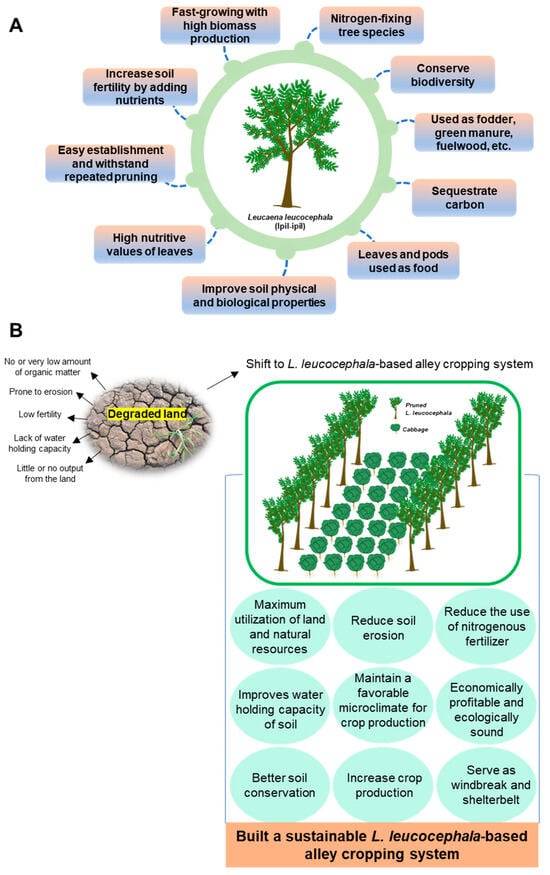
Figure 5.
Representation of the L. leucocephala-based alley cropping system showing the possible ways for improving soil properties and cabbage yield. A leguminous tree provides multiple benefits that can boost the soil fertility, conservation of biodiversity, and ability to carbon fixation, and can be used for fodder and fuelwood (A). Additionally, the L. leucocephala-based alley cropping system has the potential to convert degraded land into arable land and facilitates a sustainable farming system (B).
Microbial biomass C and biomass N exhibited a correlation between bacteria and fungi that decompose the crop residues and organic matter content in the soil. The study found that plots without adding pruned biomass (control) displayed the lowest BC compared with alley cropping plots and responded unevenly with N levels (Figure 2A,B). With the increase in N rates, soil bacteria may have had an important impact on the decline in microbial BC [65], but this is yet unknown and has to be thoroughly assessed. However, BN was augmented under different treatment combinations in the alley system and responded positively with N levels (Table 5). Post-harvest soil from the L. leucocephala alley cropping system exhibited significant increments in BC and BN. Compared to the initial levels, BC was increased by 7.65%, 4.88%, and 4.47% in the 3.0 m, 4.5 m, and 6.0 m alleys, respectively, while the increments were 4.90%, 3.42%, and 2.24%, for BN (Table 4). In narrower alley widths, a large amount of biomass from L. leucocephala might have created a habitat and facilitated the activity of microbes in soil, ultimately increasing BC and BN. Our research is supported by Yadav et al. [66] and Tian et al. [67], who reported that tree-based agroforestry provides higher microbial BC and BN compared to no tree and/or monoculture. Microbial BC and BN are considered as easily accessible and dynamic soil carbon and nitrogen fractions, which are the key factors regulating the microbial community in soil that influence the mineralization of organic C [68,69]. The microbial BC/BN ratio is an important benchmark of the activity of microbes in soil. We observed comparatively lower BC/BN ratio in most of the alley-cropped plots compared to control, where the ratios decreased with the increase in N level irrespective of alley widths (Table 5). The low C:N ratio of microbial biomass promotes nitrogen mineralization, and the balanced ratio favors the decomposition of OM [70,71]. Therefore, these findings suggest that alley cropping is a promising approach for enhancing nutrient availability and increasing soil fertility compared to open field conditions.
In our experiment, pruned biomass from L. leucocephala trees manifested itself as a contributor of microbial BC and BN in soil. Organic matter addition and decomposition might have increased the accumulation of organic carbon in the top soil layer, which favors the growth and development of microbes in soil, and ultimately augmented the content of BC and BN (Figure 5). Soil microbial BC, a responsive index of soil fertility and biotic attributes, plays a role in the biogeochemical processes [72,73]. Biomass C is employed as an indication of changing physical and chemical qualities of soils which are influenced by the addition of different organic and inorganic fertilizers. In addition, BN is a sensitive marker that upholds ecological stability and environmental strength and determines the soil quality in response to soil fertility. According to Rahman et al. [73], BN is reliant on soil physicochemical characteristics, microbial diversity, and soil–crop management techniques, including the use of fertilizer from both organic and inorganic sources.
Results revealed a remarkable variation in cabbage yields among the alley widths and N levels, where 6.0 m alley width and N100 showed the best result (Figure 3). Cabbage grown in the maximum alley width encountered less competition with L. leucocephala trees for the resources viz. light, water, and nutrients which contributed to the highest yield of cabbage. Barreto et al. [15] reported that maize productivity rose with the increase in pruning frequencies in a 5.0 m width alley due to a reduction in competition between maize and gliricidia plants. In other ways, L. leucocephala trees might have provided sufficient N through N2 fixation, and supplied other plant nutrients through biomass addition. Hence, all of these might have favored the soil environment for higher cabbage production in the alley cropping system (Figure 5). Since plants require more N than any other nutrient, biological N fixation supplies a substantial amount of N to the standing crop as well as to the next crop [74,75]. Chintu et al. [76] and Akinnifesi et al. [77] stated that it is conceivable that alley cropping systems with reduced N fertilizer inputs may be able to offer a greater advantage. However, cabbage competed highly in the 3.0 and 4.5 m alley resulting in lower yield due to strong competition with trees (Figure 3A,B) and even received more pruned materials with similar N doses and intercultural operations. Researchers have also acknowledged that trees and crops compete with each other under alley cropping systems [78,79].
Furthermore, to examine what combination of alley width and N doses supports the reduction in external N usages, our study revealed that the 6.0 m × N100 treatment combination is statistically identical to the control × N100 and 6.0 m × N75 treatment combinations (Table 6). It was proven that using 75% inorganic N and pruned biomass from a 6.0 m alley reduces competition with trees while increasing crop yield and lowering inorganic N consumption. Moreover, our results also aligned with other findings when maize, tomato, brinjal, cabbage, rice, finger millet, peanut, pigeon pea, and wheat were grown with N2-fixing legume trees, ultimately reducing the use of chemical fertilizer [9,13,30,80,81]. However, to maintain sustainability and achieve the best possible outcomes, alley cropping requires the right selection of trees, crops, and the arrangement of trees with intense care. The study results indicate that the L. leucocephala-based alley cropping system with an alley width of 6.0 m will achieve the desired crop yield even using 25% less inorganic N fertilizer in the silty clay loam terrace soil of the Madhupur Tract of Bangladesh.
5. Conclusions
The L. leucocephala-based alley cropping system was found to be promising for increasing soil fertility and cabbage yield. Our findings revealed that supplementary biomass application from L. leucocephala augmented soil properties. Improvement of soil pH, organic C, different essential plant nutrients, microbial biomass C, and biomass N in the L. leucocephala-based alley cropping might help to rejuvenate the degraded land. Consequently, a comparable cabbage yield was noticed between the addition of pruned materials in the 6.0 m alley with 75% N and 100% N without pruned materials, suggesting that synthetic N fertilizer usage can be reduced up to 25% without compromising cabbage yield. Such reduction in N fertilizer might be economically viable for agriculture and environmentally sustainable. Therefore, alley cropping could be a potential sustainable system for farmers to improve the health of intensively cultivated soils and increase crop yields. Nevertheless, a comprehensive economic analysis and a systematic process of sharing resources between trees and crops still require evaluation. Additionally, conducting experiments on farmers’ fields is needed for wider dissemination of such green technology among farming communities.
Author Contributions
Conceptualization, T.A.; methodology, T.A., M.A.R., M.M.R. and M.S.; formal analysis and investigation, M.S.; software and visualization: A.K.D. and M.S.; writing—original draft preparation, M.S. and A.K.D.; writing—review and editing, A.K.D., T.A. and M.M.R.; resources and supervision, T.A. and M.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restriction.
Acknowledgments
The authors desire to express their sincere thanks to the Department of Agroforestry and Environment and Department of Soil Science, Gazipur Agricultural University, Bangladesh for providing necessary facilities and resources for completion of this research work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- BBS. Area, population and household characteristics. In Statistical Year Book Bangladesh 2022, 42nd ed.; Bose, P.C., Miah, M.A.K., Azad, A.A., Alam, M.M., Ahmed, K.U., Ahmed, Z., Hossain, M.D., Hossain, M.A., Ahmed, M., Haque, M.E., Eds.; Bangladesh Bureau of Statistics—Ministry of Planning, Govt. of Peoples Republic of Bangladesh: Dhaka, Bangladesh, 2023; pp. 49–86. [Google Scholar]
- BBS. Land use statistics. In Yearbook of Agricultural Statistics 2022, 34th ed.; Halim, M.A., Islam, M.S., Khan, M.A.H., Islam, W., Zohara, F., Faria, T., Chowdhury, D.K., Nuri, M.A.M., Hossain, M.I., Hossain, S., Eds.; Bangladesh Bureau of Statistics—Ministry of Planning, Govt. of Peoples Republic of Bangladesh: Dhaka, Bangladesh, 2023; pp. 421–429. [Google Scholar]
- Ferdush, J.; Karim, M.M.; Noor, I.J.; Jui, S.A.; Ahamed, T.; Saha, S.R. Impact of alley cropping system on soil fertility. Int. J. Adv. Geosci. 2019, 7, 173–178. [Google Scholar] [CrossRef]
- Al Riyadh, Z.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Hoque, M.A.; Rahman, M.M. Performance of spices as lower–storey crop in jackfruit–papaya multistorey agroforestry system in Bangladesh. J. Fac. Agric Kyushu Univ. 2020, 65, 223–231. [Google Scholar] [CrossRef]
- Ahmmed, S.; Jahiruddin, M.; Razia, M.S.; Begum, R.A.; Biswas, J.C.; Rahman, A.S.M.M.; Ali, M.M.; Islam, K.M.S.; Hossain, M.M.; Gani, M.N.; et al. Soil organic matter management. In Fertilizer Recommendation Guide—2018; Ahmmed, S., Jahiruddin, M., Eds.; Bangladesh Agricultural Research Council: Dhaka, Bangladesh, 2018; pp. 53–58. [Google Scholar]
- Rahman, M.M.; Rahman, M.M.; Tanu, T.A.; Rahman, M.M. Spatial environmental impact on land degradation in Bangladesh. Asian J. Appl. Sci. Eng. 2012, 1, 84–90. [Google Scholar]
- Das, A.K.; Rahman, M.A.; Rahman, M.M.; Saha, S.R.; Keya, S.S.; Suvoni, S.S.; Rizvi, J. Scaling up of jujube-based agroforestry practice and management innovations for improving efficiency and profitability of land uses in Bangladesh. Agrofor. Syst. 2022, 96, 249–263. [Google Scholar] [CrossRef]
- Das, A.K.; Rahman, M.A.; Mitra, P.; Sukhwani, V.; Shaw, R.; Mitra, B.K.; Morey, B. Up-scaling organic agriculture to enhance food and water security in South Asia. Org. Agric. 2022, 12, 475–494. [Google Scholar] [CrossRef]
- Ferdush, J.; Karim, M.M.; Himel, R.; Saha, S.; Ahamed, T. Impact of alley cropping on wheat productivity. IOSR J. Agric. Vet. Sci. 2018, 11, 17–25. [Google Scholar]
- Tuan, V.D.; Hilger, T.; MacDonald, L.; Clemens, G.; Shiraishi, E.; Vien, T.D.; Stahr, K.; Cadisch, G. Mitigation potential of soil conservation in maize cropping on steep slopes. Field Crops Res. 2014, 156, 91–102. [Google Scholar] [CrossRef]
- Hussain, K.; Wongleecharoen, C.; Hilger, T.; Ahmad, A.; Kongkaew, T.; Cadisch, G. Modelling resource competition and its mitigation at the crop-soil-hedge interface using WaNuLCAS. Agrofor. Syst. 2016, 90, 1025–1044. [Google Scholar] [CrossRef]
- Hombegowda, H.C.; Adhikary, P.P.; Jakhar, P.; Madhu, M. Alley cropping agroforestry system for improvement of soil health. In Soil Health and Environmental Sustainability; Shit, P.K., Adhikary, P.P., Bhunia, G.S., Sengupta, D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 529–549. [Google Scholar]
- Rahman, M.A.; Miah, M.G.; Yahata, H. Maize production and soil properties change in alley cropping system at different nitrogen levels. Agriculturists 2009, 7, 41–49. [Google Scholar] [CrossRef]
- Ahmed, S.; Hasan, S.; Chowdhury, A.H.M.R.H.; Chowdhury, A.K.M.M.A.; Hossain, M.A. Performance of growth and soil properties change by four multipurpose tree species in alley cropping system. Int. J. Sustain. Crop. Prod. 2010, 5, 21–27. [Google Scholar]
- Barreto, A.C.; Chaer, G.M.; Fernandes, M.F. Hedgerow pruning frequency effects on soil quality and maize productivity in alley cropping with Gliricidia sepium in Northeastern Brazil. Soil Tillage Res. 2012, 120, 112–120. [Google Scholar] [CrossRef]
- Swieter, A.; Langhof, M.; Lamerre, J.; Greef, J.M. Long-term yields of oilseed rape and winter wheat in a short rotation alley cropping agroforestry system. Agrofor. Syst. 2019, 93, 1853–1864. [Google Scholar] [CrossRef]
- Sirohi, C.; Dhillon, R.S.; Chavan, S.B.; Handa, A.K.; Balyan, P.; Bhardwaj, K.K.; Ahlawat, K.S. Development of poplar-based alley crop system for fodder production and soil improvements in semi-arid tropics. Agrofor. Syst. 2022, 96, 731–745. [Google Scholar] [CrossRef]
- Koyejo, A.O.; Okpara, D.A.; Agugo, B.A.C. Effect of alley cropping on soil, maize and mungbean grown under different maize spatial arrangements and mungbean spacing’s in south east Nigeria. Agrofor. Syst. 2023, 97, 1337–1346. [Google Scholar] [CrossRef]
- Firoz, Z.A.; Rashid, M.H.; Huda, M.S. Effect of alley size and hedgerow pruning interval on phenology and yield of okra (Abelmoschus esculentus L. Moench) in hill slope. Bangladesh J. Agric. Res. 2011, 36, 143–150. [Google Scholar] [CrossRef]
- Conrad, K.A.; Dalal, R.C.; Dalzell, S.A.; Allen, D.E.; Fujinuma, R.; Menzies, N.W. Soil nitrogen status and turnover in subtropical Leucaena-grass pastures as quantified by δ15N natural abundance. Geoderma 2018, 313, 126–134. [Google Scholar] [CrossRef]
- Peng, S.; Chen, A.; Fang, H.; Wu, J.; Liu, G. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef]
- Adhikary, P.P.; Hombegowda, H.C.; Barman, D.; Jakhar, P.; Madhu, M. Soil erosion control and carbon sequestration in shifting cultivated degraded highlands of eastern India: Performance of two contour hedgerow systems. Agrofor. Syst. 2017, 91, 757–771. [Google Scholar] [CrossRef]
- Gusha, J.; Chiuta, T.; Katsande, S.; Zvinorova, P.I.; Kagande, S.M. Performance of cattle reared on rangelands supplemented with farm-formulated diets during the dry season in Zimbabwe. Anim. Prod. Sci. 2016, 57, 1163–1169. [Google Scholar] [CrossRef]
- Leketa, K.; Donkin, E.F.; Hassen, A.; Akanmu, A.M. Effect of Leucaena leucocephala, as a protein source in a total mixed ration, on milk yield and composition of Saanen milk goats. S. Afr. J. Anim. Sci. 2019, 49, 301–309. [Google Scholar] [CrossRef]
- Santana, A.A.; Cheng, L.; Verdecia, D.M.; Ramírez, J.L.; López, S.; Cisneros, M.V.; Al-Marashdeh, O. Effect of a mixed silage of king grass (Cenchrus purpureus) and forage legumes (Leucaena leucocephala or Gliricidia sepium) on sheep intake, digestibility and nitrogen balance. Anim. Prod. Sci. 2019, 59, 2259–2264. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, A.; Yan, S.; Rafay, L.; Du, K.; Wang, D.; Ge, Y.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sediments 2019, 19, 511–521. [Google Scholar] [CrossRef]
- De Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A multipurpose leguminous plant for the mediterranean countries: Leucaena leucocephala as an alternative protein source: A review. Animals 2021, 11, 2230. [Google Scholar] [CrossRef] [PubMed]
- Atta-Krah, A.N. Alley farming with Leucaena: Effect of short grazed fallows on soil fertility and crop yields. Exp. Agric. 1990, 26, 1–10. [Google Scholar] [CrossRef]
- Kang, B.T.; Mulongoy, K. Nitrogen contribution of woody legumes in alley cropping systems. In Biological Nitrogen Fixation and Sustainability of Tropical Agriculture; Mulonogy, K., Gueye, M., Spencer, D.S.C., Eds.; Willey: Hoboken, NJ, USA, 1992; pp. 367–375. [Google Scholar]
- Ahmed, S.; Chowdhury, A.H.M.R.H.; Ghosh, S.C.; Islam, S.M.A.S.; Parven, A. Performance of tomato, brinjal and cabbage in alley cropping system as affected by four tree species and levels of nitrogen in upland ecosystem. J. Soil Nat. 2010, 4, 17–24. [Google Scholar]
- Rahman, M.M.; Islam, M.A.; Rahman, H.M.S.; Wadud, M.A. Performance of three winter vegetables in alley cropping system. J. Agrofor. Environ. 2013, 7, 55–58. [Google Scholar]
- Hasan, M.M.; Islam, M.M.; Rahman, H.M.S. Performance of kangkong and indian spinach in ipil-ipil based alley cropping system. J. Agrofor. Environ. 2014, 8, 99–103. [Google Scholar]
- Saha, T.R.; Rahman, M.M.; Rahman, R.; Wadud, M.A.; Rahman, G.M.M. Performance of spinach under ipil-ipil based alley cropping system. J. Agrofor. Environ. 2015, 9, 7–10. [Google Scholar]
- Imogie, A.E.; Udosen, C.V.; Ugbah, M.M.; Utulu, S.N. Long term effect of Leucaena leucocephala on soil physico-chemical properties and fresh fruit bunch (FFB) production of oil palm. Afr. J. Plant Sci. 2008, 2, 129–132. [Google Scholar]
- Fernández, M.; Alaejos, J.; Andivia, E.; Madejón, P.; Díaz, M.J.; Tapias, R. Short rotation coppice of leguminous tree Leucaena spp. improves soil fertility while producing high biomass yields in Mediterranean environment. Ind. Crops Prod. 2020, 157, 112911. [Google Scholar] [CrossRef]
- Soreng, M.K.; Kerketta, N.S. Effect of organic manures on different plant varieties of chilli (Capsicum annum) under subabul (Leucaena leucocephala) based Horti-silviculture system. J. Med. Plant Stud. 2017, 5, 273–276. [Google Scholar]
- Bageel, A.; Honda, M.D.; Carrillo, J.T.; Borthakur, D. Giant Leucaena (Leucaena leucocephala subsp. glabrata): A versatile tree-legume for sustainable agroforestry. Agrofor. Syst. 2020, 94, 251–268. [Google Scholar]
- Srinivasarao, C.; Lal, R.; Kundu, S.; Babu, M.P.; Venkateswarlu, B.; Singh, A.K. Soil carbon sequestration in rainfed production systems in the semiarid tropics of India. Sci. Total Environ. 2014, 487, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.A.; Dalal, R.C.; Dalzell, S.A.; Allen, D.E.; Menzies, N.W. The sequestration and turnover of soil organic carbon in subtropical Leucaena-grass pastures. Agric. Ecosyst. Environ. 2017, 248, 38–47. [Google Scholar] [CrossRef]
- Akter, A.; Hoque, F.; Mukul, A.Z.A.; Kamal, M.R.; Rasha, R.K. Financial analysis of winter vegetables production in a selected area of Brahmanbaria district in Bangladesh. Int. Res. J. Agric. Food Sci. 2016, 1, 120–127. [Google Scholar]
- Chatterjee, R.; Jana, J.C.; Paul, P.K. Enhancement of head yield and quality of cabbage (Brassica oleracea) by combining different sources of nutrients. Indian J. Agric. Sci. 2012, 82, 324–327. [Google Scholar] [CrossRef]
- Reza, M.S.; Islam, A.K.M.S.; Rahman, M.A.; Miah, M.Y.; Akhter, S.; Rahman, M.M. Impact of organic fertilizers on yield and nutrient uptake of cabbage (Brassica oleracea var. capitata). J. Sci. Technol. Environ. Inform. 2016, 3, 231–244. [Google Scholar] [CrossRef]
- Sajib, K.; Dash, P.K.; Adhikary, B.; Mannan, M.A. Yield performance of cabbage under different combinations of manures and fertilizers. World J. Agric. Sci. 2015, 11, 411–422. [Google Scholar]
- Brammer, H. The Geography of the Soils of Bangladesh, 1st ed.; University Press Limited: Dhaka, Bangladesh, 1996; p. 287. [Google Scholar]
- Azad, A.K.; Goshwami, B.K.; Rahman, M.L.; Malakar, P.K.; Hasan, M.S.; Rahman, M.H.H. Handbook on Agro-Technology, 7th ed.; Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2017; p. 171. [Google Scholar]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Melbourne, Australia, 1992; p. 330. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Organic Carbon, Walkley-Black Method: Titration and Colorimetric Method, 1st ed.; Global Soil Partnership: Rome, Italy, 2019; pp. 1–25. [Google Scholar]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; pp. 1–771. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis: Part I; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass carbon. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wickham, H. Data Analysis, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Miah, M.G.; Islam, M.M.; Rahman, M.A.; Ahamed, T.; Islam, M.R.; Jose, S. Transformation of jackfruit (Artocarpus heterophyllus Lam.) orchard into multistory agroforestry increases system productivity. Agrofor. Syst. 2018, 92, 1687–1697. [Google Scholar] [CrossRef]
- Dollinger, J.; Jose, S. Correction to: Agroforestry for soil health. Agrofor. Syst. 2019, 93, 1205. [Google Scholar] [CrossRef]
- Rizvi, R.H.; Handa, A.K.; Sridhar, K.B.; Singh, R.K.; Dhyani, S.K.; Rizvi, J.; Dongre, G. Spatial analysis of area and carbon stocks under Populus deltoides based agroforestry systems in Punjab and Haryana states of Indo-Gangetic Plains. Agrofor. Syst. 2020, 94, 2185–2197. [Google Scholar] [CrossRef]
- Nair, P.R.; Kumar, B.M.; Nair, V.D. An Introduction to Agroforestry: Four Decades of Scientific Developments; Springer: Cham, Switzerland, 2021; pp. 1–382. [Google Scholar]
- Rahman, M.M.; Alam, M.S.; Islam, M.M.; Kamal, M.Z.U.; Rahman, G.M.; Haque, M.M.; Biswas, J.C. Potential of legume-based cropping systems for climate change adaptation and mitigation. In Advances in Legumes for Sustainable Intensification; Academic Press: Cambridge, MA, USA, 2022; pp. 381–402. [Google Scholar]
- Alam, A.S.M.J.; Saha, S.R.; Suhag, M.; Miah, M.G.; Rahman, M.M.; Islam, M.R.; Riyadh, Z.A.; Mahmud, A. Supplementary Biomass Addition Enhances the Crop Productivity: Evidence from a Gliricidia sepium—Based Alley Cropping Practices in Gazipur District of Bangladesh. Am. J. Agric. For. 2025, 13, 38–48. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Zhang, D.; Li, L.; Li, W.; Sheng, L. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Macedo, R.S.; Moro, L.; Sousa, C.S.; Carneiro, K.A.A.; Campos, M.C.C.; de Bakker, A.P.; Beirigo, R.M. Agroforestry can improve soil fertility and aggregate-associated carbon in highland soils in the Brazilian northeast. Agrofor. Syst. 2023, 98, 1167–1179. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.Y.; Xu, R.K. Use of agricultural by-products to study the pH effects in an acid tea garden soil. Soil Use Manag. 2009, 25, 128–132. [Google Scholar] [CrossRef]
- Mondal, S.; Miah, M.G.; Saleque, M.A.; Rahman, A.; Hossain, T.A.M. Effect of alley widths of Gliricidia sepium and nitrogen levels on soil properties after harvesting of rice. J. Eco-Friendly Agric. 2012, 5, 01–05. [Google Scholar]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Yadav, R.S.; Yadav, B.L.; Chhipa, B.R.; Dhyani, S.K.; Ram, M. Soil biological properties under different tree based traditional agroforestry systems in a semi-arid region of Rajasthan, India. Agrofor. Syst. 2011, 81, 195–202. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, F.; Wang, G. Soil microbiological properties and enzyme activity in Ginkgo-tea agroforestry compared with monoculture. Agrofor. Syst. 2013, 87, 1201–1210. [Google Scholar] [CrossRef]
- Toosi, E.R.; Doane, T.A.; Horwath, W.R. Abiotic solubilization of soil organic matter, a less-seen aspect of dissolved organic matter production. Soil Biol. Biochem. 2012, 50, 12–21. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, L.; Ouyang, Z.; Li, B.; Xu, Y. Different effects of plant-derived dissolved organic matter (DOM) and urea on the priming of soil organic carbon. Environ. Sci. Process. Impacts 2016, 18, 330–341. [Google Scholar] [CrossRef]
- Muhammad, I.; Lv, J.Z.; Wang, J.; Ahmad, S.; Farooq, S.; Ali, S.; Zhou, X.B. Regulation of soil microbial community structure and biomass to mitigate soil greenhouse gas emission. Front. Microbiol. 2022, 13, 868862. [Google Scholar] [CrossRef]
- Gaudel, G.; Xing, L.; Raseduzzaman, M.; Poudel, M.; Dong, W.; Hu, C. Soil microbes, carbon, nitrogen, and the carbon to nitrogen ratio indicate priming effects across terrestrial ecosystems. J. Soils Sediments 2024, 24, 307–322. [Google Scholar] [CrossRef]
- Černý, J.; Balík, J.; Kulhánek, M.; Nedvěd, V. The changes in microbial biomass C and N in long-term field experiments. Plant Soil Environ. 2008, 54, 212–218. [Google Scholar] [CrossRef]
- Rahman, G.M.; Rahman, M.M.; Alam, M.S.; Kamal, M.Z.; Mashuk, H.A.; Datta, R.; Meena, R.S. Biochar and organic amendments for sustainable soil carbon and soil health. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 45–85. [Google Scholar]
- Mahboob, W.; Yang, G.; Irfan, M. Crop nitrogen (N) utilization mechanism and strategies to improve N use efficiency. Acta Physiol. Plant. 2023, 45, 52. [Google Scholar] [CrossRef]
- Alam, M.S.; Khanam, M.; Rahman, M.M. Environment-friendly nitrogen management practices in wetland paddy cultivation. Front. Sustain. Food Syst. 2023, 7, 1020570. [Google Scholar] [CrossRef]
- Chintu, R.; Mafongoya, P.L.; Chirwa, T.S.; Kuntashula, E.; Phiri, D.; Matibini, J. Propagation and management of Gliricidia sepium planted fallows in sub-humid eastern Zambia. Exp. Agric. 2004, 40, 341–352. [Google Scholar] [CrossRef]
- Akinnifesi, F.K.; Makumba, W.; Kwesiga, F.R. Sustainable maize production using gliricidia/maize intercropping in southern Malawi. Exp. Agric. 2006, 42, 441–457. [Google Scholar] [CrossRef]
- Friday, J.B.; Fownes, J.H. Competition for light between hedgerows and maize in an alley cropping system in Hawaii, USA. Agrofor. Syst. 2002, 55, 125–137. [Google Scholar] [CrossRef]
- Wanvestraut, R.H.; Jose, S.; Nair, P.R.; Brecke, B.J. Competition for water in a pecan (Carya illinoensis K. Koch)-cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. Agrofor. Syst. 2004, 60, 167–179. [Google Scholar]
- Mondal, S.; Miah, M.G.; Elahi, N.E.; Saleque, M.A.; Rahman, A. Effect of nitrogen levels and Gliricidia sepium alley widths on rice based agroforestry systems. Bangladesh Rice J. 2013, 17, 26–32. [Google Scholar] [CrossRef]
- Balakrishna, A.N.; Lakshmipathy, R.; Bagyaraj, D.J.; Ashwin, R. Influence of alley copping system on AM fungi, microbial biomass C and yield of finger millet, peanut and pigeon pea. Agrofor. Syst. 2017, 91, 487–493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).