Co-Inoculation of Soybean Seeds with Azospirillum and/or Rhizophagus Mitigates the Deleterious Effects of Waterlogging in Plants under Enhanced CO2 Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Gas Exchange, Plant Growth, Biomass Accumulation, and Total Chlorophyll Content
2.3. Hydrogen Peroxide Levels, Lipid Peroxidation, and Antioxidant Enzyme Activity
2.4. Fermentative Enzymes and Ala-AT
2.5. Total Soluble Sugar Content
2.6. Experimental Design and Statistical Analyses
3. Results
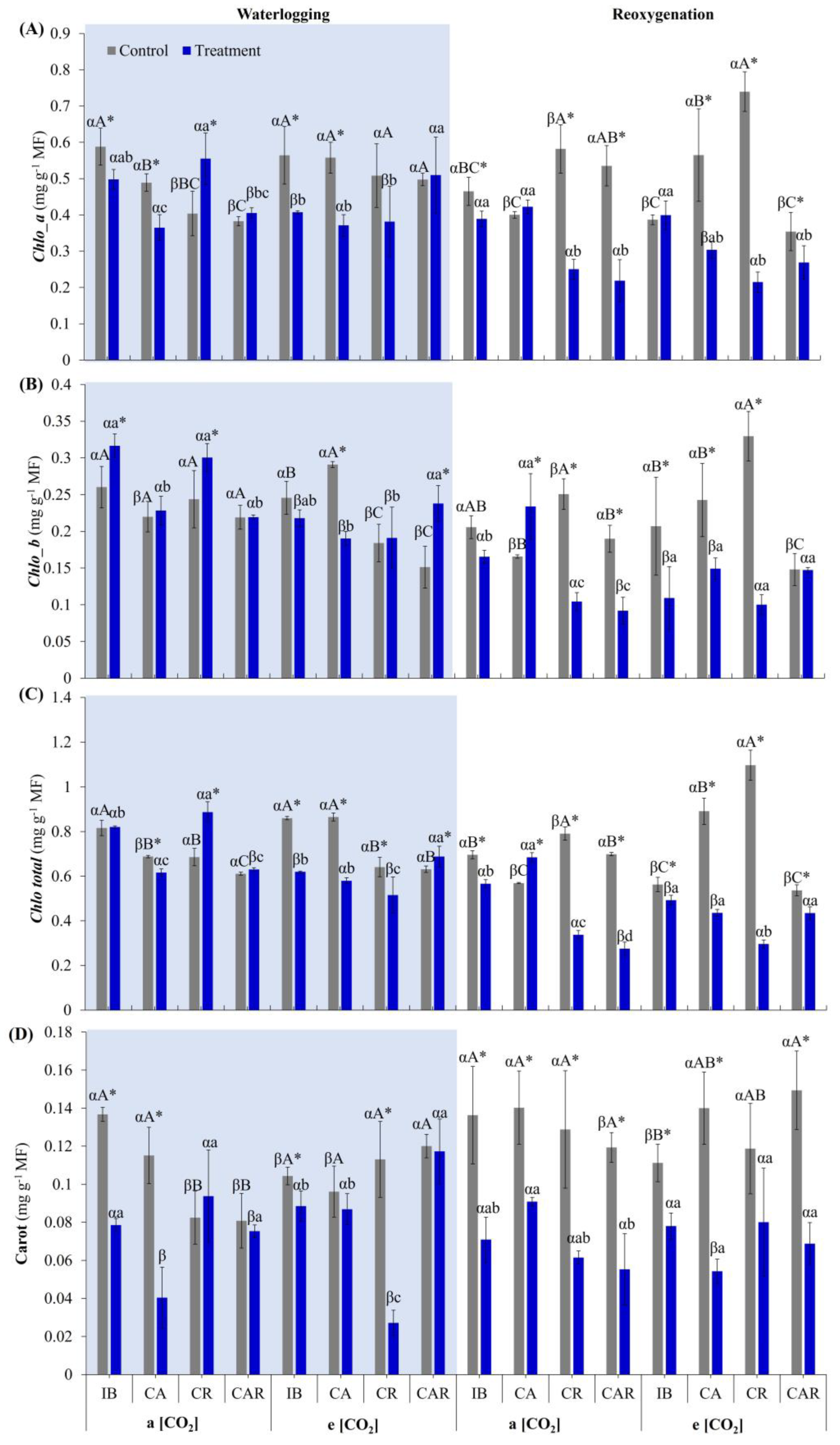
3.1. Effect of e[CO2] and Co-Inoculation on Gas Exchange and Photosynthetic Pigments
3.2. Effect of e[CO2] and Co-Inoculation on H2O2 Production and Lipid Peroxidation
3.2.1. H2O2 Content and Lipid Peroxidation in Leaves
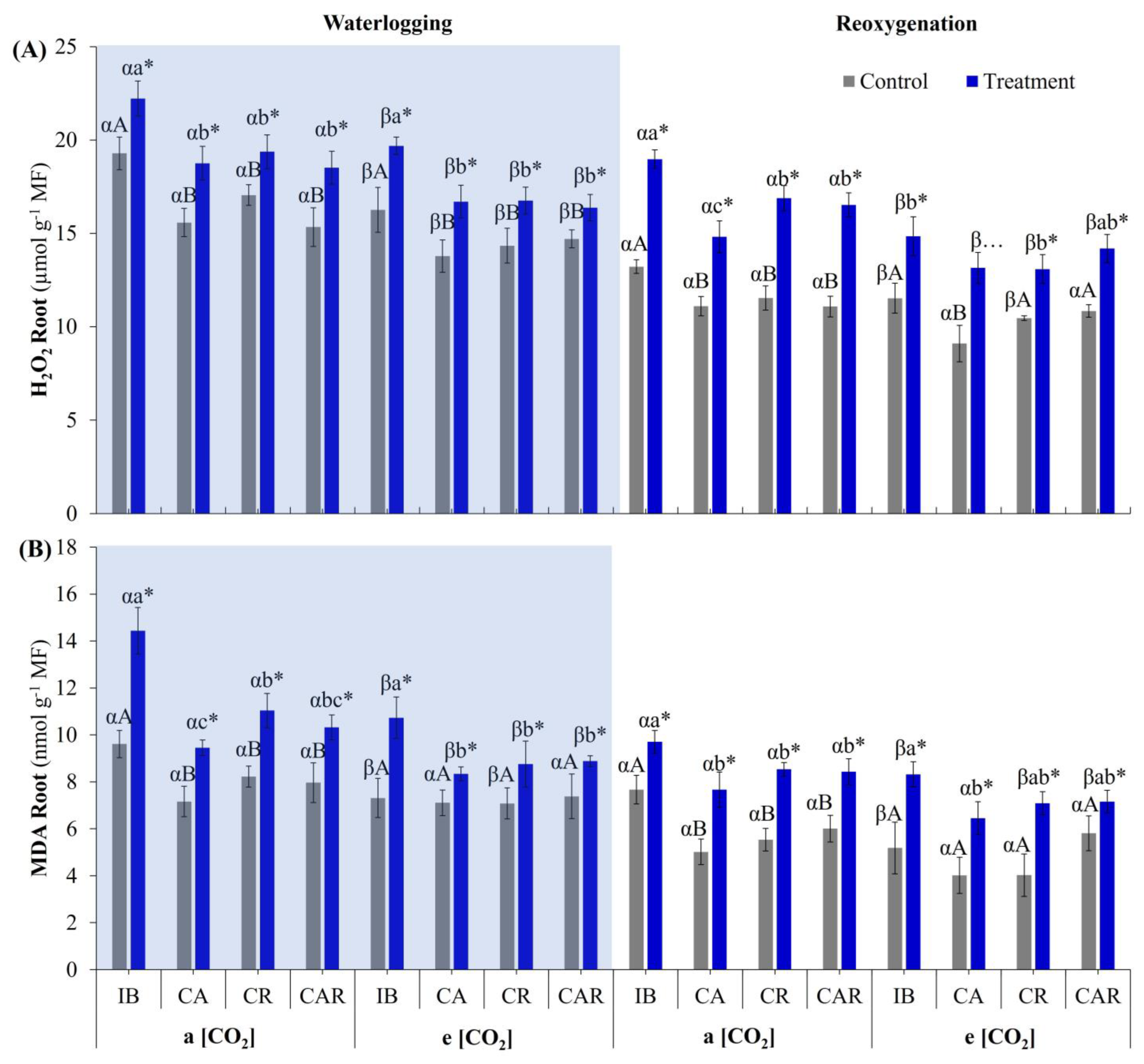
3.2.2. H2O2 Content and Lipid Peroxidation in Roots
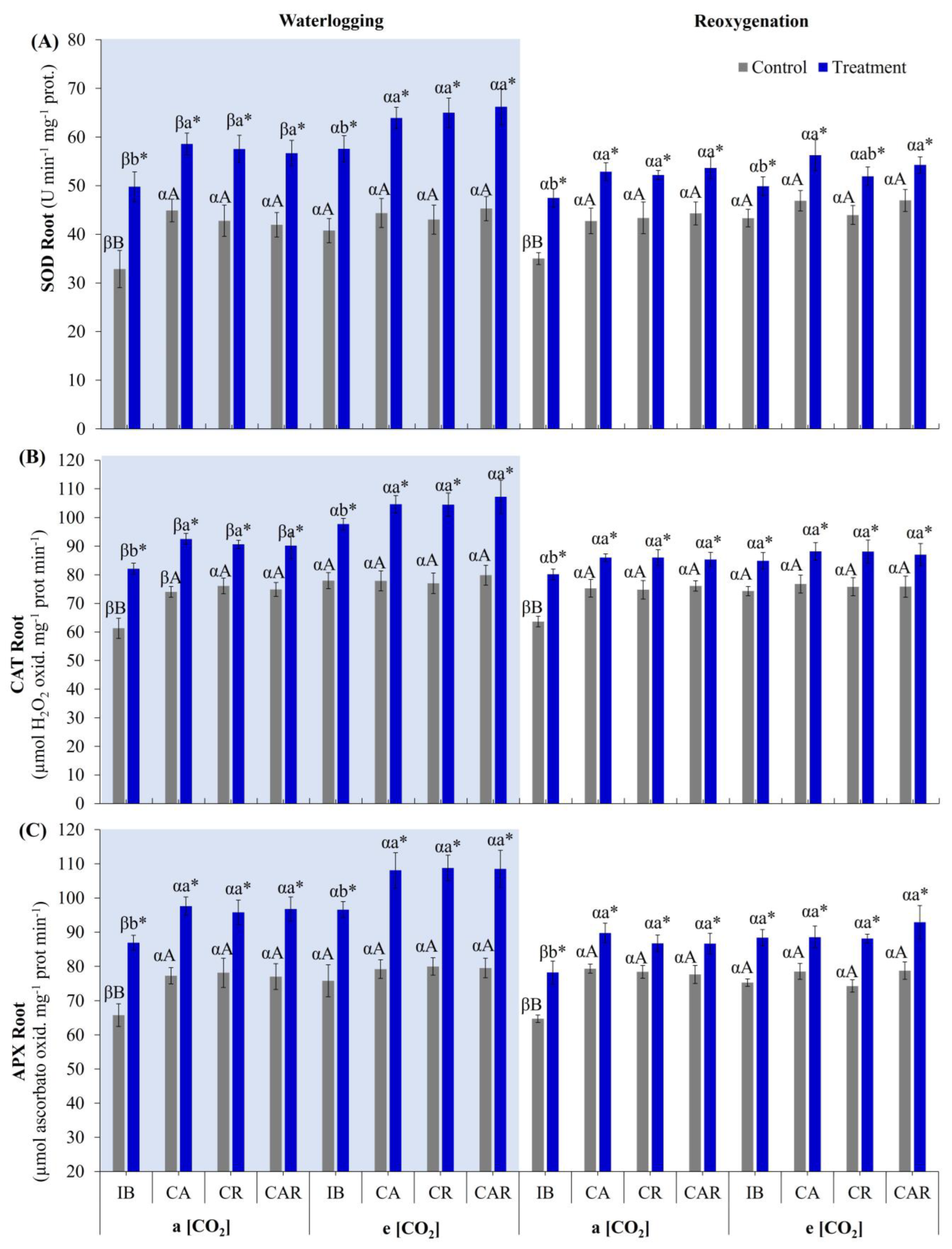
3.3. Effect of e[CO2] and Co-Inoculation on Antioxidant Enzyme Activity
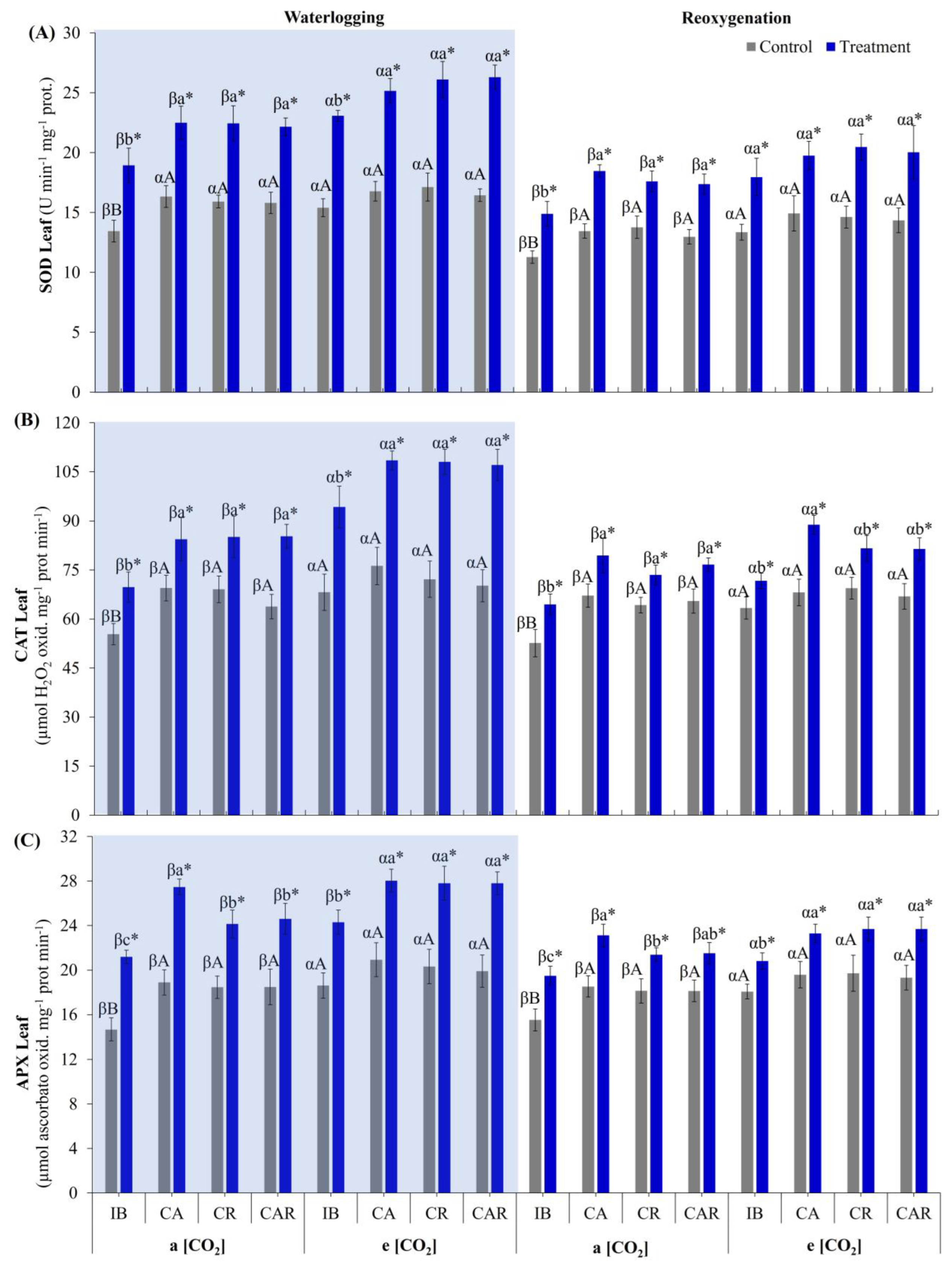
3.3.1. Antioxidant Enzyme System in Leaves
3.3.2. Antioxidant Enzyme System in Roots
3.4. Effect of e[CO2] and Co-Inoculation on Fermentative Metabolism
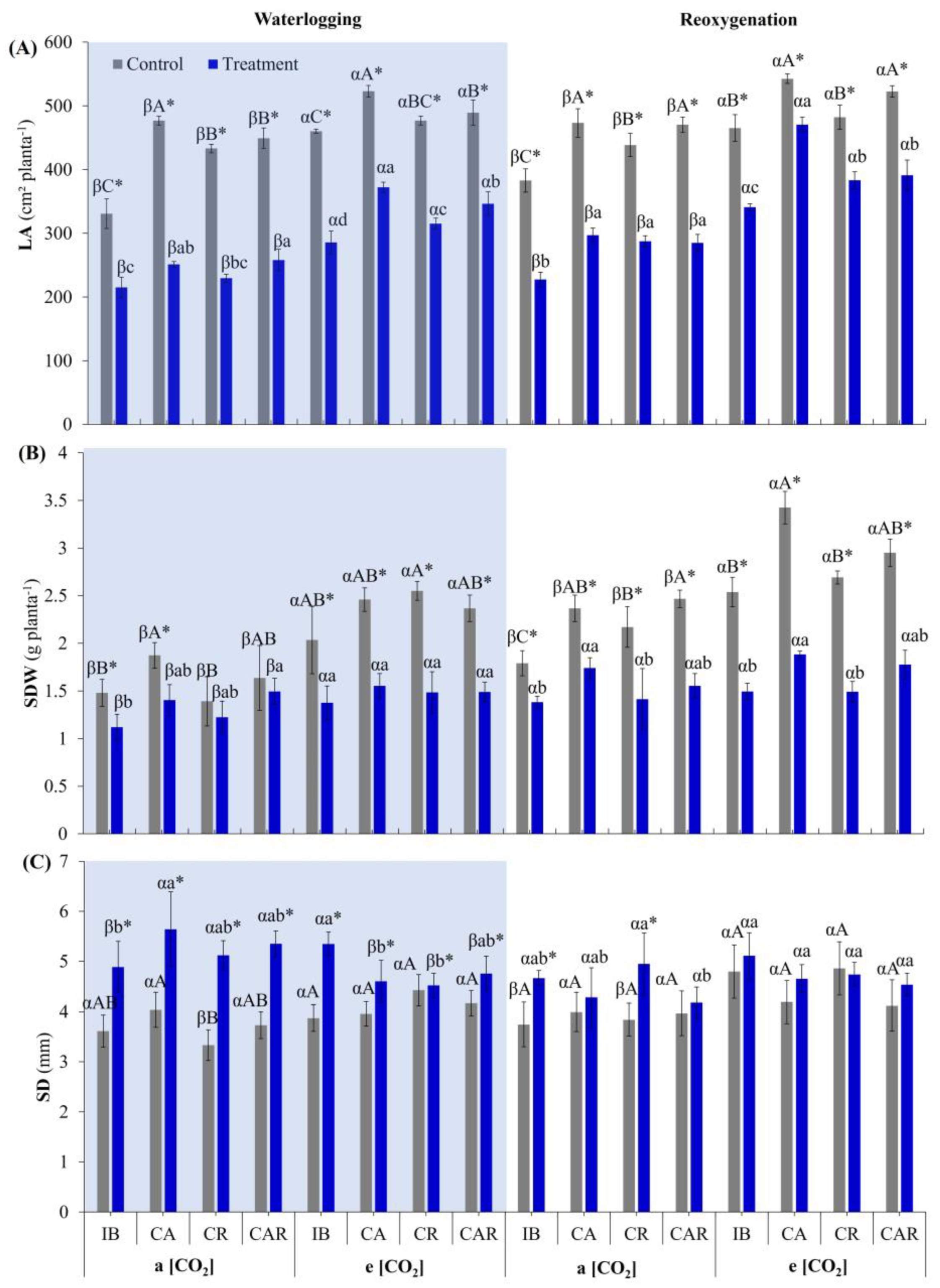
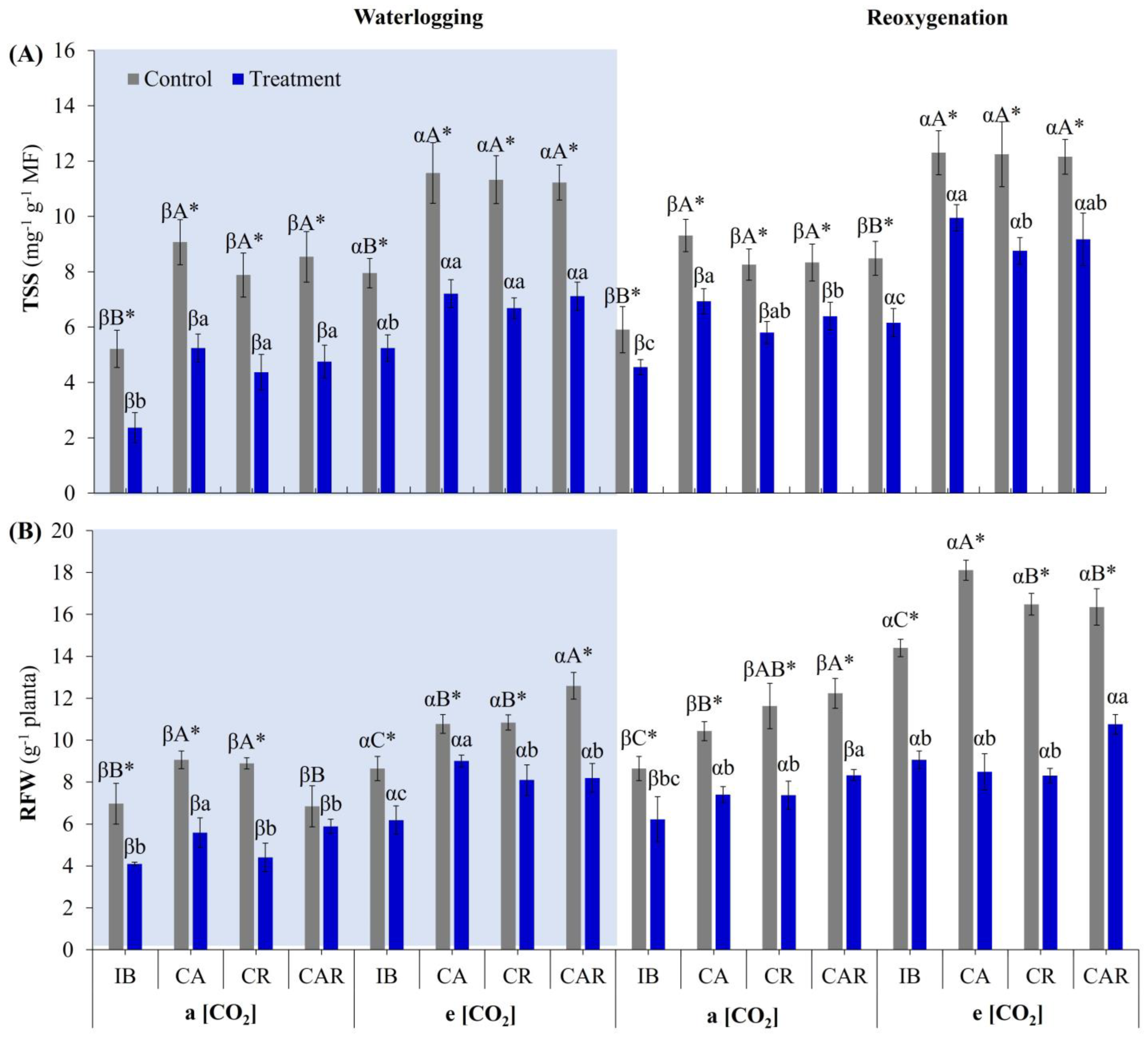
3.5. Effect of e[CO2] and Co-Inoculation on Biometric Parameters and Total Soluble Sugars
3.5.1. Biometric Parameters
3.5.2. Total Soluble Sugars and Root Fresh Weight
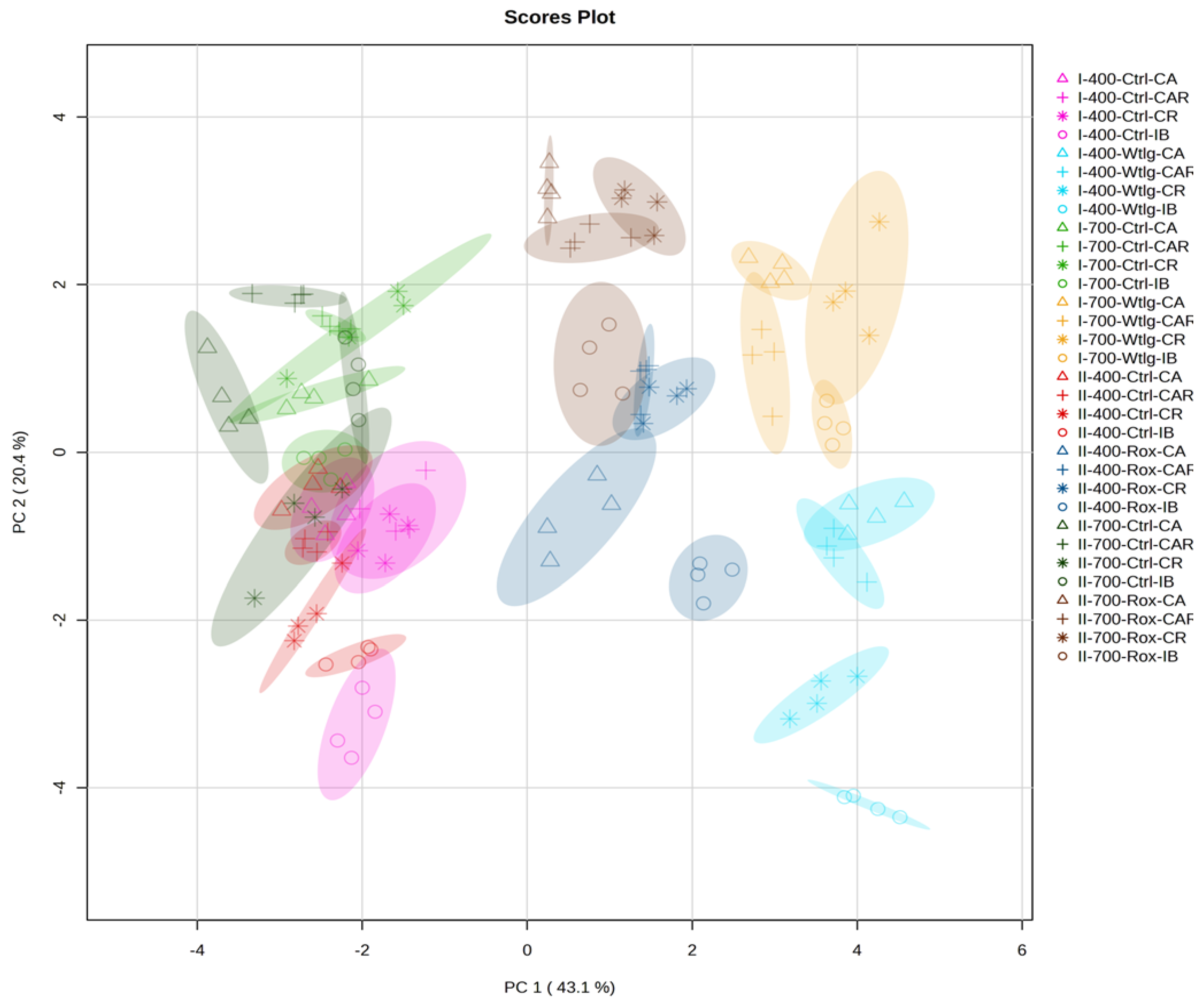
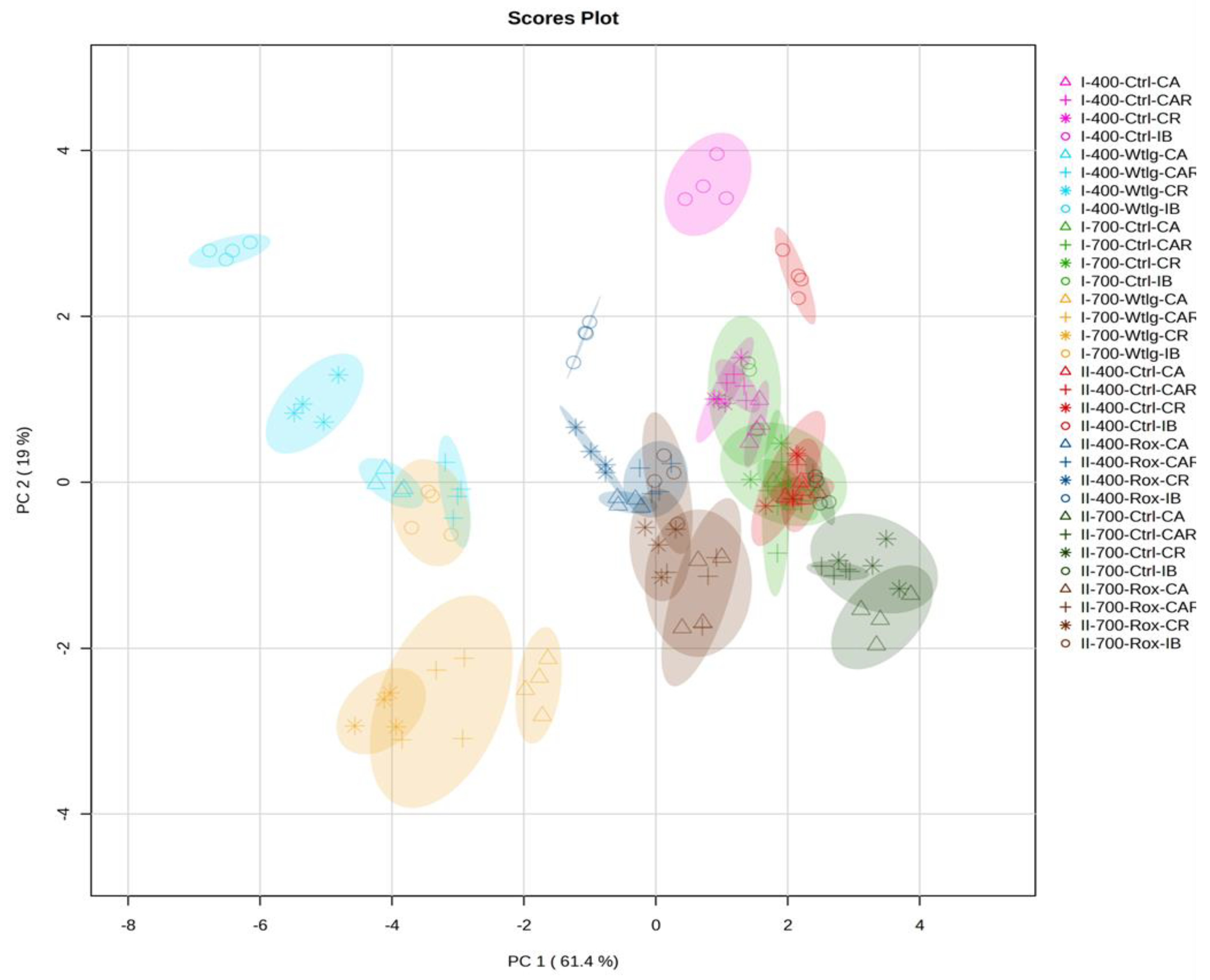
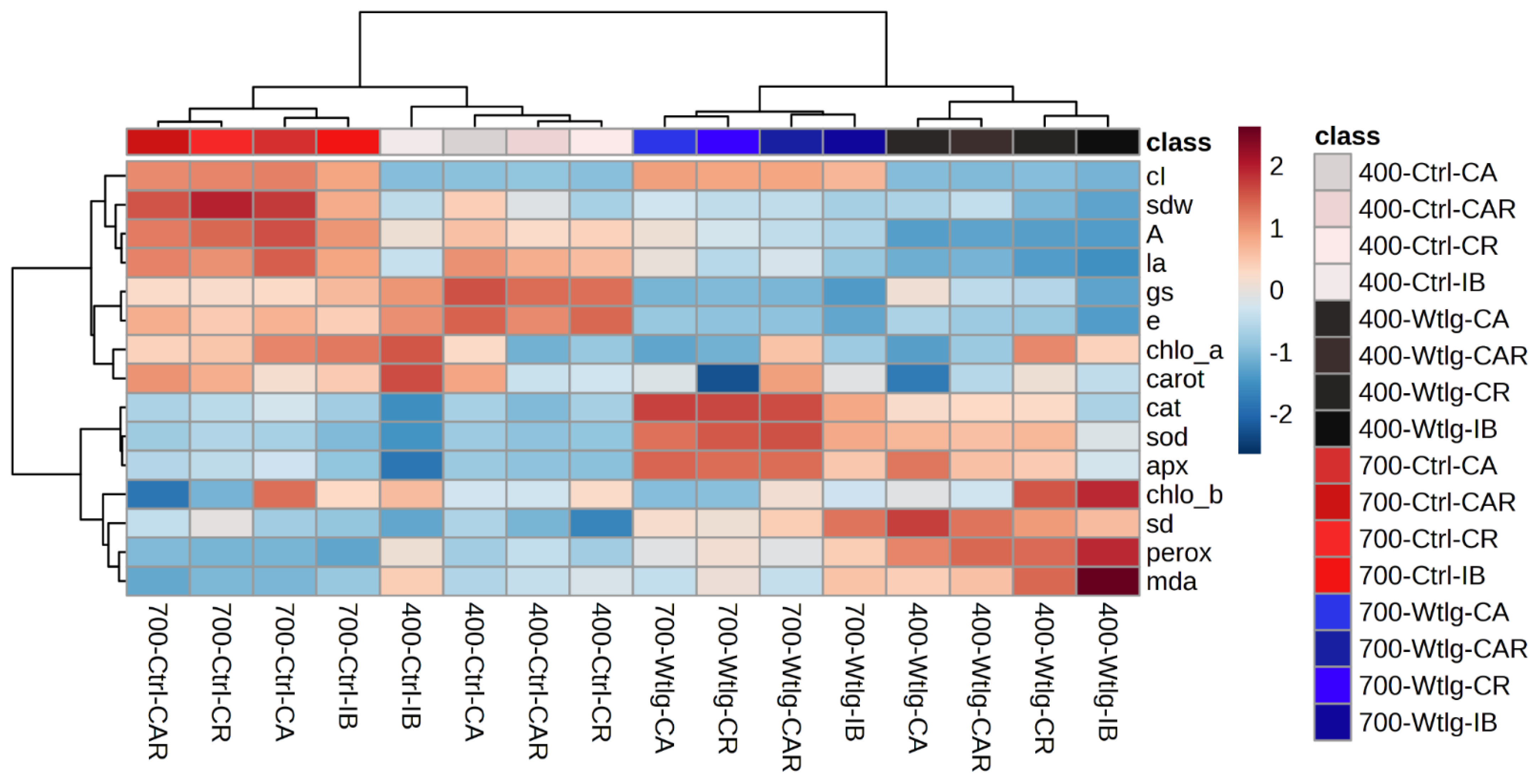
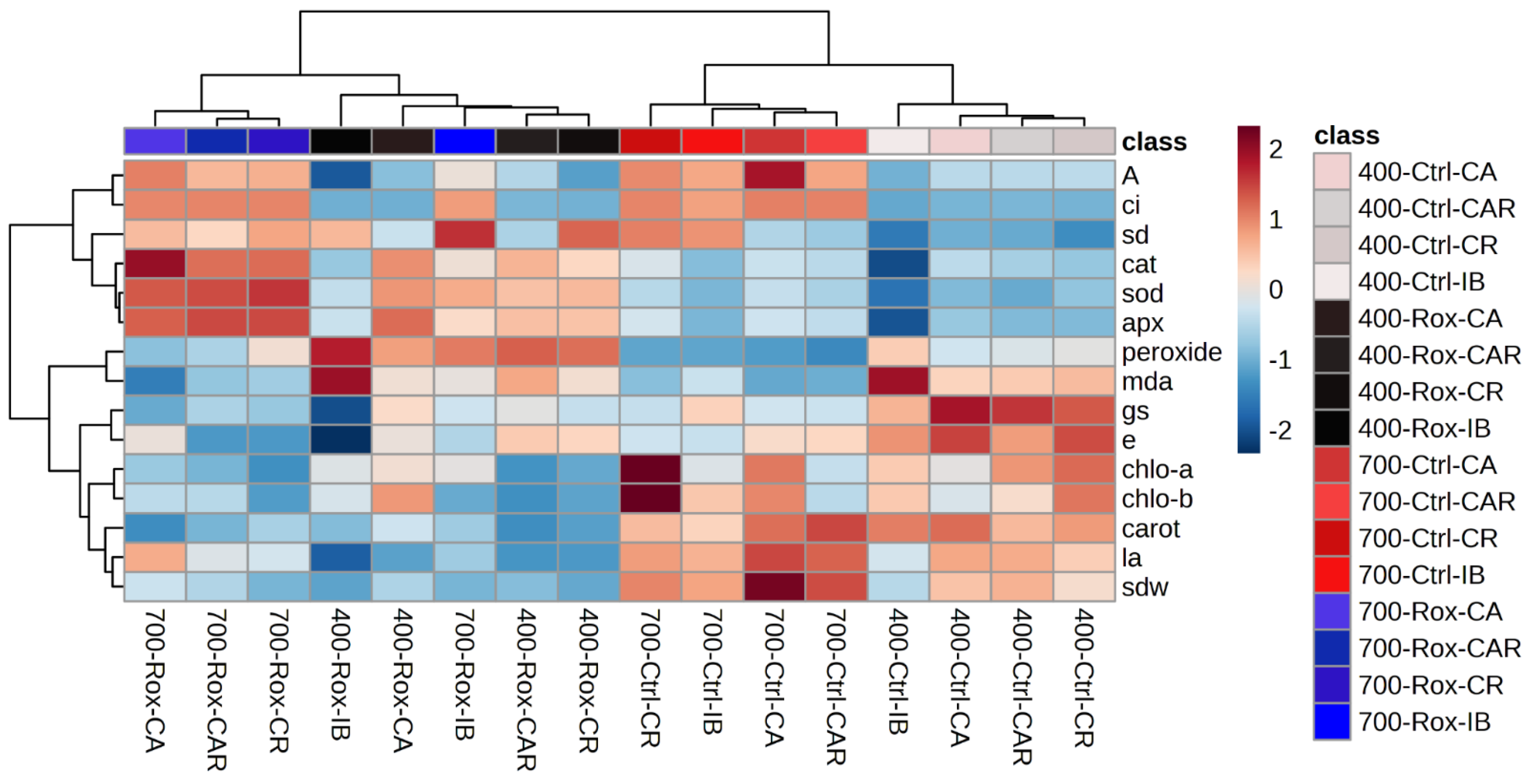
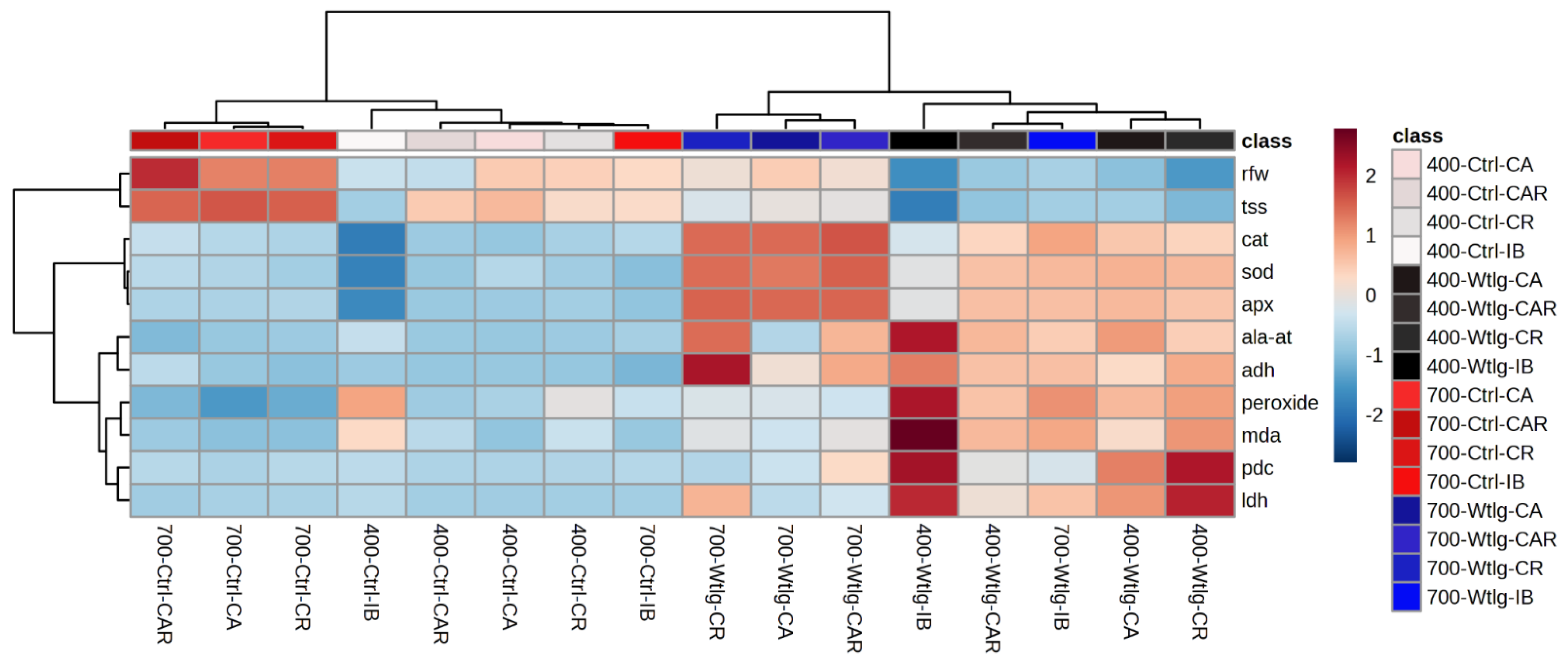
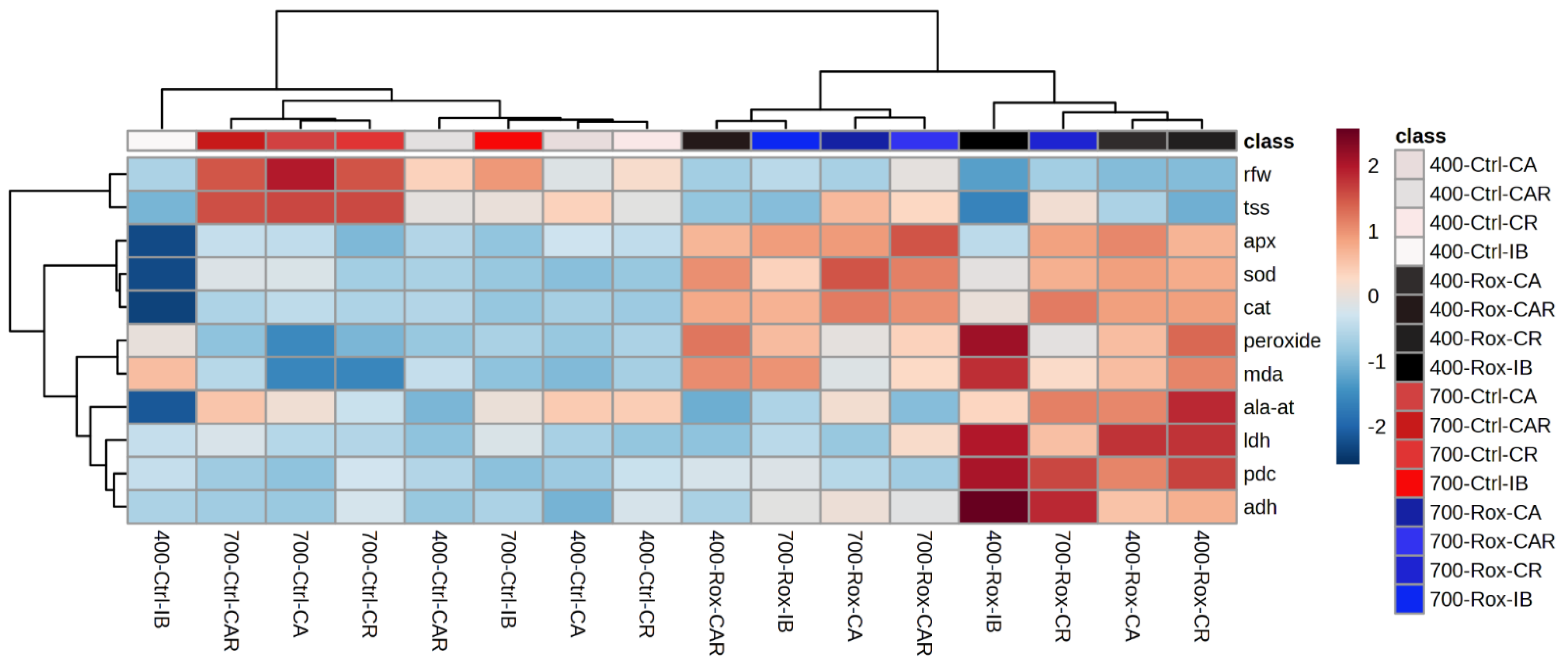
3.5.3. Principal Component Analysis and Hierarchical Clustering Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.B.; Le, Q.C.; Andrew, R.M.; Canadell, J.G.; Peters, G.P.; Roy, J.; Wu, L. Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 2017, 12, 110202. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Le, Q.C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Thomas, J.C.; Nathan, P.G.; Houghton, R.A.; Gregg, M. Contributions to accelerate the growth of atmospheric CO2 in economic activity, carbon intensity and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide. NOAA. 2016. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends (accessed on 10 March 2024).
- Bernacchi, C.J.; Leakey, A.D.B.; Heady, L.E.; Morgan, P.B.; Dohleman, F.G.; Mcgrath, J.M.; Gillespie, K.M.; Wittig, V.E.; Rogers, A.; Long, S.P.; et al. Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant Cell Environ. 2006, 29, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
- Drag, D.W.; Slattery, R.; Siebers, M.; DeLucia, E.H.; Ort, D.R.; Bernacchi, C.J. Soybean photosynthetic and biomass responses to carbon dioxide concentrations ranging from pre-industrial to the distant future. J. Exp. Bot. 2020, 71, 3690–3700. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, J.; Xie, Z.; Liu, G.; Zeng, Q.; Han, Y. Responses of rice and winter wheat to free-air CO2 enrichment (China FACE) at rice/wheat rotation system. Plant Soil 2007, 294, 137–146. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.A.; Wang, X.; Philpott, S.M. A metaanalysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 2012, 169, 1–13. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Bourgault, M.; Roessner, U.; Tausz-Posch, S.; Armstrong, R.; O’Leary, G.; Fitzgerald, G.; Tausz, M. Water availability moderates N2 fixation benefit from elevated [CO2]: A 2-year free-air CO2 enrichment study on lentil (Lens culinaris Medik.) in a water limited agroecosystem. Plant Cell Environ. 2018, 41, 2418–2434. [Google Scholar] [CrossRef]
- Rogers, A.; Ainsworth, E.A.; Leakey, A.D. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 2009, 151, 1009–1016. [Google Scholar] [CrossRef]
- Kant, S.; Seneweera, S.; Rodin, J.; Materne, M.; Burch, D.; Rothstein, S.J.; Spanenberg, G. Improving yield potential in crops under elevated CO2: Integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule performance within a changing environmental context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Chen, D.; Norton, R.; Armstrong, R. Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop Pasture Sci. 2012, 63, 53–62. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Liu, X.; Mathesius, U.; Wang, G.; Tang, C.; Jin, J. Elevated CO2 increases nitrogen fixation at the reproductive phase contributing to various yield responses of soybean cultivars. Front. Plant Sci. 2017, 8, 1546. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Stiling, P.D.; Dijkstra, P.; Johnson, D.W.; Ketterer, M.E.; Hymus, G.J.; Hinkle, R.C.; Drake, B.G. CO2 elicits long-term decline in nitrogen fixation. Science 2004, 304, 1291. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.D.; Tilman, J.M.H.; Knops, S.; Naeem, J. TrostNitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef]
- van Groenigen, K.J.; Six, J.; Hungate, B.A.; de Graaff, M.A.; Van Breemen, N.; Van Kessel, C. Element interactions limit soil carbon storage. Proc. Natl. Acad. Sci. USA 2006, 103, 6571–6574. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Momesso, L.; Garcia, A.; Kuramae, E.E.; Hungria, M. Bacterial Consortium and Microbial Metabolites Increase Grain Quality and Soybean Yield. J. Soil Sci. Plant Nutr. 2020, 20, 1923–1934. [Google Scholar] [CrossRef]
- Musyoka, D.M.; Njeru, E.M.; Nyamwange, M.M.; Maingi, J.M. Arbuscular mycorrhizal fungi and coinoculation of Bradyrhizobium increase nitrogen fixation and the growth of green grasses (Vigna radiata L.) under water stress. J. Plant Nutr. 2020, 43, 1036–1047. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Souza, S.R.D.; Schultz, N.; Saggin Júnior, O.J.; Sperandio, M.V.L.; Zilli, J.É. Interação da planta com fungo micorrízico e sua resposta à inoculação com diferentes fungos promotores de crescimento. Pesqui. Agropecu. Bras. 2018, 54, e25140. [Google Scholar] [CrossRef]
- Jilkova, V.; Sim, A.; Thornton, B.; Jandová, K.; Cajthaml, T.; Paterson, E. Impact of plant species and atmospheric CO2 concentration on rhizodeposition and soil microbial activity and community composition. J. Soil Sci. Plant Nutr. 2020, 183, 327–337. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Sasidharan, R. Ethylene–and oxygen signalling–drive plant survival during waterlogging. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; da-Silva, C.J.; Cocco, K.L.T.; Pomagualli, D.; de Oliveira, F.K.; da Silva, J.V.L.; de Oliveira, A.C.B.; do Amarante, L. Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environ. Exp. Bot. 2020, 172, 103975. [Google Scholar] [CrossRef]
- Fukami, J.; de la Osa, C.; Ollero, F.J.; Megías, M.; Hungria, M. Co-inoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct. Plant Biol. 2018, 45, 328–339. [Google Scholar] [CrossRef]
- Palit, P.; Kudapa, H.; Zougmore, R.; Kholova, J.; Whitbread, A.; Sharma, M.; Varshney, R.K. An integrated research framework combining genomics, systems biology, physiology, modelling and breeding for legume improvement in response to elevated CO2 under climate change scenario. Curr. Plant Biol. 2020, 22, 100149. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Richter, G.L.; Zanon, A.; Streck, N.A.; Guedes, J.V.C.; Kräulich, B.; da Rocha, T.S.M.; Winck, J.E.M.; Cera, J.C. Estimating leaf area of modern soybean cultivars by a non-destructive method. Bragantia 2014, 73, 416–425. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total Carotenoids, using various solvents with spectrophotometers of diferente resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidante enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 66, 280–292. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K.H. Peroxide is Scavenged by Ascorbatespecific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hanson, A.D.; Jacobsen, J.V.; Zwar, J.A. Regulated Expression of Three Alcohol Dehydrogenase Genes in Barley Aleurone Layers. Plant Physiol. 1984, 75, 573–581. [Google Scholar] [CrossRef]
- Hanson, A.D.; Jacobsen, J.V. Control of Lactate Dehydrogenase, Lactate Glycolysis, and α-Amylase by O2 Deficit in Barley Aleurone Layers. Plant Physiol. 1984, 75, 566–572. [Google Scholar] [CrossRef]
- Good, A.G.; Muench, D.G. Purification and Characterization of an Anaerobically Induced Alanine Aminotransferase from Barley Roots. Plant Physiol. 1992, 99, 1520–1525. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Graham, D.; Smydzuk, J. Use of anthrone in the quantitative determination of hexose phosphates. Anal. Biochem. 1965, 11, 246–255. [Google Scholar] [CrossRef]
- Posso, D.A.; da-Silva, C.J.; Shimoia, E.P.; da Silva Martins, T.; Reissig, G.N.; de Oliveira, A.C.B.; Borela, J.; van Dogen, T.J.; do Amarante, L. Root-hypoxia tolerance in soybean sister-lines plants indicates a better balance in energy use/dissipation and oxidative stress control. Plant Stress 2023, 10, 100225. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.S. The end game(s) of photosynthetic carbon metabolism. Plant Physiol. 2024, 195, 67–78. [Google Scholar] [CrossRef]
- do Amarante, L.; Lima, J.D.; Sodek, L. Alterations of xylem transport of key metabolic products of assimilatory activity in soybean: Do similar alterations occur in roots and nodules? Acta Physiol. Plant 2022, 44, 11. [Google Scholar] [CrossRef]
- Vitor, S.C.; Sodek, L. Products of anaerobic metabolism in waterlogged roots 978 of soybean are exported in the xylem. Plant Sci. 2019, 284, 82–90. [Google Scholar] [CrossRef] [PubMed]
- da Silva Martins, T.; Da-Silva, C.J.; Shimoia, E.P.; Posso, D.A.; Carvalho, I.R.; de Oliveira, A.C.B.; do Amarante, L. Nitrate supply decreases fermentation and alleviates oxidative and ionic stress in nitrogen-fixing soybean exposed to saline waterlogging. Funct. Plant Biol. 2023, 50, 416–433. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Shimoia, E.P.; Posso, D.A.; Cardoso, A.A.; Batz, T.A.; Oliveira, A.C.B.; do Amarante, L. Nitrate nutrition increases foliar levels of nitric oxide and waterlogging tolerance in soybean. Acta Physiol. Plant. 2021, 43, 116. [Google Scholar] [CrossRef]
- da-Silva, C.J.; do Amarante, L. Short-term nitrate supply decreases fermentation and oxidative stress caused by waterlogging in soybean plants. Environ. Exp. Bot. 2020, 176, 104078. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Soybean production under waterlogging stress and its mitigation using plant growth-promoting microbes. In Environmental Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: Oxford, UK, 2016; Volume 2, pp. 23–40. [Google Scholar] [CrossRef]
- Shimoia, E.P.; Da-Silva, C.J.; Posso, D.A.; da Silva Martins, T.; Agualongo, D.A.P.; de Oliveira, A.C.B.; do Amarante, L. Co-inoculation of Seeds with Bradyrhizobium, Azospirillum, and Rhizophagus Improves Nitrogen Assimilation and Growth in Soybean Plants Subjected to Waterlogging. Russ. J. Plant Physiol. 2023, 70, 146. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Zawoznik, M.S.; Ameneiros, M.; Benavides, M.P.; Vázquez, S.; Groppa, M.D. Response to saline stress and aquaporin expression in Azospirillum-inoculated barley seedlings. Appl. Microbiol. Biotechnol. 2011, 90, 1389–1397. [Google Scholar] [CrossRef]
- Chibeba, A.M.; de Fátima Guimarães, M.; Brito, O.R.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Co-Inoculation of Soybean with Bradyrhizobium and Azospirillum Promotes Early Nodulation. Am. J. Plant Sci. 2015, 6, 1641–1649. [Google Scholar] [CrossRef]
- Younesi, O.; Moradi, A.; Namdari, A. Influence of arbuscular mycorrhiza on osmotic adjustment compounds and antioxidant enzyme activity in nodules of salt-stressed soybean (Glycine max). Acta Agric. Slov. 2013, 101, 219. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Stoffel, S.C.G.; Meyer, E.; Lovato, P.E. Yield increase of soybean inoculated with a commercial arbuscular mycorrhizal inoculant in Brazil. Afr. J. Agric. Res. 2020, 16, 702–713. [Google Scholar]
- Fukami, J.; Ollero, F.J.; de la Osa, C.; Valderrama-Fernández, R.; Nogueira, M.A.; Megías, M.; Hungria, M. Antioxidant activity and induction of mechanisms of resistance to stresses related to the inoculation with Azospirillum brasilense. Arch. Microbiol. 2018, 200, 1191–1203. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; do Amarante, L. Time-course biochemical analyses of soybean plants during waterlogging and reoxygenation. Environ. Exp. Bot. 2020, 180, 104242. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, M.J.; Kim, J.M.; Lee, T.; Heo, J.H.; Jeong, J.Y.; Lee, J.; Moon, J.K.; Kang, S. Growth response and developing simple test method for waterlogging stress tolerance in soybean. J. Crop Sci. Biotechnol. 2019, 22, 371–378. [Google Scholar] [CrossRef]
- Juge, C.; Prévost, D.; Bertrand, A.; Bipfubusa, M.; Chalifour, F.P. Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium, Azospirillum and arbuscular mycorrhizae. Appl. Soil Ecol. 2012, 61, 147–157. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Cornero, M.; Chiocchio, V.; Lavado, R.S.; Roberts, I.N. Arbuscular mycorrhiza protects soybean plants against Macrophomina phaseolina even under nitrogen fertilization. Eur. J. Plant Pathol. 2020, 156, 839–849. [Google Scholar] [CrossRef]
- Agualongo, D.A.P.; Da-Silva, C.J.; Garcia, N.; de Oliveira, F.K.; Shimoia, E.P.; Posso, D.A.; de Oliveira, A.C.B.; Colares, D.S.O.; do Amarante, L. Waterlogging priming alleviates the oxidative damage, carbohydrate consumption, and yield loss in soybean (Glycine max) plants exposed to waterlogging. Funct. Plant Biol. 2022, 49, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A. Plant stress physiology. In Encyclopaedia of Life Sciences; Nature Publishing Group: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Hu, Y.; Zhang, T.; Guo, J.; Shi, L.; Li, M. Comparative study of leaf nutrient reabsorption by two different ecotypes of wild soybean under low-nitrogen stress. PeerJ 2023, 11, e15486. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Produtores microbianos de estimuladores de crescimento de plantas e seu uso prático: Uma revisão. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Syvash, O.O.; Zolotareva, O.K. Regulation of chlorophyll degradation in plant tissues. Biotechnol. Acta 2017, 10, 20–30. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef]
- Gong, Z.; Duan, Y.; Liu, D.; Zong, Y.; Zhang, D.; Shi, X.; Hao, X.; Li, P. Physiological and transcriptome analysis of response of soybean (Glycine max) to cadmium stress under elevated CO2 concentration. J. Hazard. Mater. 2023, 448, 130950. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, W. Impacts of CO2 elevation on the physiology and seed quality of soybean. Plant Divers. 2020, 42, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shabbaj, I.I.; AbdElgawad, H.; Balkhyour, M.A.; Tammar, A.; Madany, M.M. Elevated CO2 differentially mitigated oxidative stress induced by indium oxide nanoparticles in young and old leaves of C3 and C4 crops. Antioxidants 2022, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.K.; Da-Silva, C.J.; Garcia, N.; Agualongo, D.A.P.; de Oliveira, A.C.B.; Kanamori, N.; Takasaki, H.; Urano, K.; Shinozaki, K.; Nakashima, K.; et al. The overexpression of NCED results in waterlogging sensitivity in soybean. Plant Stress 2022, 3, 100047. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Abo-Ghalia, H.H. Effect of arbuscular mycorrhizal fungi on the metabolic products and activity of antioxidant system in wheat plants subjected to short-term water stress, followed by recovery at different growth stages. J. Appl. Sci. Res. 2008, 4, 559–569. [Google Scholar]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Rizobactérias promotoras de crescimento de plantas. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Glick, B.R. The Use of Microorganisms to Facilitate the Growth of Plants in Saline Soils. In Microbial Strategies for Crop Improvement; Khan, M., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]
- Zakikhani, H.; Ardakani, M.R.; Rejali, F.; Gholamhoseini, M.; Joghan, A.K.; Dolatabadian, A. Influence of diazotrophic bacteria on antioxidant enzymes and some biochemical characteristics of soybean subjected to water stress. J. Integr. Agric. 2012, 11, 1828–1835. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.C.; De Camargo, R.; Lana, R.M.Q.; Franco, M.H.R.; Stanger, M.C.; Pereira, V.J.; Lemes, E.M. Azospirillum brasilense and organomineral fertilizer co-inoculated with Bradyrhizobium japonicumon oxidative stress in soybean. Int. J. Recycl. Org. Waste Agric. 2022, 11, 229–245. [Google Scholar] [CrossRef]
- Pucciariello, C.; Boscari, A.; Tagliani, A.; Brouquisse, R.; Perata, P. Exploring legume-rhizobia symbiotic models for waterlogging tolerance. Front. Plant Sci. 2019, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed]
- António, C.; Päpke, C.; Rocha, M.; Diab, H.; Limami, A.M.; Obata, T.; Fernie, A.R.; van Dongen, J.T. Regulation of primary metabolism in response to low oxygen availability as revealed by carbon and nitrogen isotope redistribution. Plant Physiol. 2016, 170, 43–56. [Google Scholar] [CrossRef]
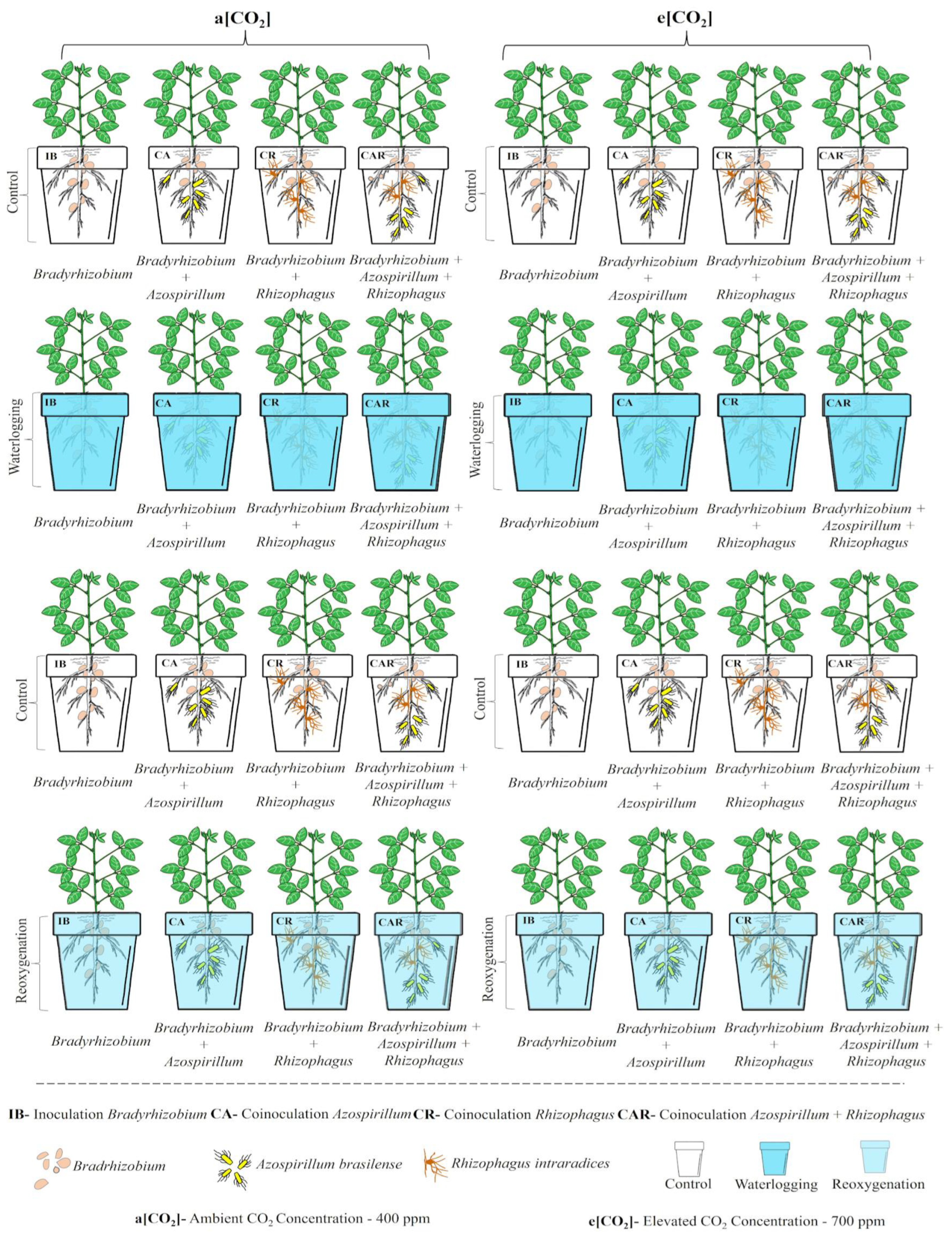



| Treatment | Abbreviation | Microorganisms * | ||
|---|---|---|---|---|
| Bradyrhizobium elkanii SEMIA 5019 and Bradyrhizobium Japonicum SEMIA 5079 | Azospirillum brasilense Strains Ab-V5 and Ab-V6 | Rhizophagus intraradices ROOTELLA BR® (Joinville, SC, Brazil) | ||
| Bradyrhizobium Inoculation | IB | 4 mL seeds kg−1 with 5 × 109 CFU mL−1 | - | - |
| Co-inoculation Bradyrhizobium + Azospirillum | CA | 4 mL seeds kg−1 with 5 × 109 CFU mL−1 | 2 mL seeds kg−1 with 2 × 108 CFU mL−1 | - |
| Co-Inoculation Bradyrhizobium + Rhizophagus | CR | 4 mL seeds kg−1 with 5 × 109 CFU mL−1 | - | 1.35 g seeds kg−1 with 20,800 propagules g−1 |
| Co-Inoculation Bradyrhizobium + Azospirillum + Rhizophagus | CAR | 4 mL seeds kg−1 with 5 × 109 CFU mL−1 | 2 mL seeds kg−1 with 2 × 108 CFU mL−1 | 1.35 g seeds kg−1 with 20,800 propagules g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoia, E.P.; Posso, D.A.; da-Silva, C.J.; Bester, A.U.; Bernardi, N.D.C.; Carvalho, I.R.; de Oliveira, A.C.B.; Avila, L.A.d.; do Amarante, L. Co-Inoculation of Soybean Seeds with Azospirillum and/or Rhizophagus Mitigates the Deleterious Effects of Waterlogging in Plants under Enhanced CO2 Concentrations. Nitrogen 2024, 5, 941-976. https://doi.org/10.3390/nitrogen5040061
Shimoia EP, Posso DA, da-Silva CJ, Bester AU, Bernardi NDC, Carvalho IR, de Oliveira ACB, Avila LAd, do Amarante L. Co-Inoculation of Soybean Seeds with Azospirillum and/or Rhizophagus Mitigates the Deleterious Effects of Waterlogging in Plants under Enhanced CO2 Concentrations. Nitrogen. 2024; 5(4):941-976. https://doi.org/10.3390/nitrogen5040061
Chicago/Turabian StyleShimoia, Eduardo Pereira, Douglas Antônio Posso, Cristiane Jovelina da-Silva, Adriano Udich Bester, Nathalia Dalla Corte Bernardi, Ivan Ricardo Carvalho, Ana Cláudia Barneche de Oliveira, Luis Antonio de Avila, and Luciano do Amarante. 2024. "Co-Inoculation of Soybean Seeds with Azospirillum and/or Rhizophagus Mitigates the Deleterious Effects of Waterlogging in Plants under Enhanced CO2 Concentrations" Nitrogen 5, no. 4: 941-976. https://doi.org/10.3390/nitrogen5040061
APA StyleShimoia, E. P., Posso, D. A., da-Silva, C. J., Bester, A. U., Bernardi, N. D. C., Carvalho, I. R., de Oliveira, A. C. B., Avila, L. A. d., & do Amarante, L. (2024). Co-Inoculation of Soybean Seeds with Azospirillum and/or Rhizophagus Mitigates the Deleterious Effects of Waterlogging in Plants under Enhanced CO2 Concentrations. Nitrogen, 5(4), 941-976. https://doi.org/10.3390/nitrogen5040061






