New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Growth Conditions
2.2. Experimental Design and Treatments
- i = Bean plant organs (Split)
- j = Molybdenum Source (Sub-split)
- k = Nitrogen doses (Sub-sub-split)
- l = Molybdenum doses (Sub-sub-sub-split)
- m = Repetition
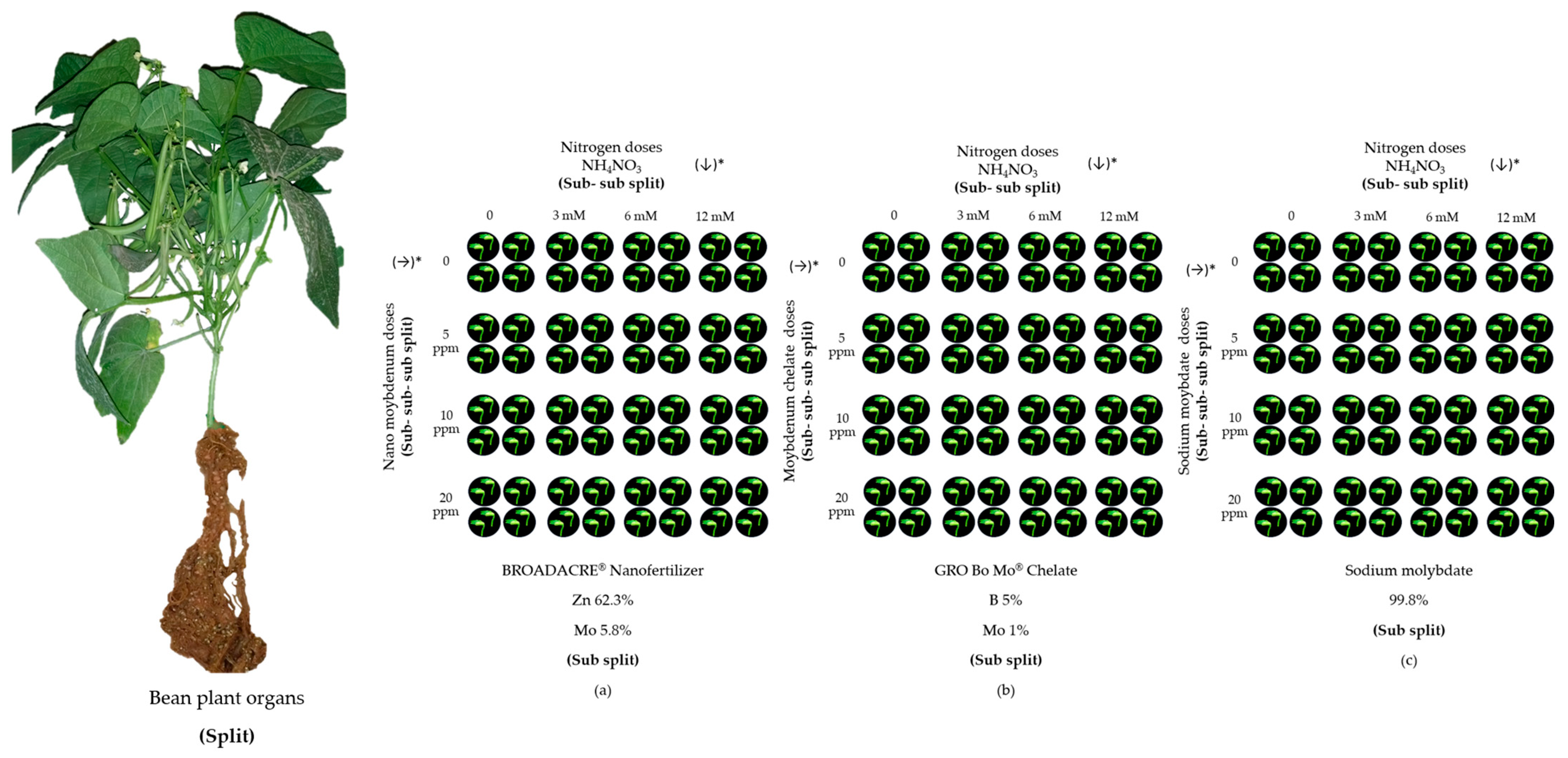
2.3. Plant Analysis
2.3.1. Total Biomass
2.3.2. Total Yield
2.3.3. Total Nitrogen Content
2.4. Efficiency Indices of Absorption, Distribution and Utilization of Nitrogen for the Formation of Plant Biomass
- Total weight (TW)
- Relative physiological contribution (RPC).
- Physiological nitrogen requirement (PNRmg).
- Relative nitrogen contribution (RNC%).
- Nitrogen fixation efficiency (NFiE).
- Nitrogen conduction (translocation) efficiency (NCoE).
- Nitrogen absorption efficiency (NAbE).
- Productivity.
2.5. Statistical Analysis
3. Results
3.1. Effect of Nitrogen Fertilization Supplemented with Molybdenum Foliar Nanofertilizer on NUE Indices for Biomass Formation
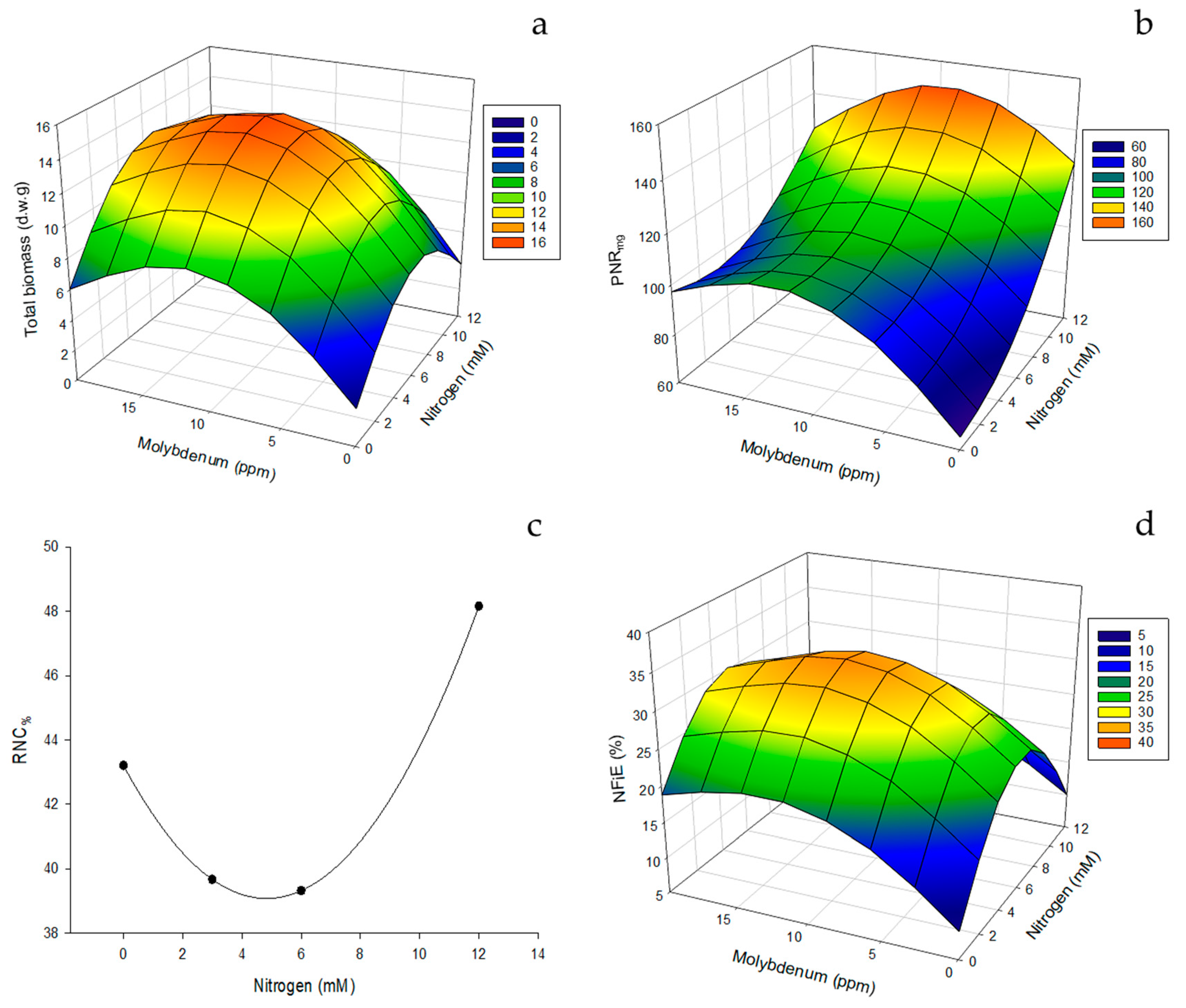
3.1.1. NUE Indices in Leaves
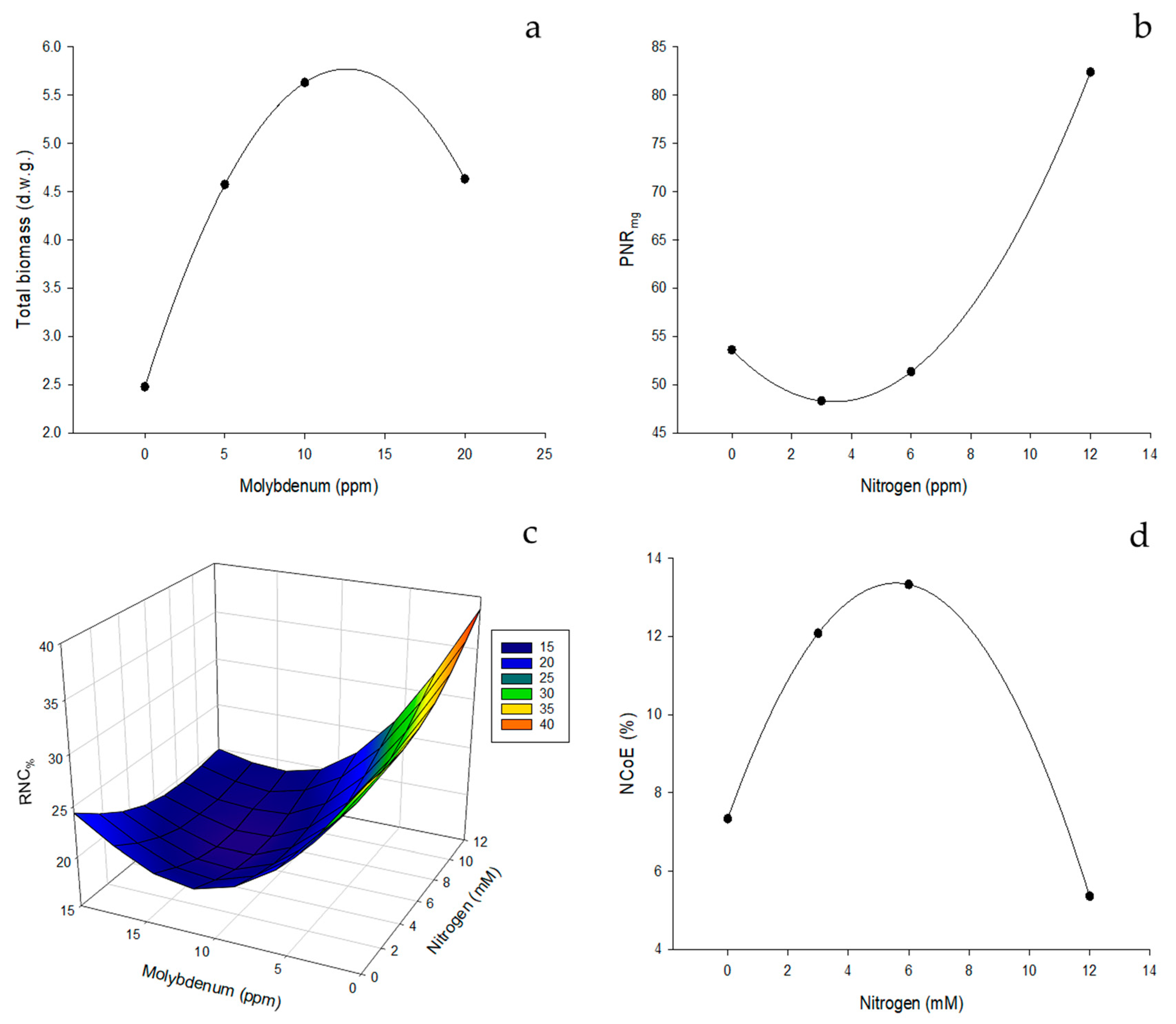
3.1.2. NUE Indices for Stems
3.1.3. NUE Indices for Roots
3.1.4. Indices NUE for Fruit
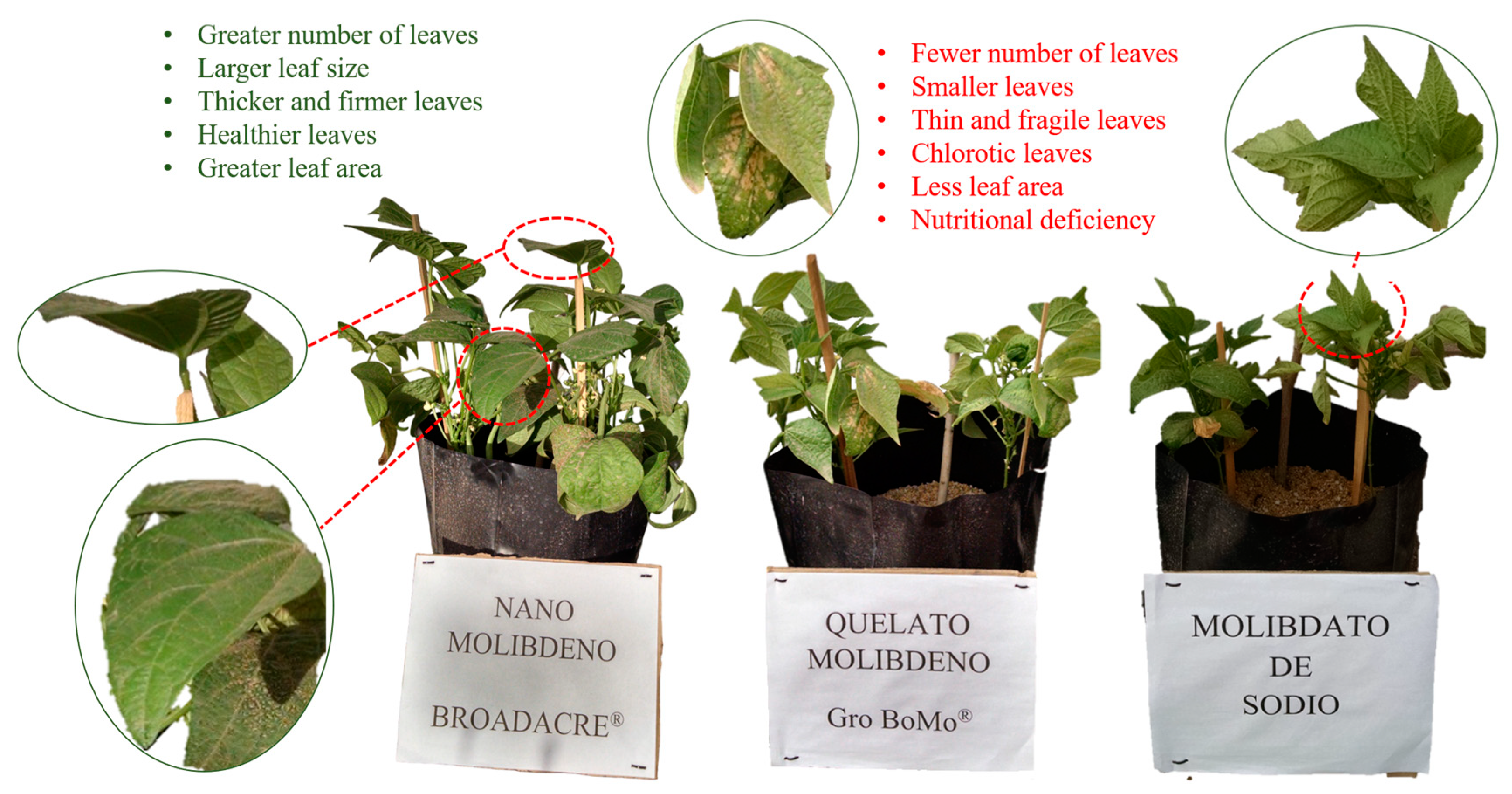
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doi, M.; Higuchi, K.; Saito, A.; Sato, T.; Ohyama, T.N. Absorption, Transport, and Recycling in Nodulated Soybean Plants by Split-Root Experiment Using 15N-Labeled Nitrate. Nitrogen 2022, 3, 636. [Google Scholar] [CrossRef]
- Poultney, D.M.N.; Thuriès, L.; Versini, A. Importance of Overlooked Crop Biomass Components in Sugarcane Nitrogen Nutrition Studies. Nitrogen 2024, 5, 62–78. [Google Scholar] [CrossRef]
- Escalante-Estrada, J.A.S.; Rodríguez-González, M.T.; Escalante-Estrada, Y.I. Rendimiento de frijol y la aplicación de nitrógeno. Explor. Rev. Real. Glob. 2021, 10, 106–112. [Google Scholar]
- Rojas-Victoria, N.J.; Escalante-Estrada, J.A.S.; Aguilar-Carpio, C. Análisis de crecimiento, rendimiento de frijol ayocote (Phaseolus coccineus L.) en un sistema de fertilización nitrogenada. Trop. Subtrop. Agroecosyst. 2023, 26, 12. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; Vadivel, R.T.K.D.; Raj, R.; Pooniya, V.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Marschner, H. Functions of mineral nutrients: Micronutrients. In Mineral Nutrition of Higher Plants 1995, 2nd ed.; Academic Press: London, UK, 1995; pp. 313–404. [Google Scholar]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, P.X.; Liu, Y.; Li, R.Y. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Hu, C.X. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 2019, 20, 3009. [Google Scholar] [CrossRef]
- Muñoz-Márquez, E.; Soto-Parra, J.M.; Noperi-Mosqueda, L.C.; Sánchez, E. Application of molybdenum nanofertilizer on the nitrogen use efficiency, growth and yield in green beans. Agronomy 2022, 12, 3163. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil; California Agricultural Experiment Station, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Sánchez, E.; Romero, L.; Ruíz, J.M. Caracterización del Estado Nutricional y Fisiológico en Plantas de Judía (Phaseolus vulgaris L. cv. Strike) Sometidas a un Estrés Por Nitrógeno; Editorial de la Universidad de Granada: Granada, Spain, 2006; ISBN 84-338-4168-8. [Google Scholar]
- Ponce-García, C.O.; Soto-Parra, J.M.; Sánchez, E.; Muñoz-Márquez, E.; Piña-Ramírez, F.J.; Flores-Córdova, M.A.; Pérez-Leal, R.; Yáñez Muñoz, R.M. Efficiency of Nanoparticle, Sulfate, and Zinc-Chelate Use on Biomass, Yield, and Nitrogen Assimilation in Green Beans. Agronomy 2019, 9, 128. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Dumas. In A History of Chemistry; Palgrave: London, UK, 1964; pp. 337–375. [Google Scholar]
- SAS Institute Inc. SAS/STATR User’s Guide, Version 6, Fourth Edition, Volume 2; SAS Institute Inc.: Cary, NC, USA, 1989; pp. 1457–1478. [Google Scholar]
- Vargas, H.M.; Zarate, D.L.G.P.; Burguete, H.F. Factoriales fraccionados y superficie de respuesta, uso de paquetes estadísticos para microcomputadoras. Monogr. Man. Estadíst. Cómp. 1991, 10, 1. [Google Scholar]
- Yadav, M.R.; Kumar, S.; Lal, M.K.; Kumar, D.; Kumar, R.; Yadav, R.K.; Kumar, S.; Nanda, G.; Singh, J.; Udawat, P.; et al. Mechanistic Understanding of Leakage and Consequences and Recent Technological Advances in Improving Nitrogen Use Efficiency in Cereals. Agronomy 2023, 13, 527. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; White, J.C.; Franklin, F.A.; Melse-Boonstra, A.; Koele, N.; Pandey, R.; Rodenburg, J.; Senthilkumar, K.; Demokritou, P.; et al. Safeguarding Human and Planetary Health Demands a Fertilizer Sector Transformation. Plants People Planet 2020, 2, 302–309. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Echevarría-Machado, I. El tamaño sí importa: Los nanofertilizantes en la era de la agricultura de precisión. Desde El Herb. CICY 2019, 11, 69–75. [Google Scholar]
- Rademacher, I.; Nelson, C. Nitrogen Effects on Leaf Anatomy within the Intercalary Meristems of Tall Fescue Leaf Blades. Ann. Bot. 2001, 88, 893–903. [Google Scholar] [CrossRef][Green Version]
- Crook, M.J.; Ennos, A.R. The effect of nitrogen and growth regulators on stem and root characteristics associated with lodging in two cultivars of winter wheat. J. Exp. Bot. 1995, 46, 931–938. [Google Scholar] [CrossRef]
- Rodríguez, S.M.; Flórez, J.V.R. Ferti-Riego: Tecnologías y Programación en Agroplasticultura; Guzmán, M., López Gálvez, J., Eds.; CYTED: Cambridge, UK, 2004; pp. 25–36. ISBN 84-96023-27-3. DL: Al-290-2004; Available online: http://www.cyted.org (accessed on 14 June 2024).
- Mora-García, E.Y.; Reyes-Matamoros, J.; Martínez-Moreno, D.; Tenorio-Arvide, M.G. Eficiencia de nitrógeno en plántulas de maíz. RD-ICUAP 2019, 5, 14. [Google Scholar] [CrossRef]
- Monsalve, J.; Escobar, R.; Acevedo, M.; Sánchez, M.; Coopman, R. Efecto de la concentración de nitrógeno sobre atributos morfológicos, potencial de crecimiento radical y estatus nutricional en plantas de Eucalyptus globulus producidas a raíz cubierta. Bosque 2009, 30, 88–94. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Guo, Z.; Shi, Y.; Zhang, Y.; Yu, Z. Impact of tillage practices on nitrogen accumulation and translocation in wheat and soil nitrate-nitrogen leaching in drylands. Soil Tillage Res. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Kant, S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2017, 74, 89–96. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Liang, Z.; Guo, H.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Genetic improvement of nitrogen uptake and utilization of winter wheat in the Yangtze River Basin of China. Field Crops Res. 2016, 196, 251–260. [Google Scholar] [CrossRef]
- Namiki, S.; Takashi, O.; Yutaka, M.; Nobuyasu, S.; Takashi, I. Differential uptake and translocation of organic chemicals by several plant species from soil. J. Pestic. Sci. 2018, 43, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Effects of nitrogen application level on the physiological characteristics, yield and fruit quality of blackberry. Sci. Hortic. 2023, 313, 111915. [Google Scholar] [CrossRef]
- Sete, P.B.; Comin, J.J.; Nara Ciotta, M.; Almeida Salume, J.; Thewes, F.; Brackmann, A.; Brunetto, G. Nitrogen fertilization affects yield and fruit quality in pear. Sci. Hortic. 2019, 258, 108782. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Wei, Z.; Yang, H.; Fan, S.; Wu, W.; Lyu, L.; Li, W. Effects of different nitrogen forms on blackberry fruit quality. Foods 2023, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Cardeñosa, V.; Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Cuevas, F.; Pradas, I. Effects of salinity and nitrogen supply on the quality and health-related compounds of strawberry fruits (Fragaria × ananassa cv. Primoris). J. Sci. Food Agric. 2015, 95, 2924–2930. [Google Scholar] [CrossRef]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Basheer, L.; Yermiyahu, U. Olive (Olea europaea L.) tree nitrogen status is a key factor for olive oil quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef]






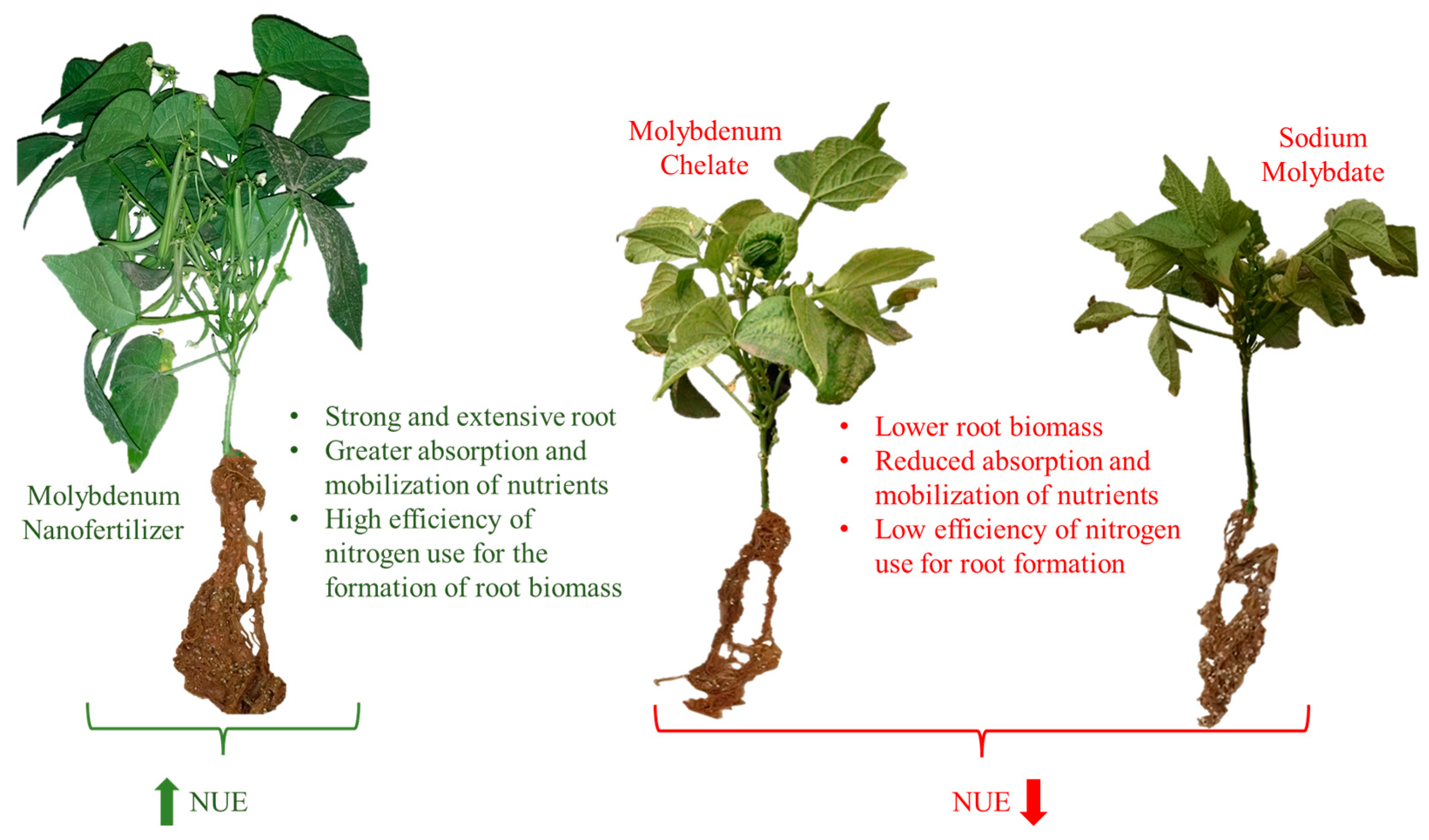

| Indices NUE (Leaf) | |||||
|---|---|---|---|---|---|
| TW | RPC | PNRmg | RCN% | NFiE | |
| Mo Source | <0.0001 U | <0.0001 | 0.0008 | 0.0719 | 0.6624 |
| Nano Mo | 8.93 a V | 52.50 a | 103.92 a | 42.58 a | 21.54 a |
| Mo Chelate | 5.21 b | 54.05 a | 77.46 b | 48.06 a | 23.49 a |
| Na Molybdate | 5.64 b | 43.52 b | 51.81 c | 40.44 a | 22.24 a |
| LSD | 1.35 W | 3.91 | 24.51 | 8.19 | 5.95 |
| Nitrogen X | <0.0001 | 0.0649 | <0.0001 | 0.0064 | <0.0001 |
| 0 | 4.74 c | 46.25 b | 51.76 b | 40.36 b | 30.90 a |
| 3 | 7.22 ab | 51.17 ab | 53.65 b | 39.71 b | 27.11 a |
| 6 | 8.20 a | 49.32 ab | 101.23 a | 44.14 ab | 17.27 b |
| 12 | 6.19 bc | 53.35 a | 104.26 a | 50.57 a | 14.42 b |
| LSD | 1.62 | 7.07 | 15.64 | 8.54 | 7.49 |
| Molybdenum Y | <0.0001 | 0.1327 | 0.9602 | 0.1924 | 0.0009 |
| 0 | 5.34 c | 50.34 a | 75.89 a | 44.68 a | 17.86 b |
| 5 | 6.07 bc | 52.35 a | 79.30 a | 46.80 a | 22.43 ab |
| 10 | 7.76 a | 47.88 a | 77.49 a | 40.99 a | 26.77 a |
| 20 | 7.20 ab | 49.56 a | 78.30 a | 43.30 a | 22.64 ab |
| LSD | 1.16 | 4.94 | 16.90 | 7.48 | 5.51 |
| SoMo*N | 0.0817 | 0.5016 | 0.0001 | 0.0926 | <0.0001 |
| SoMo*Mo | <0.0001 | 0.9408 | 0.0025 | 0.3620 | 0.0378 |
| N*Mo | 0.0675 | 0.2929 | 0.0256 | 0.0415 | 0.0327 |
| SoMo*N*Mo | 0.2956 | 0.0519 | 0.0085 | 0.0022 | <0.0001 |
| µ | 6.59 | 50.02 | 77.73 | 43.69 | 22.42 |
| C.V. | 33.08 | 18.57 | 40.83 | 32.17 | 46.18 |
| R2 | 0.7872 | 0.6217 | 0.7704 | 0.5990 | 0.7705 |
| Indices NUE (Stems) | |||||
|---|---|---|---|---|---|
| TW | RPC | PNRmg | RCN% | NCoE | |
| Mo Source | 0.0004 U | 0.2506 | 0.0003 | 0.4423 | 0.0074 |
| Nano Mo | 4.32 a V | 23.29 a | 58.91 a | 24.82 a | 9.52 b |
| Mo Chelate | 2.44 c | 23.88 a | 45.49 b | 27.26 a | 9.89 b |
| Na Molybdate | 3.29 b | 25.53 a | 34.25 c | 26.24 a | 12.90 a |
| LSD | 0.80 W | 3.59 | 10.31 | 5.10 | 2.45 |
| Nitrogen X | 0.0149 | 0.0104 | <0.0001 | <0.0001 | <0.0001 |
| 0 | 2.68 b | 22.52 b | 24.23 b | 17.65 b | 14.47 a |
| 3 | 3.53 ab | 25.41 ab | 33.98 b | 26.79 a | 13.31 a |
| 6 | 4.10 a | 25.86 a | 62.06 a | 29.45 a | 9.07 b |
| 12 | 3.09 ab | 23.14 ab | 64.59 a | 30.55 a | 6.24 c |
| LSD | 1.15 | 2.98 | 14.04 | 5.53 | 1.73 |
| Molybdenum Y | <0.0001 | 0.4451 | 0.1723 | 0.0065 | 0.0078 |
| 0 | 2.77 b | 25.39 a | 50.77 a | 29.29 a | 9.14 b |
| 5 | 2.85 b | 24.00 a | 47.76 a | 27.59 ab | 10.40 ab |
| 10 | 4.08 a | 23.57 a | 43.34 a | 22.77 b | 12.29 a |
| 20 | 3.70 a | 23.97 a | 42.99 a | 24.78 ab | 11.26 ab |
| LSD | 0.74 | 3.10 | 10.59 | 5.15 | 2.41 |
| SoMo*N | 0.6515 | 0.8421 | 0.0004 | <0.0001 | <0.0001 |
| SoMo*Mo | <0.0001 | 0.4362 | 0.2316 | 0.0287 | 0.9227 |
| N*Mo | 0.0857 | 0.3859 | 0.0111 | 0.1252 | 0.4571 |
| SoMo*N*Mo | 0.2070 | 0.8777 | 0.0022 | 0.0001 | 0.1112 |
| µ | 3.35 | 24.23 | 46.21 | 26.11 | 10.77 |
| C.V. | 41.40 | 24.03 | 43.01 | 37.06 | 42.09 |
| R2 | 0.7113 | 0.4407 | 0.7834 | 0.6900 | 0.7300 |
| Indices NUE (Roots) | |||||
|---|---|---|---|---|---|
| TW | RPC | PNRmg | RCN% | NAbE | |
| Mo Source | 0.0141 U | <0.0001 | <0.0001 | 0.3945 | <0.0001 |
| Nano Mo | 1.31 a V | 15.22 c | 47.30 a | 21.84 a | 5.75 c |
| Mo Chelate | 0.97 b | 18.74 b | 21.91 b | 19.42 a | 8.40 b |
| Na Molybdate | 1.28 a | 24.22 a | 21.83 b | 21.66 a | 13.24 a |
| LSD | 0.27 W | 3.00 | 7.52 | 5.24 | 2.39 |
| Nitrogen X | 0.0006 | <0.0001 | 0.0002 | <0.0001 | <0.0001 |
| 0 | 1.31 a | 25.80 a | 31.75 a | 35.50 a | 16.70 a |
| 3 | 1.34 a | 18.39 b | 32.14 a | 23.40 b | 9.30 b |
| 6 | 1.34 a | 18.26 b | 36.09 a | 16.02 c | 6.36 c |
| 12 | 0.85 b | 15.13 b | 21.40 b | 8.98 d | 4.15 c |
| LSD | 0.31 | 3.39 | 7.95 | 5.87 | 2.29 |
| Molybdenum Y | 0.0995 | 0.8598 | 0.1130 | 0.2469 | 0.0318 |
| 0 | 1.12 a | 20.11 a | 28.25 a | 19.98 a | 7.57 b |
| 5 | 1.05 a | 19.34 a | 27.97 a | 19.19 a | 8.54 ab |
| 10 | 1.31 a | 19.40 a | 33.34 a | 23.04 a | 10.54 a |
| 20 | 1.26 a | 18.73 a | 31.83 a | 21.70 a | 9.88 ab |
| LSD | 0.30 | 4.14 | 6.86 | 5.39 | 2.81 |
| SoMo*N | 0.1897 | 0.0274 | 0.5581 | <0.0001 | <0.0001 |
| SoMo*Mo | 0.0719 | 0.1580 | 0.0004 | 0.3757 | 0.4295 |
| N*Mo | 0.0264 | 0.6066 | 0.0079 | 0.0027 | 0.3090 |
| SoMo*N*Mo | 0.0006 | 0.0922 | 0.0001 | 0.0403 | 0.2646 |
| µ | 1.19 | 19.39 | 30.35 | 20.98 | 9.13 |
| C.V. | 48.65 | 40.10 | 42.49 | 48.24 | 57.94 |
| R2 | 0.6052 | 0.6114 | 0.7714 | 0.7642 | 0.7653 |
| Indices NUE (Fruit) | |||||
|---|---|---|---|---|---|
| TW | RPC | PNRmg | RCN% | Productivity | |
| Mo Source | 0.0020 U | 0.0145 | 0.0923 | 0.0334 | 0.0059 |
| Nano Mo | 2.25 a V | 8.97 a | 28.78 a | 10.73 ab | 0.0510 a |
| Mo Chelate | 1.22 b | 3.31 b | 11.45 a | 5.25 b | 0.0280 b |
| Na Molybdate | 1.85 a | 6.72 ab | 25.49 a | 12.00 a | 0.0407 ab |
| LSD | 0.55 W | 4.24 | 20.5 | 6.28 | 0.0147 |
| Nitrogen X | 0.5207 | 0.3797 | 0.0476 | 0.5260 | 0.0005 |
| 0 | 1.62 a | 5.41 a | 7.91 a | 6.47 a | 0.0543 a |
| 3 | 1.72 a | 5.01 a | 15.76 a | 10.09 a | 0.0490 a |
| 6 | 2.05 a | 6.54 a | 29.40 a | 10.38 a | 0.0356 ab |
| 12 | 1.70 a | 8.36 a | 34.56 a | 10.37 a | 0.0209 b |
| LSD | 0.84 | 5.62 | 27.33 | 8.47 | 0.0203 |
| Molybdenum Y | 0.0001 | 0.0030 | 0.0095 | 0.0030 | 0.0008 |
| 0 | 1.43 bc | 1.18 b | 13.73 b | 6.04 b | 0.0229 b |
| 5 | 1.39 c | 4.30 b | 12.80 b | 6.40 b | 0.0357 ab |
| 10 | 2.28 a | 9.13 a | 33.16 a | 13.19 a | 0.0524 a |
| 20 | 1.99 ab | 7.72 ab | 27.93 ab | 11.68 ab | 0.0488 a |
| LSD | 0.58 | 1.13 | 18.82 | 6.05 | 0.0201 |
| SoMo*N | 0.7188 | 0.5879 | 0.6899 | 0.8514 | 0.0011 |
| SoMo*Mo | 0.0097 | 0.0011 | 0.0224 | 0.0048 | 0.0094 |
| N*Mo | 0.3347 | 0.7546 | 0.5063 | 0.9849 | 0.2249 |
| SoMo*N*Mo | 0.1443 | 0.9891 | 0.9834 | 0.9164 | 0.0687 |
| µ | 1.77 | 6.33 | 21.91 | 9.33 | 0.03 |
| C.V. | 61.29 | 122.40 | 161.29 | 121.71 | 94.54 |
| R2 | 0.62 | 0.56 | 0.56 | 0.54 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Márquez, E.; Soto-Parra, J.M.; Pérez-Leal, R.; Sanchez, E. New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer. Nitrogen 2024, 5, 667-687. https://doi.org/10.3390/nitrogen5030044
Muñoz-Márquez E, Soto-Parra JM, Pérez-Leal R, Sanchez E. New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer. Nitrogen. 2024; 5(3):667-687. https://doi.org/10.3390/nitrogen5030044
Chicago/Turabian StyleMuñoz-Márquez, Ezequiel, Juan Manuel Soto-Parra, Ramona Pérez-Leal, and Esteban Sanchez. 2024. "New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer" Nitrogen 5, no. 3: 667-687. https://doi.org/10.3390/nitrogen5030044
APA StyleMuñoz-Márquez, E., Soto-Parra, J. M., Pérez-Leal, R., & Sanchez, E. (2024). New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer. Nitrogen, 5(3), 667-687. https://doi.org/10.3390/nitrogen5030044






