Abstract
Nitrogen fertilization can provide greater nutritional support and mitigate salt stress in the millet crop. The aim of this study was to evaluate the physiological responses and agronomic performance of millet crop subjected to nitrogen fertilization and irrigation water salinity. The study was carried out in a greenhouse, using a completely randomized design in a 5 × 2 factorial scheme, with four replications, with five doses of nitrogen (40; 60; 80; 100 and 120 kg ha−1 of N), and two levels of electrical conductivity for the irrigation water: 0.3 and 4.0 dS m−1. We concluded that salt stress increased leaf sodium levels and had a negative impact on stalk and panicle dry mass, leaf gas exchange, mineral element concentrations (K, P, and Ca), and water use efficiency. The use of lower-salinity water associated with increased nitrogen fertilization provides greater stalk and panicle dry mass, photosynthesis, water use efficiency, chlorophyll index, leaf potassium concentration, and biomass production. The adverse effects of salt stress were evident in decreased transpiration and stomatal conductance, alongside reductions in leaf phosphorus and calcium levels, coupled with elevated leaf sodium concentrations, particularly as nitrogen fertilization rates increased in potted millet plants. These findings offer insights for devising strategies aimed at mitigating the detrimental effects of salt stress on millet plant nutrition through targeted nitrogen fertilization approaches.
1. Introduction
Millet (Pennisetum glaucum L.) is an annual forage crop belonging to the Poaceae botanical family and originally from the African continent. It can be used for human and animal food (forage and grains) due to its nutritional composition. When compared to other forage plants, millet has a simple cultivation, low production costs, and greater tolerance to water deficit. This crop is also an alternative for conservation soil management systems, such as no-till, used in crop rotation and succession, as well as having low soil-fertility requirements [1,2,3].
In many regions of the world, the use of irrigation is the only way to guarantee agricultural production safely, especially in tropical regions with hot, dry, or sub-humid climates [4]. However, the water resources available for irrigating crops have become increasingly scarce, and there is competition with the consumption of populations and other economic activities in the semi-arid northeast of Brazil. In addition, in many regions, including northeast Brazil, some water sources, especially those of underground origin, have a high concentration of soluble salts, which often represent the only source for irrigation [4,5,6].
Salinity is one of the main abiotic factors that negatively affect crop yield and can severely affect agricultural production. High concentrations of soluble salts and high Na+ saturation are commonly observed in soils in arid and semi-arid regions, or in regions where soils have imperfect drainage conditions [7,8]. In these regions, agricultural production is limited by water scarcity, high soil salinity, and low fertility [9,10].
The application of mineral fertilizers is an important practice for promoting crop development and production. To ensure high yields, producers make intensive use of fertilizers, especially nitrogen fertilizers [10,11,12], as nitrogen (N) is an essential macronutrient for plant growth, development, and yield [13]. However, although N is one of the nutrients most absorbed by the millet crop, care must be taken not to supply it in excess, since excessive doses of N reduce root growth and harvest index and favor the growth of the vegetative part [14,15].
However, in saline environments, the complex ionic interactions impact the viability, absorption, and transportation of nutrients. This complexity arises primarily from variations in concentration and ionic composition, resulting in a heightened accumulation of Na+ and Cl− ions. Consequently, the absorption of essential nutrients such as N, P, K, Ca, and Mg may be limited [16,17,18].
Thus, the objective of this work was to evaluate the physiological responses and agronomic performance of the millet crop subjected to nitrogen fertilization and brackish waters.
2. Materials and Methods
2.1. Location and Characterization of the Experimental Area
The experiment took place during the dry season, spanning from September to November 2020, within the experimental area of the Auroras Seedling Production Unit (UPMA), which is part of the University of the International Integration of Afro-Brazilian Lusophony (UNILAB), located in Redenção, Ceará, Brazil. Redenção sits at a latitude of 04°13′33″ S and a longitude of 38°43′50″ W, with an average altitude of 88 m. Characterized by a sub-humid and hot climate, the region experiences rainfall primarily during the summer and fall seasons. Meteorological data throughout the experiment period were recorded using a data logger (HOBO® U12-012 Temp/RH/Light/Ext), Onset Computer Corporation; Bourne, MA, USA (Figure 1), revealing a total rainfall of only 18 mm.
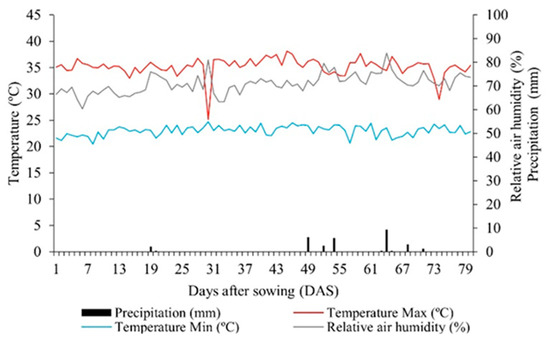
Figure 1.
Mean values of maximum (Max) and minimum (Min) temperatures, precipitation, and relative air humidity observed during the experimental period.
2.2. Experimental Design and Treatments
A randomized completely experimental design with four replications was used in a 5 × 2 factorial arrangement consisting of five nitrogen doses (40; 60; 80; 100 and 120 kg ha−1 of N) and two electrical conductivities of the irrigation water (ECw): 0.5 and 4.0 dS m−1.
2.3. Plant Material and Fertilization
The crop used was millet (Pennisetum glaucum L.), specifically cultivar BRS-1501, sown in rows (five per pot), using an average of 45 seeds to ensure a minimum plant stand in each experimental unit. Thinning was carried out 15 days after emergence, resulting in three plants remaining per pot.
Mineral fertilization was recommended by the authors of [19] with 120 kg ha−1 of N, 30 kg ha−1 of P2O5, and 40 kg ha−1 of K2O. For pot fertilization purposes, a stand of 10,000 plants ha−1 was considered, where each pot received 3.0 g of P2O5 and 4.0 g of K2O, using single superphosphate and potassium chloride, respectively. For nitrogen fertilization, doses of 4.8, 7.2, 9.6, 12, and 14.4 g of N per pot were used, corresponding to 40, 60, 80, 100, and 120 kg ha−1, respectively, using urea (45%). Fertilization with NPK was divided into two doses, 50% at planting and 50% at 21 days after sowing.
The pots used had a volumetric capacity of 25 L and were filled with a substrate comprising a blend of arisco (a light-textured sandy material commonly used in constructions in northeast Brazil), sand, and cattle manure in a ratio of 4:3:1, respectively. Detailed characteristics of the substrate can be found in Table 1.

Table 1.
Chemical attributes of the substrate used to fill the pots.
2.4. Irrigation Management
The plants were irrigated manually with a daily watering frequency and a leaching fraction set at 0.15, as suggested by the authors of [20], following the drainage lysimeter methodology outlined in [21], while ensuring the substrate remained at field capacity. The volume of water applied during irrigation was calculated using the equation provided below (Equation (1)):
where
- VI—volume of water to be applied in the irrigation event (mL);
- Vp—volume of water applied in the previous irrigation event (mL);
- Vd—volume of water drained (mL); and,
- LF—leaching fraction of 0.15.
To prepare the water with an electrical conductivity of 4.0 dS m−1, a mixture of soluble salts including NaCl, CaCl2.2H2O, and MgCl2.6H2O was used in a ratio of 7:2:1 for Na, Ca, and Mg, respectively, following the methodology outlined in [22]. Saline solution irrigation commenced 10 days after sowing (DAS), once the plants were established. The electrical conductivity of the water was regularly assessed using a benchtop conductivity meter (AZ® 86505 pH/Cond./TDS/Salt), AZ Instrument Corp; Taichung City, Taiwan. Water samples were sent to a laboratory to determine their chemical characteristics, employing the methodology described in [23], and classified using the approach detailed in [24]. The chemical attributes of the irrigation water are presented in Table 2.

Table 2.
Chemical characteristics and classification of the irrigation water used in the experiment.
2.5. Gas Exchange and Chlorophyll Index
At 76 DAS, leaf gas exchange measurements were conducted on fully expanded leaves in the morning (between 9:00 h and 11:00 h) using an infrared gas analyzer (LI 6400 XT from LI-COR), LI-COR Biosciences, Inc.; Lincoln, NT, USA. in an open system, with an air flow rate of 300 mL min−1, under ambient air temperature and CO2 concentration conditions. The evaluated gas exchange variables were net photosynthesis rate (A), transpiration (E), stomatal conductance (gs), internal CO2 concentration (Ci), and leaf temperature (LT). These gas exchange data were utilized to calculate physiological indices such as instantaneous water use efficiency (WUE). Simultaneously, measurements of the relative chlorophyll index (RCI) were obtained from the same leaves using a non-destructive method with a portable meter (SPAD—502 Plus), Konica Minolta Inc.; Tokyo, Japan.
2.6. Biomass Production
At 76 days after sowing (DAS), two plants were collected from each experimental unit, then separated into leaves, stalks, and roots, packed in paper bags, and kept in a forced-air oven at 65 °C for 72 h to dry and then determine the dry mass of the leaves (LDM), stalk (SDM), and panicle (PDM).
2.7. Mineral Element Concentration
For mineral element concentration assessment, oven-dried leaf samples underwent grinding in a Wiley-type mill for analysis. Nitrogen (N) concentration was determined using the Kjeldahl method [25], involving wet digestion, steam distillation, and titration to quantify NH4+. Other elements (P, K, Mg, Ca, and Na) were determined through dry digestion in a muffle furnace using a 1% HNO3 solution as an extractant. A 500 mg sample of leaf tissue was placed in an electric muffle furnace and incinerated at temperatures between 500 and 550 °C. The resulting ash was dissolved in a nitric acid solution, and the extract obtained was utilized for determining P, K, Mg, Ca, and Na. Potassium (K) and sodium (Na) readings were measured via flame photometry, phosphorus (P) readings through molybdenum blue spectrophotometry, and magnesium (Mg) and calcium (Ca) readings via atomic absorption spectrophotometry [26].
2.8. Data Analysis
To assess the normality of the data, the variables were subjected to the Kolmogorov–Smirnov test (p ≤ 0.05). The data were then subjected to an analysis of variance and a Tukey test to compare means (p ≤ 0.05) using the ASSISTAT 7.7 BETA program [27].
3. Results and Discussion
3.1. Leaf Gas Exchange and Biomass Production
Following the analysis of variance (Table 3), a significant interaction (p ≤ 0.01 and p ≤ 0.05) was observed between nitrogen doses (D) and salinity (S) for the following variables: net photosynthesis rate (A), stomatal conductance (gs), transpiration (E), instantaneous water use efficiency (WUE), relative chlorophyll content (RCI), stalk dry mass (SDM), panicle dry mass (PDM), and total dry mass (TDM). Additionally, leaf dry mass exhibited a significant individual effect for nitrogen doses (p ≤ 0.01).

Table 3.
Analysis of variance for net photosynthesis rate (A), stomatal conductance (gs), transpiration (E), instantaneous water use efficiency (WUE), relative chlorophyll index (RCI), leaf dry mass (LDM), stalk dry mass (SDM), and panicle dry mass (PDM) in millet crops subjected to doses of nitrogen under irrigation with brackish waters.
The photosynthetic rate increased as the nitrogen dose increased in plants irrigated with 0.5 dS m−1 water. However, when irrigation water of 4.0 dS m−1 was used, there was a decrease when comparing the highest and lowest doses of nitrogen (Figure 2A).
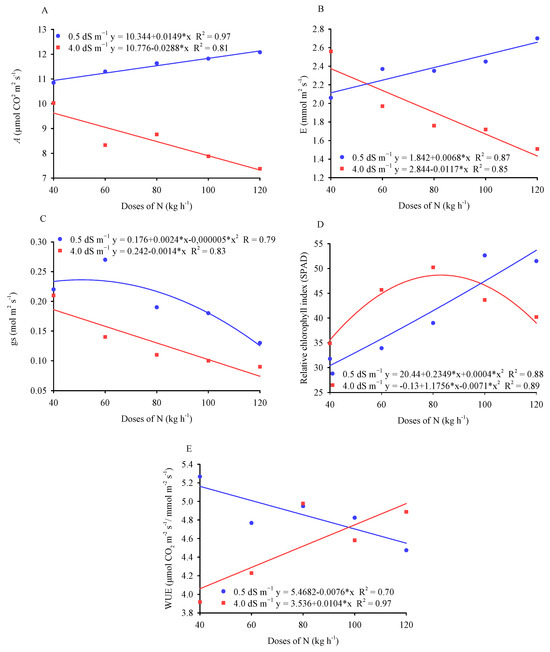
Figure 2.
Photosynthetic rate (A), transpiration (B), stomatal conductance (C), relative chlorophyll index (D), and water use efficiency (E) in millet plants as a function of different doses of nitrogen and the electrical conductivity of the irrigation water.
Increasing the amount of N fertilizer applied tends to prolong the longevity of functional leaves and increases chlorophyll content, thus improving photosynthetic capacity [28]. In our study, it was found that increasing the dose of N intensified the effect of salt stress on CO2 assimilation compared to control plants. This indicates that the increase in nitrogen concentration under conditions of salt stress may have caused a nutritional imbalance, impairing the absorption of other essential nutrients for photosynthesis such as Mg2+, as well as leading to the accumulation of Na+ and/or Cl− ions in the chloroplasts, affecting the biochemical and photochemical processes involved in photosynthesis [29,30].
The showed that nitrogen fertilizations of 60 and 120 kg ha−1 provide greater photosynthesis, transpiration, stomatal conductance, and internal CO2 concentration in millet plants using lower-salinity water throughout the cycle or using brackish water from 30 to 45 days after sowing [31]. On the other hand, the authors of [12] found no interaction between salinity and N doses in the corn crop.
The transpiration of millet plants (Figure 2B) increased linearly under water with the lowest salinity (0.5 dS m−1) and decreased under water with the highest salinity (4.0 dS m−1) as the doses of nitrogen increased, respectively, although the values were always higher in the control plants. Transpiration is correlated with stomatal conductance, which also decreased with salinity and as the dose of N increased (Figure 2C). Increasing the dose of nitrogen in the soil intensifies the osmotic effect, especially in the saline treatment, thus reducing the gradient of water potential between the soil and the plant roots and decreasing stomatal conductance, transpiration rate, and water absorption by the plants [32,33,34].
The impact of the different doses of nitrogen and the electrical conductivity of the irrigation water on the relative chlorophyll index (Figure 2D) differed from the results observed for leaf gas exchange. The RCI increased linearly in the leaves of plants irrigated with low-salinity water (0.5 dS m−1), comparing the highest and lowest doses of N applied. However, under water with higher salinity (4.0 dS m−1), there was a quadratic effect, with the maximum RCI value being observed at a dose of 82.78 kg ha−1 of N. In addition, the RCI values were higher in plants under salt stress compared to the control plants, except for the highest doses of N. The increase in the relative chlorophyll index in plants irrigated with high-salinity water is possibly related to a process of acclimatization to salt stress and the crop’s environment, in order to ensure photosynthetic rates in line with physiological and growth needs [35]. In non-stressed plants, the increase in chlorophyll concentration usually translates into greener leaves and improved photosynthetic capacity, which can result in better plant growth and development [36].
The increase in nitrogen doses associated with the use of lower-salinity water linearly reduced the instantaneous efficiency of irrigation water use but increased it when plants were irrigated with higher-salinity water (Figure 2E). Salt stress reduces plant gas exchange and therefore instantaneous water use efficiency, which is the result of the relationship between photosynthesis and transpiration, so stress translates into reduced water consumption by plants [37]
Research carried out by the authors of [31] on the same millet cultivar fertilized with 50 and 100% of the recommended dose and irrigated with brackish water from the 30th, 45th, and 65th days onwards found no mitigating effect of nitrogen fertilization on water use efficiency. On the other hand, the authors of [38] found similar results to those of this study. These same authors, when assessing the effect of nitrogen fertilization on the eggplant crop, obtained a higher water use efficiency of 6.34 (µmol CO2 mmol H2O m−2 s−1) for a dose of 250 kg ha−1 of N, at the lowest electrical conductivity of the water.
Increasing the doses of nitrogen in the substrate had a linear effect on leaf dry mass, with a maximum increase of 68% when comparing the lowest and highest doses of N (Figure 3A). The positive influence of increasing doses of nitrogen in the substrate may be related to the effect of the nutrient on the transport of assimilates from the source to the sink, enabling greater dry matter accumulation [39].
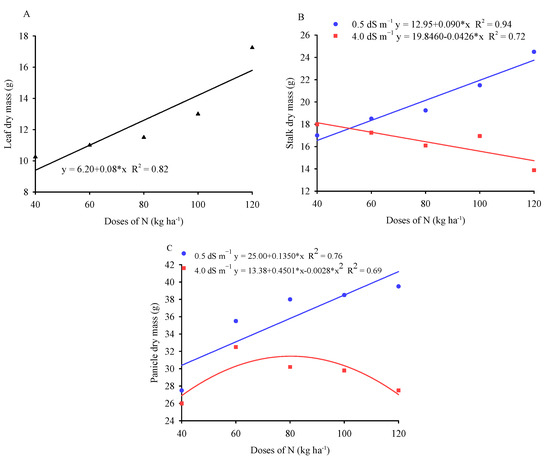
Figure 3.
Leaf dry mass (A), stalk dry mass (B), and panicle dry mass (C) in millet plants as a function of nitrogen doses and electrical conductivities of the irrigation water.
Stalk dry mass (Figure 3B) and panicle dry mass (Figure 3C), when plants were irrigated with low-salinity water, showed increasing linear responses with increasing nitrogen doses in the substrate. On the other hand, plants irrigated with brackish water (4.0 dS m−1) showed a linear decrease in stalk dry mass with increasing N dose, while panicle dry mass had a maximum estimated value of 31.47 g for a dose of 80.37 kg ha−1 of N. These results indicate that the cultivation of millet plants under high nitrogen doses and irrigated with brackish water intensifies the deleterious effects of salts on biomass production.
On the other hand, plants irrigated with brackish water (4.0 dS m−1) showed a better fit to the quadratic polynomial model, according to which stalk dry mass, panicle dry mass, and total dry mass had maximum estimated values of 35.59, 42.04, and 117.09 g for doses of 83.84, 82.63, and 79.32 kg ha−1 of N. These results possibly indicate that growing millet plants under high nitrogen doses and irrigated with brackish water intensifies the deleterious effects of salts on dry mass accumulation.
The authors of [40] found that adding NaCl to the irrigation solution significantly reduced leaf growth, in terms of dry weight, by 11% and 7% when the plants were fertilized with NO3− and NH4+, respectively. Similar to the results found in this study, [41,42] saw an increase in the dry mass of the aerial part as a result of the increase in nitrogen doses in cotton and corn plants, respectively.
A study carried out by the authors of [31] with salt stress and nitrogen fertilization at 50 and 100% of the recommended dose in the millet crop reported that there was no effect similar to that found in the present study. These same authors found that the use of brackish water from 30 days after sowing did not negatively affect leaf biomass. They differed from the use of water with lower salinity, using instead a greater biomass with water of 4.0 dS m−1 in plants fertilized with 100% of the nitrogen dose. On the other hand, [43] showed a similar trend to that found in the present study, where plants under salt stress showed greater stalk biomass accumulation. For panicle mass, the authors of [44] evaluated the use of high-salinity water associated with 100% of the recommended dose in millet cultivation and found similarity to the results observed in the present study.
3.2. Leaf Concentration of Nutrients
The leaf potassium content showed an individual effect for irrigation water, while the other nutrients were influenced by the interaction between the factors studied at a significant level of p ≤ 0.01 and p ≤ 0.05 by the F test (Table 4).

Table 4.
Summary of the analysis of variance for the leaf contents of nitrogen (N), phosphorus (P), calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K) in millet plants under doses of nitrogen in the substrate and irrigated with different electrical conductivities of the irrigation water.
The leaf N content (Figure 4A) increased linearly with the increase in N doses in the control plants. On the other hand, the leaf N content in plants irrigated with brackish water (4.0 dS m−1) responded quadratically, with a maximum value of 31.8 g kg−1 at a dose of 90.13 kg ha−1 of N.
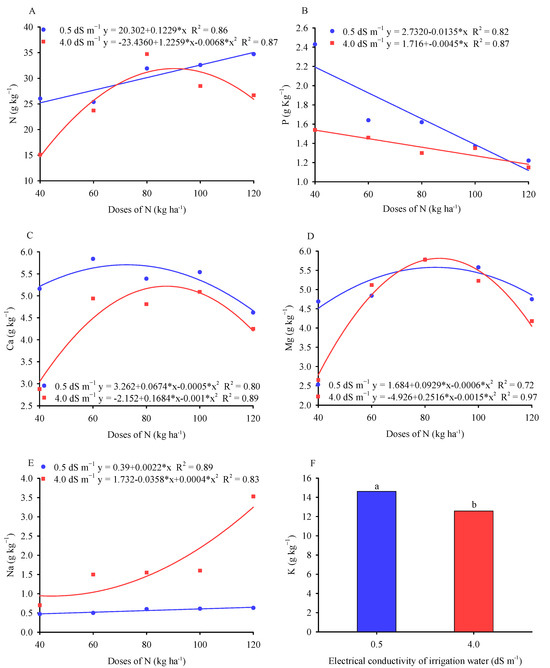
Figure 4.
Leaf nitrogen (A), phosphorus (B), calcium (C), magnesium (D), and sodium (E) contents in millet plants as a function of the interaction between nitrogen doses in the substrate and electrical conductivities of the irrigation water and leaf potassium (F) content in millet plants as a function of different electrical conductivities of the irrigation water. Averages followed by the same lowercase letters at the same electrical conductivity of the water do not differ significantly according to the Tukey test (p ≤ 0.05).
In saline environments, there is an antagonism between Cl− and NO3− which causes deleterious effects on the absorption and translocation of nitrogen to plant structures [45], which was demonstrated from the dose of 90.13 kg ha−1 of N. These results indicate that millet plants, when they receive the maximum recommended nitrogen fertilization, may develop a mechanism to use N for proline synthesis and resistance to salt stress [46].
Contrary to the results obtained in this study, [47], when irrigating the maize crop with brackish water (3.0 dS m−1), found no effect of nitrogen fertilization with 100% of the recommended dose on leaf content, i.e., there was no effect of the interaction between the nutrient and salt stress factors.
Regardless of the salt concentrations in the irrigation water, increasing the doses of N in the substrate led to a reduction in the leaf P content of millet plants (Figure 4B). The reductions, according to the regression analyses, represented 5.04 and 1.8% per unit increase in the doses of N in the substrate for ECw 0.3 and 4.0 dS m−1, respectively.
In his study, [48] describes that plants adapt to nutrient levels by altering their gene expression profile, i.e., they modulate nutrient absorption and metabolism in order to process and adapt to environmental conditions. The authors of [47] found an individual effect for ECw levels and N doses on leaf P content in maize, which decreased with increasing ECw and increased in plants that did not receive fertilization.
Figure 4C shows that the ECw equations as a function of the increase in N doses in the substrate were best fitted by the polynomial model and behaved similarly for the leaf Ca content in millet plants. For water with a low salt concentration (0.3 dS m−1), there was an estimated maximum value of 5.53 g kg−1 of Ca for a dose of 67.40 kg ha−1 of N. In plants irrigated with brackish water (4.0 dS m−1), there was an estimated maximum value of leaf Ca of 4.93 g kg−1 for a dose of 84.20 kg ha−1 of N.
The increase in N doses increased the Ca content in millet leaves in a saline environment, being slightly higher than that found in the control treatment at doses close to 100% of the recommended dose. Millet plants may have developed an antioxidant mechanism driven by the increase in N to tolerate toxic ions such as Na+ [47,49], or even increased the exchange capacity of the roots in saline environments and favored a greater absorption of bivalent ions such as Ca2+ and Mg2+ [50]. The authors of [40] studied the interactive effects of salinity and forms of nitrogen on plant growth, photosynthesis, and osmotic adjustment in maize and found that there was an increase in the concentration of Ca2+ in the presence of NaCl in the nutrient solution regardless of the N source.
With regard to the leaf Mg content in the millet crop (Figure 4D), it was found that the accumulation of Mg showed a similar response range, regardless of the ECw and doses of N in the substrate, but with the control being superiorly close to the recommended dose. A similar trend was observed by the authors of [47], who recorded an increase in leaf Mg content in maize irrigated with brackish water and fertilized with 50% of the N recommendation. One of the main side effects of salinity is nutritional deficiency, but this balances the nutritional status (N, P, K+, Mg2+, and Ca2+), and this adverse effect can be partially corrected through fertilizer management [51].
As can be seen in Figure 4E, the leaf Na content increased under irrigation water with the lowest salinity (0.3 dS m−1), showing the smallest increase compared to plants irrigated with the highest-salinity water. The results show that the increase in N doses in the substrate boosted the accumulation of leaf Na content in millet plants. In addition, the supply of N may have contributed to the synthesis of compatible solute compounds (proline and glycine-betaine), consequently adjusting the osmotic potential of the cytoplasm [31,52]. Similar effects were found by the authors of [40] in corn. These same authors observed an increase in leaf Na content in plants under salt stress and fertilized with 100% of the nitrogen fertilizer.
For leaf K content as a function of ECw (Figure 4F), the mean comparison test shows that millet plants irrigated with low-salinity water (0.3 dS m−1) were statistically superior to plants irrigated with brackish water (4.0 dS m−1). High concentrations of Na+ can affect the integrity of root cell membranes by replacing Ca2+ ions and disrupting the selectivity of transporters, affecting the absorption of K ions and translocation to the aerial part [53].
In line with the present study, the authors of [54] found that there was a reduction in leaf K content in the maize crop as the ECw increased. On the other hand, the authors of [55] found that there was no significant effect on the K content in the aerial part of the Sorghum bicolor crop, but plants grown under high soil and water salinity and without leaching showed a decrease in the K content in the roots.
4. Conclusions
We concluded that salt stress increased leaf sodium levels and had a negative impact on stalk and panicle dry mass, leaf gas exchange, mineral element concentrations (K, P, and Ca), and water use efficiency. The use of lower-salinity water associated with increased nitrogen fertilization provides greater stalk and panicle dry mass, photosynthesis, water use efficiency, chlorophyll index, leaf potassium concentration, and biomass production.
The adverse effects of salt stress were evident in decreased transpiration and stomatal conductance, alongside reductions in leaf phosphorus and calcium levels, coupled with elevated leaf sodium concentrations, particularly as nitrogen fertilization rates increased in potted millet plants. These findings offer insights for devising strategies aimed at mitigating the detrimental effects of salt stress on millet plant nutrition through targeted nitrogen fertilization approaches.
Author Contributions
Conceptualization, G.G.d.S. and F.H.R.C.; methodology, G.G.d.S., J.T.M.d.S., M.d.S.A. and S.d.O.S.; software, F.B.L.; formal analysis, C.F.d.L. and T.V.d.A.V.; investigation, G.G.d.S., F.B.d.S.J. and S.P.G., resources, G.G.d.S., M.d.S.A., S.C.R.V.L., C.F.d.L. and A.O.d.S.; data curation, G.G.d.S.; F.H.R.C. and S.P.G.; writing—original draft preparation, G.G.d.S., J.T.M.d.S. and M.d.S.A.; writing—review and editing, G.G.d.S., T.V.d.A.V. and F.B.L.; visualization, S.C.R.V.L.; supervision, G.G.d.S.; project administration, F.H.R.C.; funding acquisition, G.G.d.S., C.F.d.L., T.V.d.A.V., F.B.L. and S.C.R.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); process number: 311828/2022-1.
Data Availability Statement
The data that supported the conclusions reported in this article will be made available by the authors upon request.
Acknowledgments
Acknowledgments are given to the Conselho Nacional de Desenvolvimento. Científico e Tecnológico (CNPq).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marcante, N.C.; Camacho, M.A.; Junior, F.P.P. Teores de nutrientes no milheto como cobertura de solo. J. Biosci. 2011, 27, 196–204. [Google Scholar]
- Jacovetti, R.; França, A.F.S.; Carnevalli, R.A.; Miyagi, E.S.; Brunes, L.C.; Corrêa, D.C. Milheto como silagem comparado a gramíneas tradicionais: Aspectos quantitativos, qualitativos e econômicos. Ciênc. Anim. Bras. 2018, 19, 1–16. [Google Scholar] [CrossRef]
- Ferreira, F.N.; Oliveira, I.C.M.; Andrade, C.L.T.; Simeão, R.M.; Souza, I.R.P. Produção de Silagem de Milheto sob Diferentes Lâminas de Irrigação; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2020; 25p. [Google Scholar]
- Holanda, J.S.; Amorim, J.R.A.; Ferreira Neto, M.; Holanda, A.C.; Sá, F.V.S. Qualidade da água para irrigação. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; Gheyi, H.R., Dias, N.S., Lacerda, C.F., Gomes Filho, E., Eds.; INCTSal: Fortaleza, Brazil, 2016; pp. 35–50. [Google Scholar]
- Lima, G.S.; Moreira, B.L.; Silva, A.G.; Diniz Neto, M.L.; Oliveira, D.S.; Cavalcante, A.P. Crescimento e produtividade de algodão de fibra colorida cultivado sob estresse salino e adubação nitrogenada. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 415–420. [Google Scholar] [CrossRef]
- Sousa, G.G.D.; Sousa, H.C.; Lessa, C.I.; Goes, G.F.; Freire, M.H.D.C.; de Souza, M.V.; Schneider, F. Production of watermelon seedlings in different substrates under salt stress. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 343–351. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt Affected Soils; FAO: Rome, Italy, 2021. [Google Scholar]
- Rai, A.K.; Basak, N.; Sundha, P. Saline and Sodic Ecosystems in the Changing World. In Soil Science: Fundamentals to Recent Advances; Rakshit, A., Singh, S., Abhilash, P., Biswas, A., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Cavalcante, Í.H.; Oliveira, F.A.D.; Cavalcante, L.F.; Beckmann, M.Z.; Campos, M.C.; Gondim, S.C. Growth and production of two cotton cultivars irrigated with saline water. Rev. Bras. Eng. Agric. Ambient. 2021, 9, 108–111. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Hu, M.; Chen, Z.; Tan, J.; Liu, L.; Xiong, Y.; Huang, Q.; Huang, G. Modeling water–salt–nitrogen dynamics and crop growth of saline maize farmland in Northwest China: Searching for appropriate irrigation and N fertilization strategies. Agric. Water Manag. 2023, 282, 108271. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.; Gao, Z.; Hong, J.; Li, L.; Meng, H.; Ma, H.; Jia, J. Year-round film mulching system with monitored fertilization management improve grain yield and water and nitrogen use efficiencies of winter wheat in the dryland of the Loess Plateau, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 9524–9535. [Google Scholar] [CrossRef] [PubMed]
- Sousa, H.C.; Sousa, G.G.; Lessa, C.I.N.; Lima, A.F.S.; Ribeiro, R.M.R.; Rodrigues, F.H.C. Growth and gas exchange of corn under salt stress and nitrogen doses. Rev. Bras. Eng. Agric. Ambient. 2021, 23, 907–913. [Google Scholar] [CrossRef]
- Prado, R.d.M. Nutrição de Plantas, 2nd ed.; Editora Unesp: São Paulo, Brazil, 2020; Volume 1, 414p. [Google Scholar]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Liu, L.; Tan, Y.; Sheng, X.; Yu, D. Effect of salinity stress on rice yield and grain quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126765. [Google Scholar] [CrossRef]
- Sousa, G.G.; Lacerda, C.F.; Cavalcante, L.F.; Guimarães, F.V.A.; Bezerra, M.E.J.; Silva, G.L. Nutrição mineral e extração de nutrientes de planta de milho irrigada com água salina. Rev. Bras. Eng. Agric. Ambient. 2010, 14, 1143–1151. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Zhu, X.; Zhou, G.; Ali, A.Y.A.; Ahmad, I.; Elsiddig, A.M.I. Fertilizante nitrogenado reduz o impacto do cloreto de sódio no rendimento de trigo. J. Agron. 2018, 110, 1731–1737. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, C.; Hu, Y.; Shao, K.; Tang, X.; Zhang, L.; Gao, G.; Qin, B. Climate-induced salinization may lead to increased lake nitrogen retention. Water Res. 2023, 228, 119354. [Google Scholar] [CrossRef] [PubMed]
- Pereira Filho, I.A.; Pereira, A.S.; Coelho, A.M.; Casela, C.R.; Karam, D.; Rodrigues, J.A.S.; Cruz, J.C.; Waquil, J.M. Manejo da Cultura do Milheto; Embrapa-CNPMS. Circular Técnica, 29; Embrapa-CNPMS: Sete Lagoas, Brazil, 2003; 17p. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. A Qualidade da Água na Agricultura, 2nd ed.; Estudos FAO: Irrigação e Drenagem; UFPB: Campina Grande, Brazil, 1999; 153p. [Google Scholar]
- Bernardo, S.; Mantovani, E.C.; Silva, D.D.; Soares, A.A. Irrigation Manual; Editora UFV: Viçosa, Brazil, 2019; 545p. [Google Scholar]
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. Uso de Águas Salinas para Produção Agrícola; Estudos da FAO, Irrigação e Drenagem; UFPB: Campina Grande, Brazil, 2000; 117p. [Google Scholar]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Comunicação para Transferência de Tecnologia: Brasília, Brazil, 1999; p. 370. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; USDA Agriculture Handbook, 60; US Department of Agriculture: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.; Melo, W.J.D. Análise química de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Silva, F.C., Ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; Chapter 2; pp. 193–233. [Google Scholar]
- Silva, F.C.; Dasilva, F.C. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2009; 573p. [Google Scholar]
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 733–3740. [Google Scholar]
- Zhang, M.; Wang, Z.J.; Huang, J.C.; Sun, S.; Cui, X.; Zhou, W.; He, S. Salinity-driven nitrogen removal and its quantitative molecular mechanisms in artificial tidal wetlands. Water Res. 2021, 202, 117446. [Google Scholar] [CrossRef]
- Ribeiro, R.M.R.; de Sousa, G.G.; Barbosa, A.S.; de Lacerda, C.F.; Freire, M.H.D.C.; Moraes, J.G.L. Irrigation strategies with saline water and phosphate fertilization in cowpea culture. Rev Bras. Ciên. Agrár. 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Roque, I.A.; Soares, L.A.D.A.; Lima, G.S.D.; Lopes, I.A.P.; Silva, L.D.A.; Fernandes, P.D. Biomass, gas exchange and production of cherry tomato cultivated under saline water and nitrogen fertilization. Rev. Caatinga 2022, 35, 686–696. [Google Scholar] [CrossRef]
- Có, E.G.; de Sousa, G.G.; Gomes, S.P.; Freire, M.H.D.C.; da Silva, F.D. Strategies for the management of irrigation with saline water and nitrogen fertilization in millet crop. Rev. Caatinga 2023, 36, 424–431. [Google Scholar]
- Melo, H.F.D.; Souza, E.R.D.; Duarte, H.H.; Cunha, J.C.; Santos, H.R. Gas exchange and photosynthetic pigments in bell pepper irrigated with saline water. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 38–43. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Zhang, Z. Plant salinity stress response and nano-enabled plant salt tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar]
- Zhou, H.; Kang, S.; Li, F.; Du, T.; Shukla, M.K.; Li, X. Nitrogen application modified the effect of deficit irrigation on tomato transpiration, and water use efficiency in different growth stages. Sci. Hortic. 2020, 263, 09112. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Kim, I.D.; Shin, D.H. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J. Saudi Soc. Agric. Sci. 2020, 19, 261–269. [Google Scholar] [CrossRef]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; ArtMed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Sousa, G.G.; de Araújo Viana, T.V.; Neto, M.D.O.R.; Da Silva, G.L.; De Azevedo, B.M.; Costa, F.R.B. Características agronômicas do girassol irrigado com águas salinas em substratos com fertilizantes orgânicos. Rev Agrogeoambient. 2017, 9, 1–8. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Tao, X.; Khan, A.; Tan, D.K.; Luo, H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, Z.; Zhang, Y.; Sun, H.; Song, S.; Bai, Z.; Lu, Z.; Li, C. Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Front. Plant Sci. 2020, 11, 880. [Google Scholar] [CrossRef]
- Bianchet, P.; Sangoi, L.; Souza, C.A.D.; Klauberg Filho, O.; Panison, F. Desenvolvimento vegetativo do arroz irrigado afetado pela inoculação com Azospirillum e aplicação de nitrogênio mineral. Rev. Fac. Agron. 2015, 114, 2015. [Google Scholar]
- Sousa, G.G.D.; Rodrigues, V.D.S.; Soares, S.D.C.; Damasceno, Í.N.; Fiusa, J.N.; Saraiva, S.E. Irrigation with saline water in soybean (Glycine max (L.) Merr.) in a soil with bovine biofertilizer. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 604–609. [Google Scholar] [CrossRef]
- Costa, F.H.; Sousa, G.G.D.; Lima, J.M.D.P.; Almeida, M.D.S.; Sousa, H.C.; Gomes, S.P.; Cruz Filho, E.M.; Azevedo, B.M.D. Frequencies of irrigation in millet crop under salt stress. Rev. Bras. Eng. Agric. Ambient. 2024, 28, 272197. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.G.D.; Sousa, H.C.; Santos, M.F.D.; Lessa, C.I.N.; Gomes, S.P. Saline water and nitrogen fertilization on leaf composition and yield of corn. Rev. Caatinga 2022, 35, 191–198. [Google Scholar] [CrossRef]
- Ueda, Y.; Yanagisawa, S. Perception, transduction, and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J. Exp. Bot. 2019, 70, 3709–3717. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Esmaili, E.; Kapourchal, S.A.; Malakouti, M.J.; Homaee, M. Interactive effect of salinity and two nitrogen fertilizers on growth and composition of sorghum. Plant Soil Environ. 2008, 54, 537–546. [Google Scholar] [CrossRef]
- Nathawat, N.S.; Kuhad, M.S.; Goswami, C.L.; Patel, A.L.; Kumar, R. Interactive effects of nitrogen source and salinity on growth indices and ion content of Indian mustard. J. Plant Nutr. 2007, 30, 569–598. [Google Scholar] [CrossRef]
- Slama, I.; M’Rabet, R.; Ksouri, R.; Talbi, O.; Debez, A.; Abdelly, C. Water deficit stress applied only or combined with salinity affects physiological parameters and antioxidant capacity in Sesuvium portulacastrum. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 213, 69–76. [Google Scholar] [CrossRef]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen, Z. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirb.-Agric. 2016, 103, 229–238. [Google Scholar] [CrossRef]
- Rodrigues, V.D.S.; Sousa, G.G.D.; Soares, S.D.C.; Leite, K.N.; Ceita, E.D.; Sousa, J.T.M.D. Gas exchanges and mineral content of corn crops irrigated with saline water. Rev. Ceres. 2021, 68, 453–459. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Lambertini, C.; Speranza, M.; Vittori Antisari, L.; Vianello, G.; Barbanti, L. Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water salinity. Plants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).