Effects of Seasonal Variation on Nitrogen Use in Brazilian Cerrado Grass Communities
Abstract
1. Introduction
2. Material and Methods
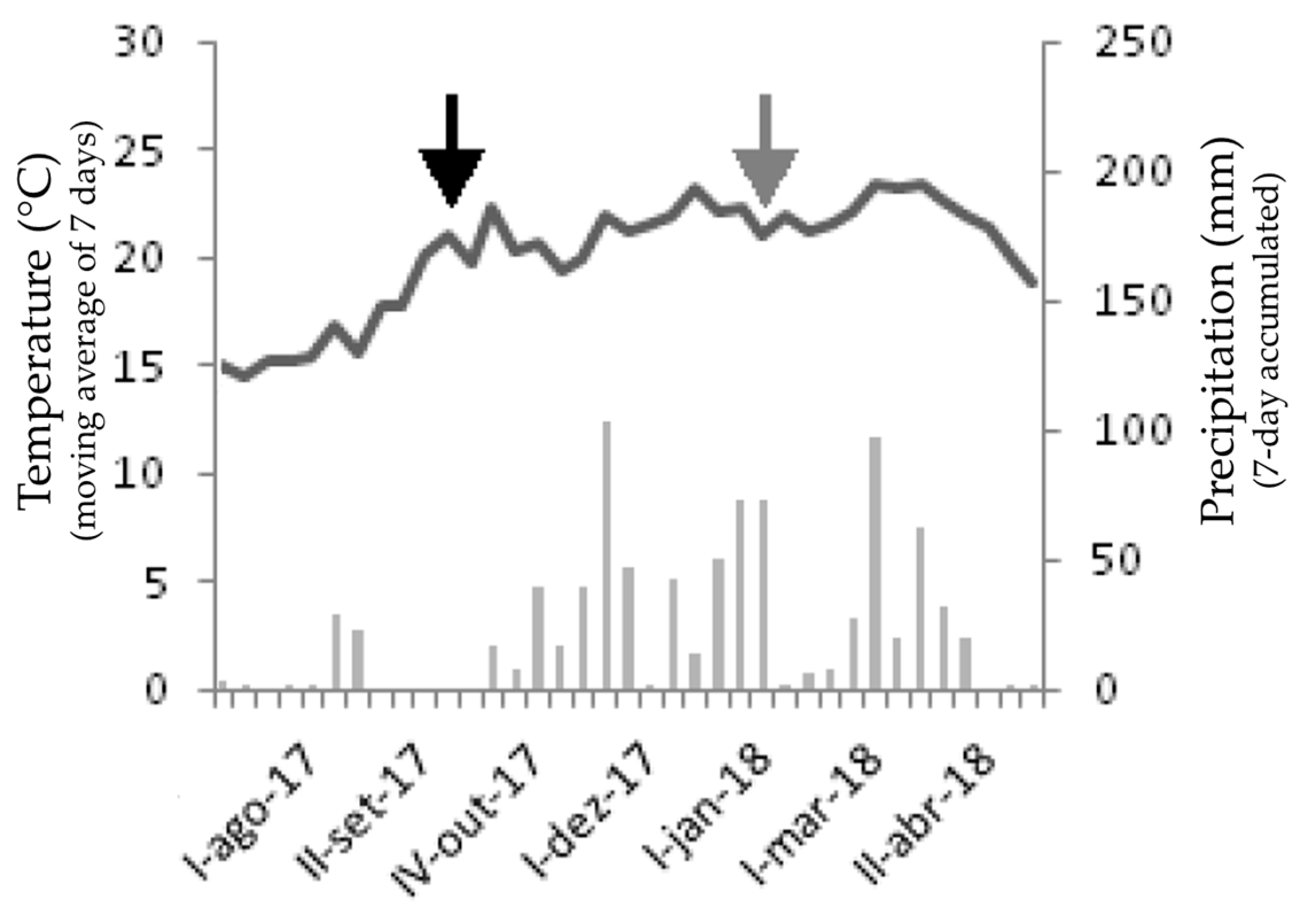
2.1. Study Site and Data Collection
2.2. Edaphoclimatic Characterization
2.3. Plant Nitrogen Analysis
2.4. Statistical Data Analysis
3. Results
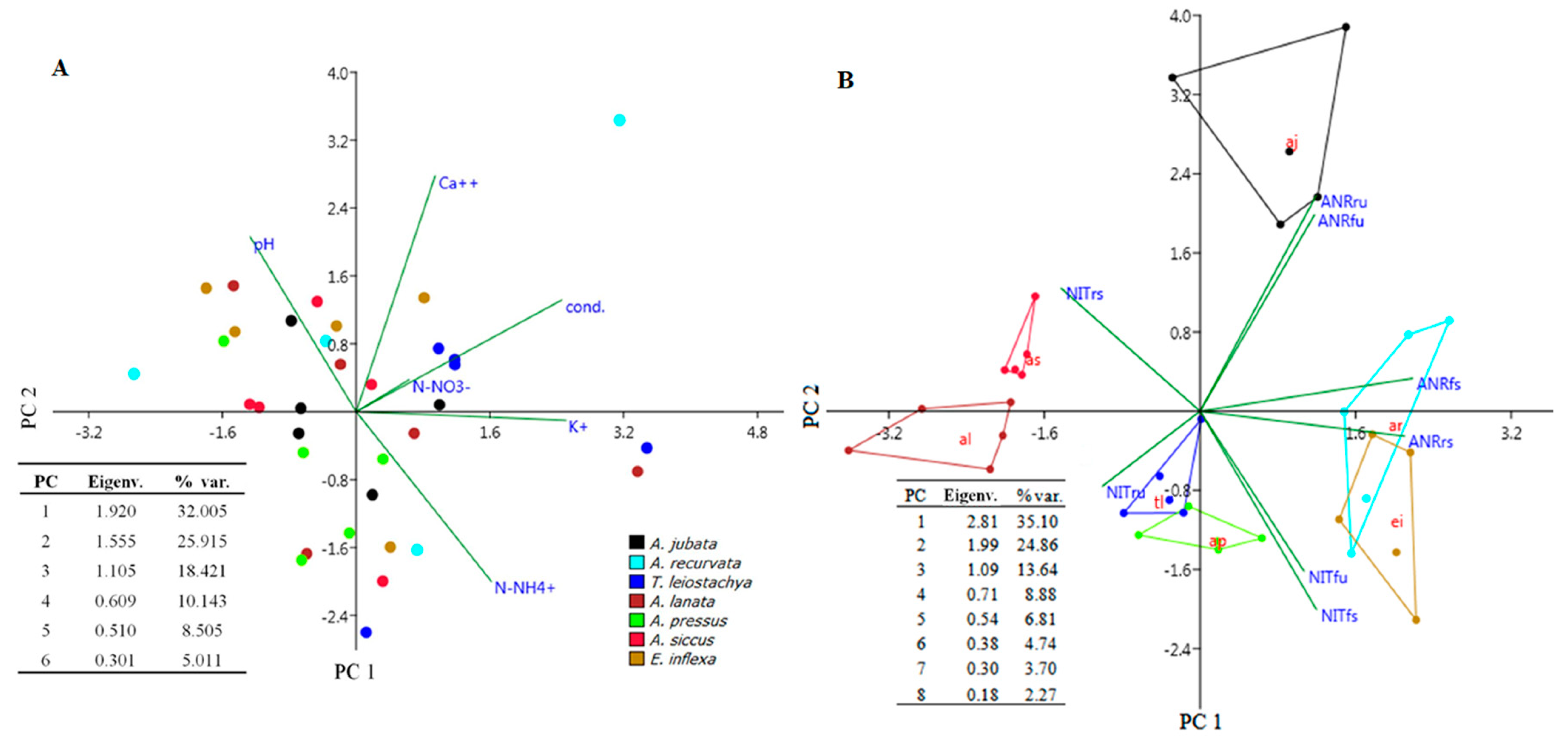
3.1. Edaphoclimatic Characterization
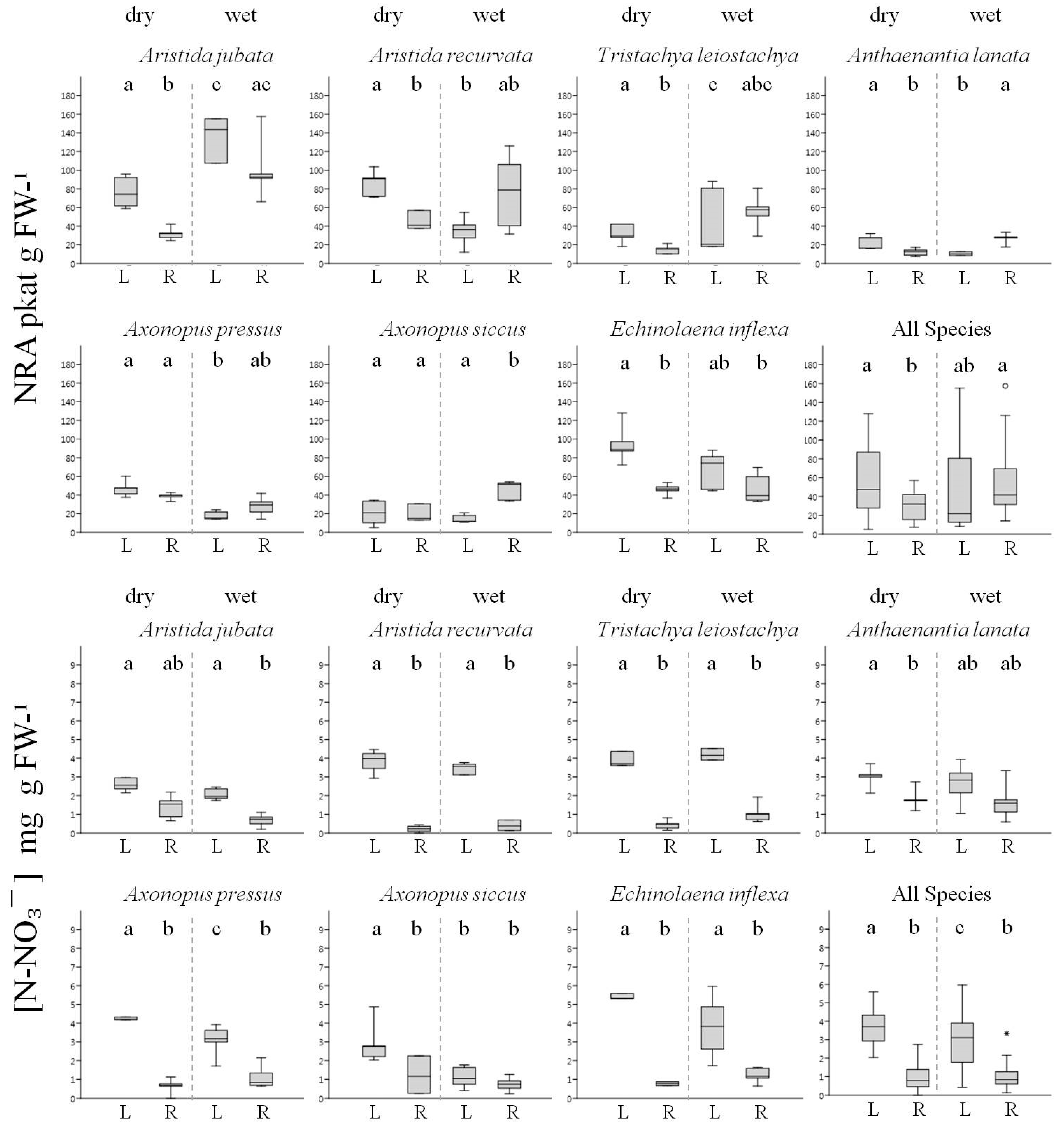
3.2. Nitrogen Usage by Native Grasses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Falkengren-Grerup, U. Interspecies differences in the preference of ammonium and nitrate in vascular plants. Oecologia 1995, 102, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Sigman, D.M.; Schuur, A.G.; Hedin, L.O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 2007, 104, 8902–8906. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.A.; Bol, R.; Bardgett, R.D. Preferences for uptake of different nitrogen forms by co-existing plant species and soil microbes. Ecology 2007, 88, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; da Silva, L.M.I.; de Freitas, L.D.; Debiasi, T.V.; Marchiori, N.M.; Aidar, M.P.M.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R. Nitrogen use strategies of seedlings from neotropical tree species of distinct successional groups. Plant Physiol. Biochem. 2017, 114, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Liu, X.; Nie, M.; Xu, X.; Zhou, S. Limited inorganic N niche partitioning by nine alpine plant species after long-term nitrogen addition. Sci. Total. Environ. 2020, 718, 137270. [Google Scholar] [CrossRef] [PubMed]
- Uscola, M.; Oliet, J.A.; Villar-Salvador, P.; Díaz-Pinés, E.; Jacobs, D.F. Nitrogen form and concentration interact to affect the performance of two ecologically distinct mediterranean forest trees. Eur. J. For. Res. 2014, 133, 235–246. [Google Scholar] [CrossRef]
- Andrews, M.; Maule, H.G.; Hodge, S.; Cherrill, A.; Raven, J.A. Seed dormancy, nitrogen nutrition and shade acclimation of impatiens glandulifera: Implications for successful invasion of deciduous woodland. Plant Ecol. Divers. 2009, 2, 145–153. [Google Scholar] [CrossRef][Green Version]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Eiten, G. The Cerrado vegetation of Brazil. Bot. Rev. 1972, 38, 201–341. [Google Scholar] [CrossRef]
- Furley, P.A.; Ratter, J.A. Soil Resources and Plant Communities of the Central Brazilian Cerrado and Their Development. J. Biogeogr. 1988, 15, 97. [Google Scholar] [CrossRef]
- Pereira-Silva, E.F.L.; Hardt, E.; dos Santos, J.E.; Aidar, M.P.M. Atividade de Nitrato Redutase e Conteúdo de Nitrogênio em Folhas de Espécies Arbóreas de Cerradão da Estação Ecológica de Jataí, Luiz Antônio, SP; EdUFSCar: São Carlos, Brazil, 2006; pp. 65–79. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 135–189. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A Re-evaluatin of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F.; Bin Sun, Q. Ammonium under solution culture alleviates aluminum toxicity in rice and reduces aluminum accumulation in roots compared with nitrate. Plant Soil 2008, 315, 107–121. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Pereira-Silva, E.; Casals, P.; Sodek, L.; Delitti, W.; Vallejo, V. Post-fire nitrogen uptake and allocation by two resprouting herbaceous species with contrasting belowground traits. Environ. Exp. Bot. 2019, 159, 157–167. [Google Scholar] [CrossRef]
- Cramer, M.D.; Hawkins, H.-J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef]
- Smirnoff, N.; Stewart, G.R. Nitrate assimilation and translocation by higher plants: Comparative physiology and ecological consequences. Physiol. Plant. 1985, 64, 133–140. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Palmborg, C.; Prinz, A.; Schulze, E.-D. The Role of Plant Diversity and Composition for Nitrate Leaching in Grasslands. Ecology 2003, 84, 1539–1552. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Salsac, L.; Chaillou, S.; Morot-Gaudry, J.-F.; Lesaint, C.; Jolivet, E. Nitrate and ammonium nutrition in plants. Plant Physiol. Biochem. 1987, 25, 805–812. [Google Scholar]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2017, 217, 35–53. [Google Scholar] [CrossRef]
- Amancio, S.; Stulen, I. Nitrogen Acquisition and Assimilation in Higher Plants, 3rd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Lu, P.; Zhai, X.; Zhang, R.; Zheng, Y.; Wang, H.; Nie, B.; Bai, W.; Niu, S.; Shi, P.; et al. An integrated belowground trait-based understanding of nitrogen-driven plant diversity loss. Glob. Chang. Biol. 2022, 28, 3651–3664. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Cleland, E.E.; Collins, S.L.; Fargione, J.E.; Gough, L.; Gross, K.L.; Pennings, S.C.; Suding, K.N.; Grace, J.B. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol. Lett. 2007, 10, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Eller, C.B.; Oliveira, R.S. Effects of nitrogen availability on the competitive interactions between an invasive and a native grass from Brazilian cerrado. Plant Soil 2017, 410, 63–72. [Google Scholar] [CrossRef]
- Boudsocq, S.; Niboyet, A.; Lata, J.C.; Raynaud, X.; Loeuille, N.; Mathieu, J.; Blouin, M.; Abbadie, L.; Barot, S. Plant Preference for Ammonium versus Nitrate: A Neglected Determinant of Ecosystem Functioning? Am. Nat. 2012, 180, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.C.; Menendez, E.; da Silva, D.L.; Bonieck, D.; Ramírez-Bahena, M.H.; Resende-Stoianoff, M.A.; Peix, A.; Velázquez, E.; Mateos, P.F.; Scotti, M.R. Invasion of the Brazilian campo rupestre by the exotic grass Melinis minutiflora is driven by the high soil N availability and changes in the N cycle. Sci. Total. Environ. 2017, 577, 202–211. [Google Scholar] [CrossRef]
- Garcia, D.B.; Xavier, R.O.; Camargo, P.B.; Vieira, S.A.; Pivello, V.R. Can an invasive African grass affect carbon and nitrogen stocks in open habitats of the Brazilian Cerrado? Flora Morphol. Distrib. Funct. Ecol. Plants 2022, 286, 151968. [Google Scholar] [CrossRef]
- Stewart, G.R.; Joly, C.A.; Smirnoff, N. Partitioning of inorganic nitrogen assimilation between the roots and shoots of cerrado and forest trees of contrasting plant communities of South East Brasil. Oecologia 1992, 91, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Rice, E.L. Differences in nitrate reductase activity between species of different stages in old field succession. Oecologia 1983, 57, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Scheurwater, I.; Koren, M.; Lambers, H.; Atkin, O.K. The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. J. Exp. Bot. 2002, 53, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Aidar, M.P.M.; Schmidt, S.; Moss, G.; Stewart, G.R.; Joly, C.A. Nitrogen use strategies of neotropical rainforest trees in threatened Atlantic Forest. Plant Cell Environ. 2003, 26, 389–399. [Google Scholar] [CrossRef]
- Pereira-Silva, E.F.L.; Hardt, E.; Joly, C.A.; Aidar, M.P.M. Sucessão Ecológica E O Uso De Nitrogênio Em Florestas Tropicais. Interciência Soc. 2011, 1, 149–159. [Google Scholar]
- Pereira-Silva, E.F.L.; Hardt, E.; Fernandes, A.O. The soil-plant relationship of nitrogen use in three tropical tree species. Web Ecol. 2012, 12, 57–64. [Google Scholar] [CrossRef]
- Baitello, J.B.; de Aguiar, O.T.; Pastore, J.A.; Arzolla, F.A.R.D.P. Parque Estadual do Juquery: Refúgio do Cerrado no Domínio Atlântico. IF Ser. Regist. 2013, 50, 1–46. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Keller, V.C.; Pereira-Silva, E.F.L.; Hardt, E. High richness, new occurrences, and threatened species in a savanna grassland remnant in the largest Brazilian metropolis. Check List 2021, 17, 507–549. [Google Scholar] [CrossRef]
- Amorim, D.G.d.A.; Zaine, J.E.; Rodrigues, F.H. Avaliação de suscetibilidade à erosão e movimentação gravitacional de massa no Parque Estadual do Juquery, Franco da Rocha (SP). Rev. Inst. Geocièncias—USP 2017, 17, 3–21. [Google Scholar] [CrossRef]
- Rossi, M. Mapa Pedológico do Estado de São Paulo: Revisado e Expandido; Instituto Florestal: São Paulo, Brazil, 2017. [Google Scholar]
- Keller, V.C. Diversidade Taxonômica e Funcional em uma Comunidade Vegetal Campestre de Cerrado na Região Metropolitana de São Paulo. Master’s Thesis, Universidade Federal de São Paulo, Diadema, Brasil. Available online: https://repositorio.unifesp.br/items/b08eb344-2633-435c-bbac-7793a6b37798 (accessed on 18 April 2024).
- McCullough, H. The determination of ammonia in whole blood by a direct colorimetric method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef]
- Stewart, G.; Lee, J.; Orebamjo, T. Nitrogen metabolism of halophytes II. Nitrate availability and utilization. New Phytol. 1973, 72, 539–546. [Google Scholar] [CrossRef]
- Stewart, G.R.; Popp, M.; Holzapfel, I.; Stewart, J.A.; Dickie-Eskew, A. Localization of nitrate reduction in ferns and its relationship to environment and physiological characteristics. New Phytol. 1986, 104, 373–384. [Google Scholar] [CrossRef]
- Saltzman, B.E. Colorimetric microdetermination of nitrogen dioxide in atmosphere. Anal. Chem. 1954, 26, 1949–1955. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry; Macmillan: New York, NY, USA, 1995. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2018. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- dos Santos, H.G.; Jacomine, P.K.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Lepsch, I.F. Formação e Conservação dos Solos; Oficina de textos: São Paulo, Brazil, 2016. [Google Scholar]
- Soares, M.P.; Reys, P.; Pifano, D.S.; de Sá, J.L.; da Silva, P.O.; Santos, T.M.; Silva, F.G. Relationship Between Edaphic Factors and Vegetation in Savannas of the Brazilian Midwest Region. Rev. Bras. Ciência Solo 2015, 39, 821–829. [Google Scholar] [CrossRef]
- Pivello, V.R.; Oliveras, I.; Miranda, H.S.; Haridasan, M.; Sato, M.N.; Meirelles, S.T. Effect of fires on soil nutrient availability in an open savanna in Central Brazil. Plant Soil 2010, 337, 111–123. [Google Scholar] [CrossRef]
- Corre, M.D.; Schnabel, R.R.; Stout, W.L. Spatial and seasonal variation of gross nitrogen transformations and microbial biomass in a Northeastern US grassland. Soil Biol. Biochem. 2002, 34, 445–457. [Google Scholar] [CrossRef]
- Pereira-Silva, E.F.L.; Hardt, E.; Biral, M.B.; Keller, V.C.; Delitti, W.B.C. Effects of recent fire on soil conditions and nutrient use of a native and an invasive grass in the Brazilian savanna. Écoscience 2019, 26, 359–370. [Google Scholar] [CrossRef]
- Sarmiento, G. Adaptive strategies of perennial grasses in South American savannas. J. Veg. Sci. 1992, 3, 325–336. [Google Scholar] [CrossRef]
- Patterson, K.; Walters, L.A.; Cooper, A.M.; Olvera, J.G.; Rosas, M.A.; Rasmusson, A.G.; Escobar, M.A. Nitrate-regulated glutaredoxins control arabidopsis primary root growth. Plant Physiol. 2016, 170, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Xi, N.; Zhu, B.-R.; Zhang, D.-Y. Contrasting grass nitrogen strategies reflect interspecific trade-offs between nitrogen acquisition and use in a semi-arid temperate grassland. Plant Soil 2017, 418, 267–276. [Google Scholar] [CrossRef]
- Luo, W.; Wang, X.; Sardans, J.; Wang, Z.; Dijkstra, F.A.; Lü, X.-T.; Peñuelas, J.; Han, X. Higher capability of C3 than C4 plants to use nitrogen inferred from nitrogen stable isotopes along an aridity gradient. Plant Soil 2018, 428, 93–103. [Google Scholar] [CrossRef]
- Weigelt, A.; Bol, R.; Bardgett, R.D. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia 2005, 142, 627–635. [Google Scholar] [CrossRef]
| Tribe | Species | Acronym | PP | RF | RC | IV |
|---|---|---|---|---|---|---|
| Aristideae | Aristida jubata (Arechav.) Herter | Aj | C4 | 0.24 | 0.18 | 0.42 |
| Aristida recurvata Kunth | Ar | C4 | 2.89 | 0.67 | 3.56 | |
| Arundinelleae | Tristachya leiostachya Nees | Tl | C4 | 4.05 | 8.21 | 12.26 |
| Paniceae | Anthaenantia lanata (Kunth) Benth. | Al | C4 | 4.62 | 7.56 | 12.18 |
| Axonopus pressus (Nees ex Steud.) Parodi | Ap | C4 | 2.89 | 4.30 | 7.19 | |
| Axonopus siccus (Nees) Kuhlm. | As | C4 | 3.47 | 8.06 | 11.53 | |
| Echinolaena inflexa (Poir.) Chase | Ei | C3 | 4.62 | 25.09 | 29.71 |
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Season | n | pH H2O | pHss | Ca2+ | K+ | N–NO3− | N–NH4+ | Condutiv |
| g/L | mg/g | µS/cm2 | ||||||
| Dry | 7 | 3.8 ± 0.1 a | 4.2 ± 0.1 a | 2.8 ± 0.2 a | 1.8 ± 0.3 a | 7.3 ± 0.5 a | 18.6 ± 3.3 a | 15.9 ± 1.8 a |
| Wet | 35 | 3.9 ± 0.1 a | 3.7 ± 0.1 a | 5.4 ± 0.6 b | 1.7 ± 0.2 a | 15.1 ± 0.8 b | 30.6 ± 3.1 A | 18.7 ± 1.9 a |
| B | ||||||||
| Species | ||||||||
| Aj | 5 | 3.8 ± 0.1 a | 3.5 ± 0.1 a | 6.6 ± 0.2 a | 1.0 ± 0.0 b | 16.4 ± 4.2 a | 39.2 ± 5.2 a | 20.4 ± 3.7 ab |
| Ar | 5 | 3.8 ± 0.1 a | 3.4 ± 0.1 a | 7.0 ± 3.2 ab | 1.8 ± 0.6 ab | 14.4 ± 3.1 a | 19.6 ± 6.0 A | 20.0 ± 5.6 ab |
| Tl | 5 | 3.6 ± 0.1 a | 3.6 ± 0.1 a | 6.6 ± 0.9 a | 2.8 ± 0.2 a | 12.8 ± 2.9 a | 38.9 ± 10.7 A | 25.3 ± 5.7 ab |
| Al | 5 | 4.0 ± 0.1 a | 3.7 ± 0.1 a | 4.4 ± 1.0 ab | 2.0 ± 0.8 ab | 14.8 ± 4.4 a | 41.1 ± 6.8 A | 24.0 ± 3.3 a |
| Ap | 5 | 3.8 ± 0.1 a | 3.7 ± 0.1 a | 3.8 ± 0.9 b | 1.6 ± 0.2 b | 15.6 ± 5.0 a | 33.1 ± 6.4 A | 15.2 ± 1.4 b |
| As | 5 | 3.9 ± 0.2 a | 3.5 ± 0.1 a | 5.2 ± 1.0 ab | 1.6 ± 0.2 b | 16.4 ± 2.8 a | 29.8 ± 8.4 A | 16.8 ± 1.2 ab |
| Ei | 5 | 3.9 ± 0.1 a | 3.6 ± 0.1 a | 6.8 ± 1.0 a | 1.6 ± 0.2 b | 11.6 ± 2.1 a | 20.7 ± 8.1 A | 20.0 ± 3.5 ab |
| All Species | Aj | Ar | Tl | Al | Ap | As | Ei | |
|---|---|---|---|---|---|---|---|---|
| NRA | ||||||||
| Period | ns | *** | ns | * | ns | *** | ns | ns |
| Compartment | ns | * | ns | ns | ns | ns | * | ** |
| Interaction | *** | ns | ** | * | *** | ns | * | ns |
| N–NO3− | ||||||||
| Period | ns | ns | ns | ns | ns | ns | ** | ns |
| Compartment | *** | *** | *** | *** | * | *** | * | *** |
| Interaction | ns | ns | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, V.C.; Pereira-Silva, E.F.L.; Meirelles, S.T.; Hardt, E. Effects of Seasonal Variation on Nitrogen Use in Brazilian Cerrado Grass Communities. Nitrogen 2024, 5, 373-385. https://doi.org/10.3390/nitrogen5020024
Keller VC, Pereira-Silva EFL, Meirelles ST, Hardt E. Effects of Seasonal Variation on Nitrogen Use in Brazilian Cerrado Grass Communities. Nitrogen. 2024; 5(2):373-385. https://doi.org/10.3390/nitrogen5020024
Chicago/Turabian StyleKeller, Victor Camargo, Erico Fernando Lopes Pereira-Silva, Sergio Tadeu Meirelles, and Elisa Hardt. 2024. "Effects of Seasonal Variation on Nitrogen Use in Brazilian Cerrado Grass Communities" Nitrogen 5, no. 2: 373-385. https://doi.org/10.3390/nitrogen5020024
APA StyleKeller, V. C., Pereira-Silva, E. F. L., Meirelles, S. T., & Hardt, E. (2024). Effects of Seasonal Variation on Nitrogen Use in Brazilian Cerrado Grass Communities. Nitrogen, 5(2), 373-385. https://doi.org/10.3390/nitrogen5020024







