Effects of Drought Stress on Red Clover-Grass Mixed Stands Compared to Grass Monoculture Stands in Nitrogen-Deficient Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
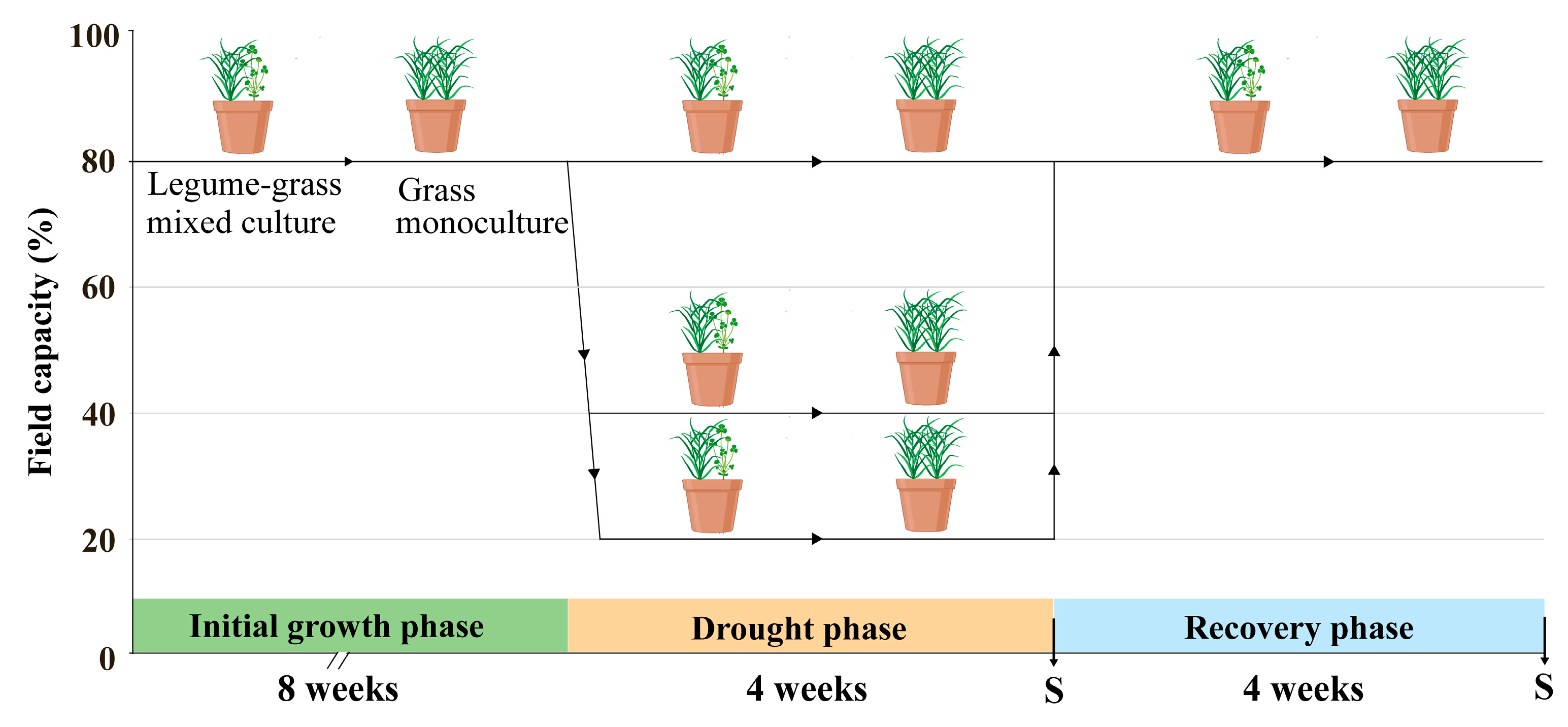
2.2. Drought Stress and Data Collection
2.3. Determination of C:N Ratio and Carbon Isotope Discrimination (CID)
2.4. Determination of Nitrogen Fixation-Related Parameters
2.5. Statistical Analysis
3. Results
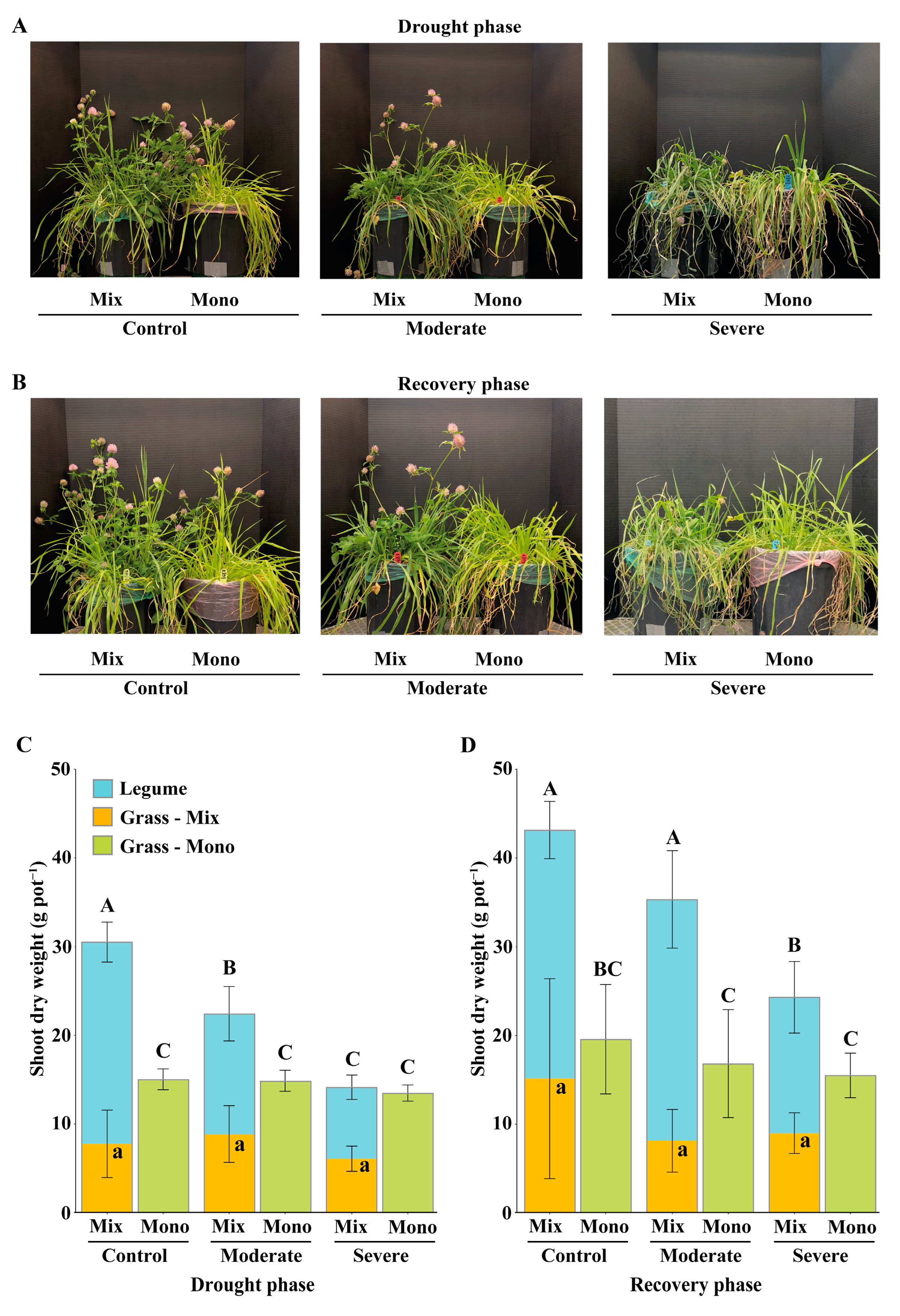
3.1. Effects of Drought Stress on Aboveground Biomass
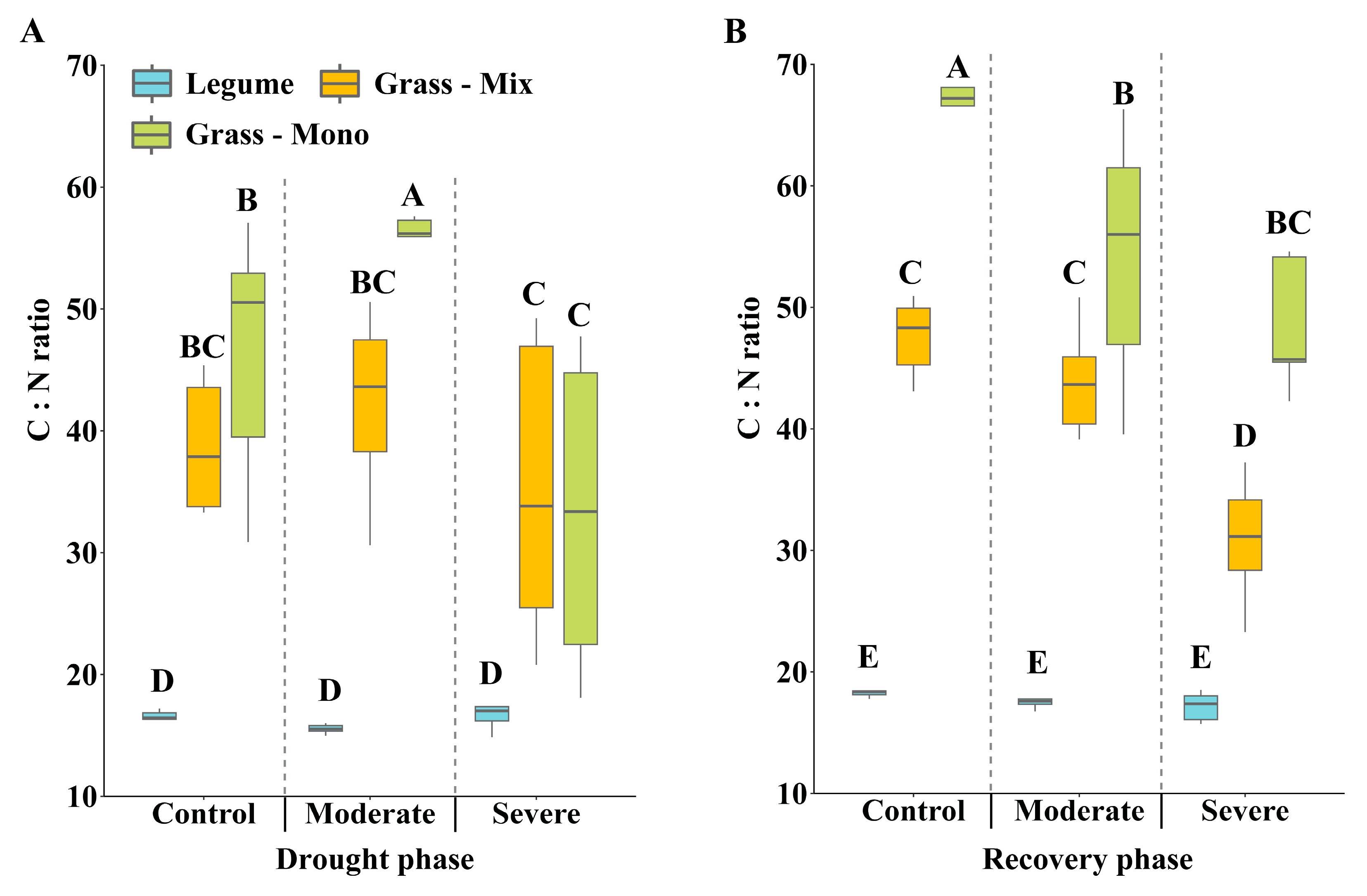
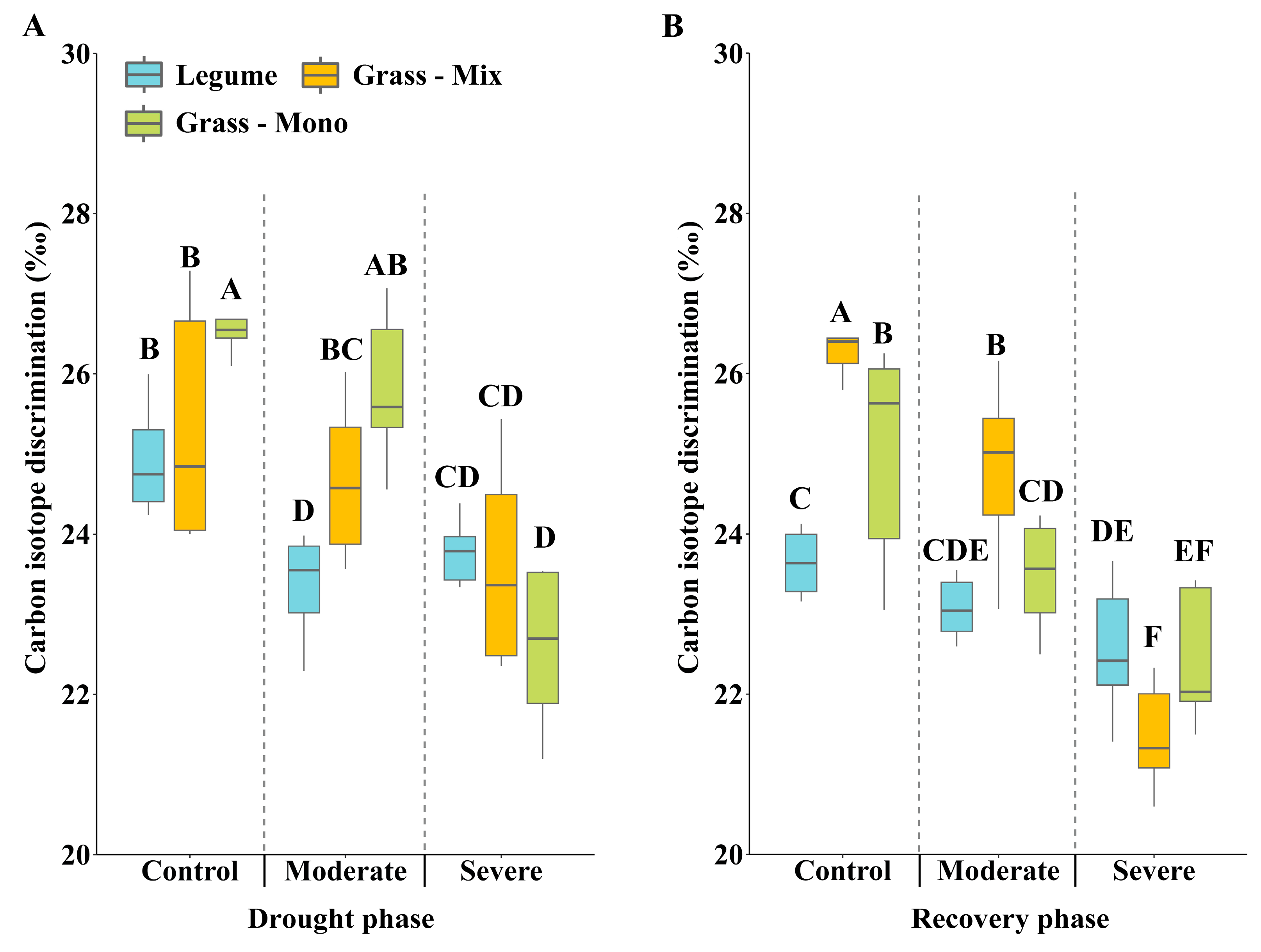
3.2. Effects of Drought Stress on C:N Ratio and Carbon Isotope Discrimination
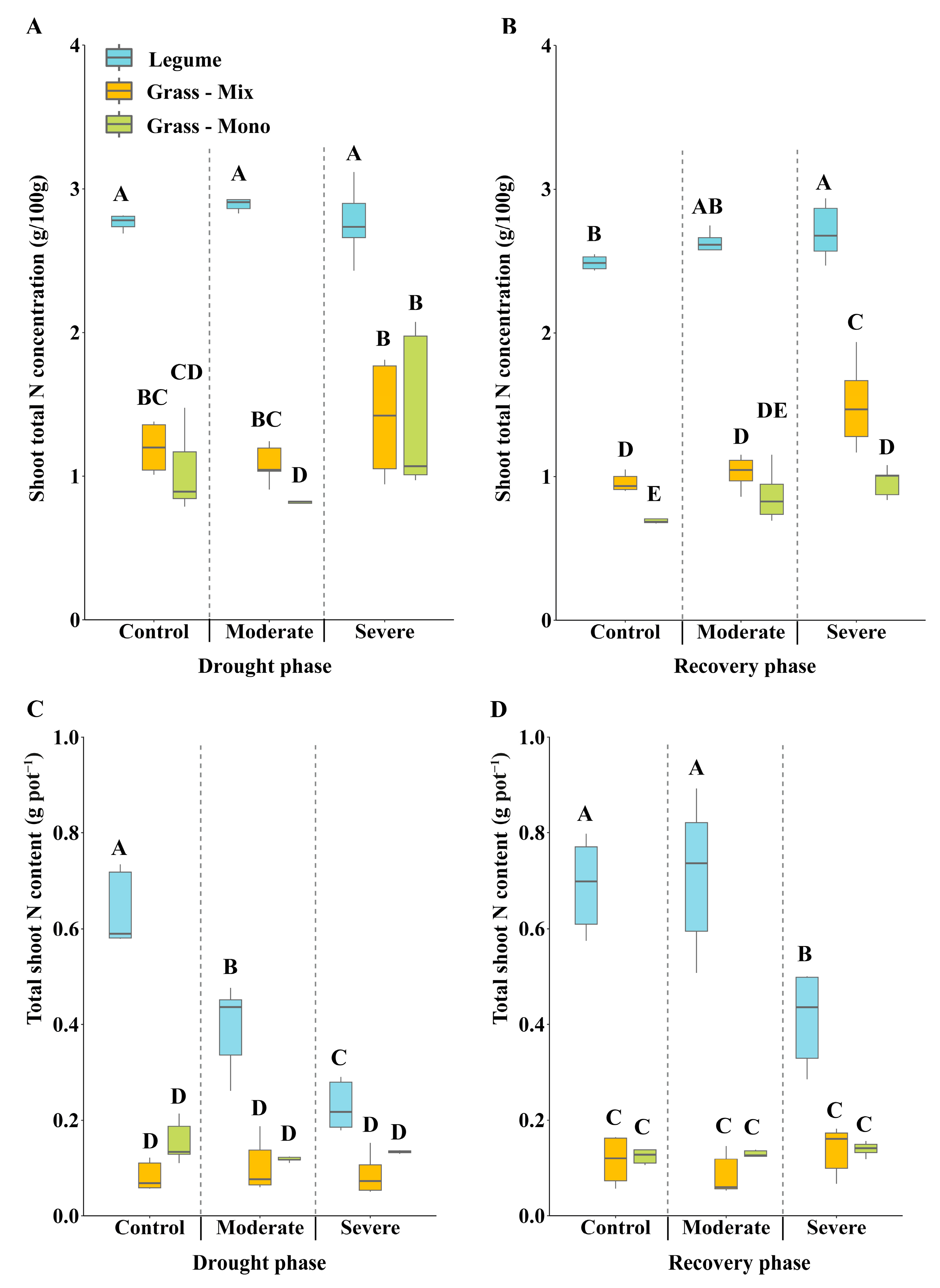
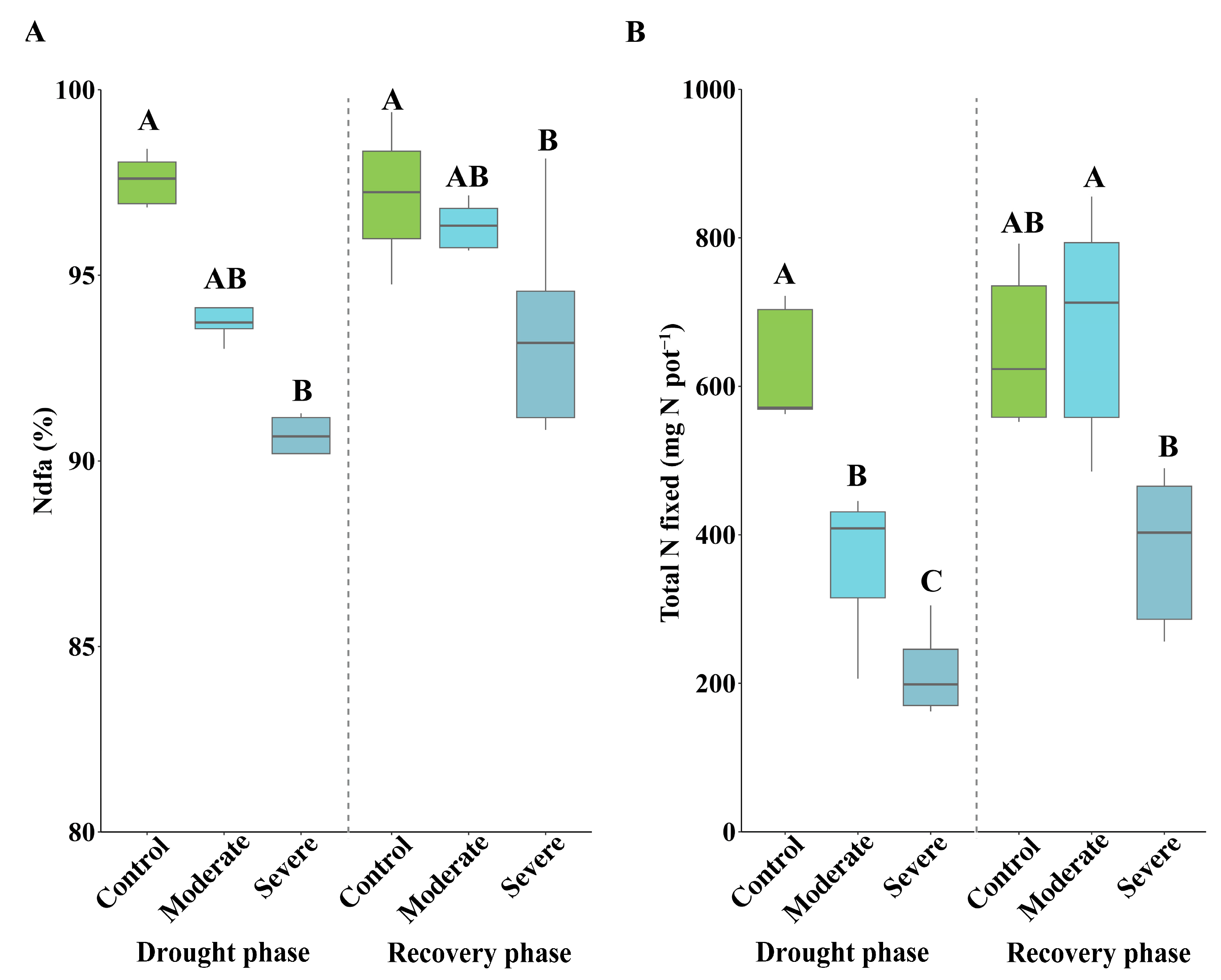
3.3. Effects of Drought on Shoot N Concentration and Symbiotic Nitrogen Fixation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem Function Enhanced by Combining Four Functional Types of Plant Species in Intensively Managed Grassland Mixtures: A 3-Year Continental-Scale Field Experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef]
- Tahir, M.; Li, C.; Zeng, T.; Xin, Y.; Chen, C.; Javed, H.H.; Yang, W.; Yan, Y. Mixture Composition Influenced the Biomass Yield and Nutritional Quality of Legume-Grass Pastures. Agronomy 2022, 12, 1449. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Caviglia, O.P.; Agnusdei, M.G.; Errecart, P.M. Forage Yield, Water- and Solar Radiation-Productivities of Perennial Pastures and Annual Crops Sequences in the South-Eastern Pampas of Argentina. Field Crops Res. 2018, 221, 19–31. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning Selection and Complementarity in Biodiversity Experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M. Biodiversity and Ecosystem Functioning: Recent Theoretical Advances. Oikos 2000, 91, 3–17. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Thomson, K.T. Plant Diversity and Ecosystem Productivity: Theoretical Considerations. Proc. Natl. Acad. Sci. USA 1997, 94, 1857–1861. [Google Scholar] [CrossRef]
- Huang, Z.; Dunkerley, D.; López-Vicente, M.; Wu, G.-L. Trade-Offs of Dryland Forage Production and Soil Water Consumption in a Semi-Arid Area. Agric. Water Manag. 2020, 241, 106349. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Yan, R.; Gao, J.; Wu, S.; Liu, Y. A Comprehensive Meta-Analysis of the Impacts of Intensified Drought and Elevated CO2 on Forage Growth. J. Environ. Manag. 2023, 327, 116885. [Google Scholar] [CrossRef]
- Taleb, M.H.; Majidi, M.M.; Pirnajmedin, F.; Maibody, S.A.M.M. Plant Functional Trait Responses to Cope with Drought in Seven Cool-Season Grasses. Sci. Rep. 2023, 13, 5285. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain; a Review of the Evidence. Glob. Food Sec. 2021, 28, 100488. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global Warming and Changes in Drought. Nature Clim. Change 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Loka, D.; Harper, J.; Humphreys, M.; Gasior, D.; Wootton-Beard, P.; Gwynn-Jones, D.; Scullion, J.; Doonan, J.; Kingston-Smith, A.; Dodd, R.; et al. Impacts of Abiotic Stresses on the Physiology and Metabolism of Cool-Season Grasses: A Review. Food Energy Secur. 2019, 8, e00152. [Google Scholar] [CrossRef]
- Lumactud, R.A.; Dollete, D.; Liyanage, D.K.; Szczyglowski, K.; Hill, B.; Thilakarathna, M.S. The Effect of Drought Stress on Nodulation, Plant Growth, and Nitrogen Fixation in Soybean during Early Plant Growth. J. Agron. Crop Sci. 2023, 209, 345–354. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, Signalling, Effects and Tolerance in Higher Plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Rathor, P.; Gorim, L.Y.; Thilakarathna, M.S. Plant Physiological and Molecular Responses Triggered by Humic Based Biostimulants—A Way Forward to Sustainable Agriculture. Plant Soil 2023, 492, 31–60. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Bahrani, M.J.; Bahrami, H.; Haghighi, A.A.K. Effect of Water Stress on Ten Forage Grasses Native or Introduced to Iran. Grassl. Sci. 2010, 56, 1–5. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal Responses to Drought Stress. In Water Stress and Crop Plants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 24–40. ISBN 978-1-119-05445-0. [Google Scholar]
- Joshi, J.; Stocker, B.D.; Hofhansl, F.; Zhou, S.; Dieckmann, U.; Prentice, I.C. Towards a Unified Theory of Plant Photosynthesis and Hydraulics. Nat. Plants 2022, 8, 1304–1316. [Google Scholar] [CrossRef]
- Johnson, D.A.; Asay, K.H.; Tieszen, L.L.; Ehleringer, J.R.; Jefferson, P.G. Carbon Isotope Dicrimination: Potential in Screening Cool-Season Grasses for Water-Limited Environments. Crop Sci. 1990, 30, 338–343. [Google Scholar] [CrossRef]
- Moghaddam, A.; Raza, A.; Vollmann, J.; Ardakani, M.R.; Wanek, W.; Gollner, G.; Friedel, J.K. Carbon Isotope Discrimination and Water Use Efficiency Relationships of Alfalfa Genotypes under Irrigated and Rain-Fed Organic Farming. Eur. J. Agron. 2013, 50, 82–89. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiong, B.; Cao, B.; Deng, X. Carbon/Nitrogen Imbalance Associated with Drought-Induced Leaf Senescence in Sorghum bicolor. PLoS ONE 2015, 10, e0137026. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and Signaling Aspects Underpinning the Regulation of Plant Carbon Nitrogen Interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Purcell, L.C.; Serraj, R.; Sinclair, T.R.; De, A. Soybean N2 Fixation Estimates, Ureide Concentration, and Yield Responses to Drought. Crop Sci. 2004, 44, 484–492. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought Effects on Soil Carbon and Nitrogen Dynamics in Global Natural Ecosystems. Earth-Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Finn, J.A.; Suter, M.; Haughey, E.; Hofer, D.; Lüscher, A. Greater Gains in Annual Yields from Increased Plant Diversity than Losses from Experimental Drought in Two Temperate Grasslands. Agric. Ecosyst. Environ. 2018, 258, 149–153. [Google Scholar] [CrossRef]
- Haughey, E.; Suter, M.; Hofer, D.; Hoekstra, N.J.; McElwain, J.C.; Lüscher, A.; Finn, J.A. Higher Species Richness Enhances Yield Stability in Intensively Managed Grasslands with Experimental Disturbance. Sci. Rep. 2018, 8, 15047. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Buchmann, N.; Lüscher, A. Nitrogen Status of Functionally Different Forage Species Explains Resistance to Severe Drought and Post-Drought Overcompensation. Agric. Ecosyst. Environ. 2017, 236, 312–322. [Google Scholar] [CrossRef]
- Liu, X.; Tahir, M.; Li, C.; Chen, C.; Xin, Y.; Zhang, G.; Cheng, M.; Yan, Y. Mixture of Alfalfa, Orchardgrass, and Tall Fescue Produces Greater Biomass Yield in Southwest China. Agronomy 2022, 12, 2425. [Google Scholar] [CrossRef]
- Thilakarathna, R.M.M.S.; Papadopoulos, Y.A.; Rodd, A.V.; Gunawardena, A.N.; Fillmore, S.A.E.; Prithiviraj, B. Characterizing Nitrogen Transfer from Red Clover Populations to Companion Bluegrass under Field Conditions. Can. J. Plant Sci. 2012, 92, 1163–1173. [Google Scholar] [CrossRef]
- Berg, C.C.; McElroy, A.R.; Kunelius, H.T. Timothy. In Cool-Season Forage Grasses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 643–664. ISBN 978-0-89118-226-9. [Google Scholar]
- Kunelius, H.T.; McRae, K.B.; Fillmore, S.A.E.; Dürr, G. Single-Cut Red Clover Combined with Late Timothy May Be Valuable in Short-Term Rotations. Can. J. Plant Sci. 2000, 80, 309–313. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground Nitrogen Transfer from Legumes to Non-Legumes under Managed Herbaceous Cropping Systems. A Review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar] [CrossRef]
- Tahir, M.; Wei, X.; Liu, H.; Li, J.; Zhou, J.; Kang, B.; Jiang, D.; Yan, Y. Mixed Legume-Grass Seeding and Nitrogen Fertilizer Input Enhance Forage Yield and Nutritional Quality by Improving the Soil Enzyme Activities in Sichuan, China. Front. Plant Sci. 2023, 14, 1176150. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Türk, M.; Yüksel, O.; Yilmaz, M. Forage Yield and the Quality of Perennial Legume-Grass Mixtures under Rainfed Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 114–118. [Google Scholar] [CrossRef]
- Liyanage, D.K.; Torkamaneh, D.; Belzile, F.; Balasubramanian, P.; Hill, B.; Thilakarathna, M.S. The Genotypic Variability among Short-Season Soybean Cultivars for Nitrogen Fixation under Drought Stress. Plants 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Torkamaneh, D.; Bruce, R.W.; Rajcan, I.; Chu, G.; Grainger, C.M.; Szczyglowski, K.; Hill, B.; Raizada, M.N. Testing Whether Pre-Pod-Fill Symbiotic Nitrogen Fixation in Soybean Is Subject to Drift or Selection over 100 Years of Soybean Breeding. Front. Agron. 2021, 3, 725813. [Google Scholar] [CrossRef]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and Nutrient Use in Four Grasses Under Drought Stress as Mediated by Silicon Fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; De Swaef, T.; Baert, J.; Pecio, Ł. Physiological and Biochemical Responses of Forage Grass Varieties to Mild Drought Stress under Field Conditions. Int. J. Plant Prod. 2020, 14, 335–353. [Google Scholar] [CrossRef]
- Staniak, M.; Kocoń, A. Forage Grasses under Drought Stress in Conditions of Poland. Acta Physiol. Plant 2015, 37, 116. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Marcinkevičienė, A.; Velička, R.; Kosteckas, R.; Rudinskienė, A.; Adamonytė, I.; Kriaučiūnienė, Z. Effects of Nitrogen Rates on the Productivity and Nutritive Value of Forage Grass Grown under Extreme Climatic Conditions. Agronomy 2021, 11, 2572. [Google Scholar] [CrossRef]
- Sleugh, B.; Moore, K.J.; George, J.R.; Brummer, E.C. Binary Legume-Grass Mixtures Improve Forage Yield, Quality, and Seasonal Distribution. Agron. J. 2000, 92, 24–29. [Google Scholar] [CrossRef]
- Sturludóttir, E.; Brophy, C.; Bélanger, G.; Gustavsson, A.-M.; Jørgensen, M.; Lunnan, T.; Helgadóttir, Á. Benefits of Mixing Grasses and Legumes for Herbage Yield and Nutritive Value in Northern Europe and Canada. Grass Forage Sci. 2014, 69, 229–240. [Google Scholar] [CrossRef]
- Kasulyte, D.; Praciak, A. Phleum pratense (Timothy Grass). In CABI Compendium; CABI: New York, NY, USA, 2015; p. 40248. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2020, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-L. Carbon and Nitrogen Nutrient Balance Signaling in Plants. Plant Signal Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological Stoichiometry of Plant Production: Metabolism, Scaling and Ecological Response to Global Change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Ranells, N.N.; Wagger, M.G. Winter Annual Grass-Legume Bicultures for Efficient Nitrogen Management in No-Till Corn. Agric. Ecosyst. Environ. 1997, 65, 23–32. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Soares, T.G.; Fernandes, A.R.; Braz, A.M.d.S. Grasses and Legumes as Cover Crop in No-Tillage System in Northeastern Pará Brazil. Acta Amaz. 2014, 44, 411–418. [Google Scholar] [CrossRef]
- Kohmann, M.M.; Sollenberger, L.E.; Dubeux, J.C.B., Jr.; Silveira, M.L.; Moreno, L.S.B.; da Silva, L.S.; Aryal, P. Nitrogen Fertilization and Proportion of Legume Affect Litter Decomposition and Nutrient Return in Grass Pastures. Crop Sci. 2018, 58, 2138–2148. [Google Scholar] [CrossRef]
- Dollete, D.; Lumactud, R.A.; Carlyle, C.N.; Szczyglowski, K.; Hill, B.; Thilakarathna, M.S. Effect of Drought Stress on Symbiotic Nitrogen Fixation, Soil Nitrogen Availability and Soil Microbial Diversity in Forage Legumes. Plant Soil 2023. [Google Scholar] [CrossRef]
- Lucero, D.W.; Grieu, P.; Guckert, A. Water Deficit and Plant Competition Effects on Growth and Water-Use Efficiency of White Clover (Trifolium repens, L.) and Ryegrass (Lolium perenne, L.). Plant Soil 2000, 227, 1–15. [Google Scholar] [CrossRef]
- Johnson, R.C.; Bassett, L.M. Carbon Isotope Discrimination and Water Use Efficiency in Four Cool-Season Grasses. Crop Sci. 1991, 31, 157–162. [Google Scholar] [CrossRef]
- More, S.J.; Ravi, V.; Raju, S. Chapter 17—Carbon Isotope Discrimination Studies in Plants for Abiotic Stress. In Climate Change and Crop Stress; Shanker, A.K., Shanker, C., Anand, A., Maheswari, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 493–537. ISBN 978-0-12-816091-6. [Google Scholar]
- Hall, A.E. Plant Adaptation to Hot and Dry Stresses in Relation to Horticultural Plant Breeding. Adv. Hortic. Sci. 1990, 4, 44–48. [Google Scholar]
- Blum, A. Effective Use of Water (EUW) and Not Water-Use Efficiency (WUE) Is the Target of Crop Yield Improvement under Drought Stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Serraj, R. Effects of Drought Stress on Legume Symbiotic Nitrogen Fixation: Physiological Mechanisms. Indian J. Exp. Biol. 2003, 41, 1136–1141. [Google Scholar]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule Performance within a Changing Environmental Context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef]
- Larrainzar, E.; Wienkoop, S.; Scherling, C.; Kempa, S.; Ladrera, R.; Arrese-Igor, C.; Weckwerth, W.; González, E.M. Carbon Metabolism and Bacteroid Functioning Are Involved in the Regulation of Nitrogen Fixation in Medicago truncatula under Drought and Recovery. Mol. Plant-Microbe Interact. 2009, 22, 1565–1576. [Google Scholar] [CrossRef]
- Molero, G.; Tcherkez, G.; Roca, R.; Mauve, C.; Cabrera-Bosquet, L.; Araus, J.L.; Nogués, S.; Aranjuelo, I. Do Metabolic Changes Underpin Physiological Responses to Water Limitation in Alfalfa (Medicago sativa) Plants during a Regrowth Period? Agric. Water Manag. 2019, 212, 1–11. [Google Scholar] [CrossRef]
- Naya, L.; Ladrera, R.; Ramos, J.; González, E.M.; Arrese-Igor, C.; Minchin, F.R.; Becana, M. The Response of Carbon Metabolism and Antioxidant Defenses of Alfalfa Nodules to Drought Stress and to the Subsequent Recovery of Plants. Plant Physiol. 2007, 144, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Hofer, D.; Suter, M.; Haughey, E.; Finn, J.A.; Hoekstra, N.J.; Buchmann, N.; Lüscher, A. Yield of Temperate Forage Grassland Species Is Either Largely Resistant or Resilient to Experimental Summer Drought. J. Appl. Ecol. 2016, 53, 1023–1034. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Keblawy, A.; Aljabi, A.E.; Aljabi, D.E.; Hafez, M.; Al Jasmi, A.; Schampoel, T.; Temperton, V.M. Nitrogen Fixation and Carbon Assimilation of the Desert Legume Tephrosia apollinea under PEG-Induced Osmotic Stress. Flora 2019, 251, 105–113. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Boldt-Burisch, K.; Fritsch, S.; Koning, L.A.; Freese, D. Carbon Allocation, Nodulation, and Biological Nitrogen Fixation of Black Locust (Robinia pseudoacacia L.) under Soil Water Limitation. Ann. For. Res. 2015, 58, 259–274. [Google Scholar] [CrossRef]
- Westra, S.V. Non-Uniform Stands of Red Clover (Trifolium pratense L.) Underseeded to Winter Wheat (Tritcum aestivum L.): A Survey Study to Identify Causes; University of Guelph: Guelph, ON, USA, 2015. [Google Scholar]
- Suter, M.; Connolly, J.; Finn, J.A.; Loges, R.; Kirwan, L.; Sebastià, M.-T.; Lüscher, A. Nitrogen Yield Advantage from Grass-Legume Mixtures is robust over a Wide Range of Legume Proportions and Environmental Conditions. Glob. Change Biol. 2015, 21, 2424–2438. [Google Scholar] [CrossRef]
- Goh, K.M. Effects of Multiple Reference Plants, Season, and Irrigation on Biological Nitrogen Fixation by Pasture Legumes Using the Isotope Dilution Method. Commun. Soil Sci. Plant Anal. 2007, 38, 1841–1860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Silva, C.; Rathor, P.; Poudel, H.P.; Thilakarathna, M.S. Effects of Drought Stress on Red Clover-Grass Mixed Stands Compared to Grass Monoculture Stands in Nitrogen-Deficient Systems. Nitrogen 2023, 4, 382-396. https://doi.org/10.3390/nitrogen4040027
De Silva C, Rathor P, Poudel HP, Thilakarathna MS. Effects of Drought Stress on Red Clover-Grass Mixed Stands Compared to Grass Monoculture Stands in Nitrogen-Deficient Systems. Nitrogen. 2023; 4(4):382-396. https://doi.org/10.3390/nitrogen4040027
Chicago/Turabian StyleDe Silva, Chathuranga, Pramod Rathor, Hari P. Poudel, and Malinda S. Thilakarathna. 2023. "Effects of Drought Stress on Red Clover-Grass Mixed Stands Compared to Grass Monoculture Stands in Nitrogen-Deficient Systems" Nitrogen 4, no. 4: 382-396. https://doi.org/10.3390/nitrogen4040027
APA StyleDe Silva, C., Rathor, P., Poudel, H. P., & Thilakarathna, M. S. (2023). Effects of Drought Stress on Red Clover-Grass Mixed Stands Compared to Grass Monoculture Stands in Nitrogen-Deficient Systems. Nitrogen, 4(4), 382-396. https://doi.org/10.3390/nitrogen4040027




