Herbicides versus Nitrogen Cycle: Assessing the Trade-Offs for Soil Integrity and Crop Yield—An In-Depth Systematic Review
Abstract
1. Introduction
2. Interaction between Herbicides and the Nitrogen Cycle: Negative Impacts on Soil Health and Crop Yield
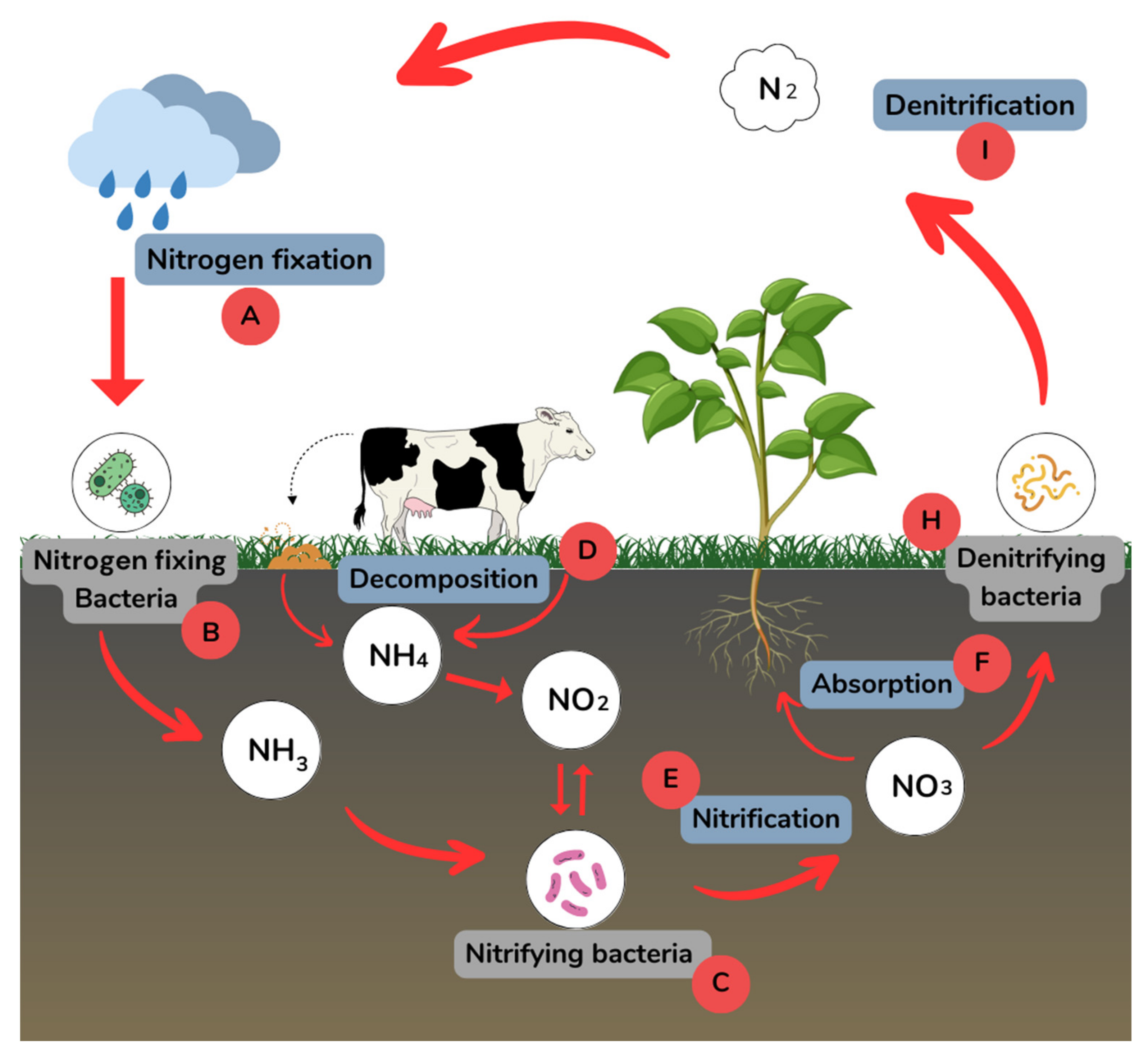
2.1. Mechanism of Interaction
2.1.1. Nitrogen Fixation Inhibition
2.1.2. Inhibition of Denitrifying Bacteria
2.1.3. Reduced Nitrogen Uptake by Plants
2.1.4. Changing the Rate of Organic Matter Decomposition
2.1.5. Change in the Rate of Nitrogen Fixation by Symbiotic Bacteria
3. Perspective of Contemporary Literature Regarding the Effects of Herbicides on Nitrogen Cycle Dynamics and Agricultural Systems
3.1. Distribution of Articles by Year and Cumulative Total
3.2. Distribution of Articles by Index Bases
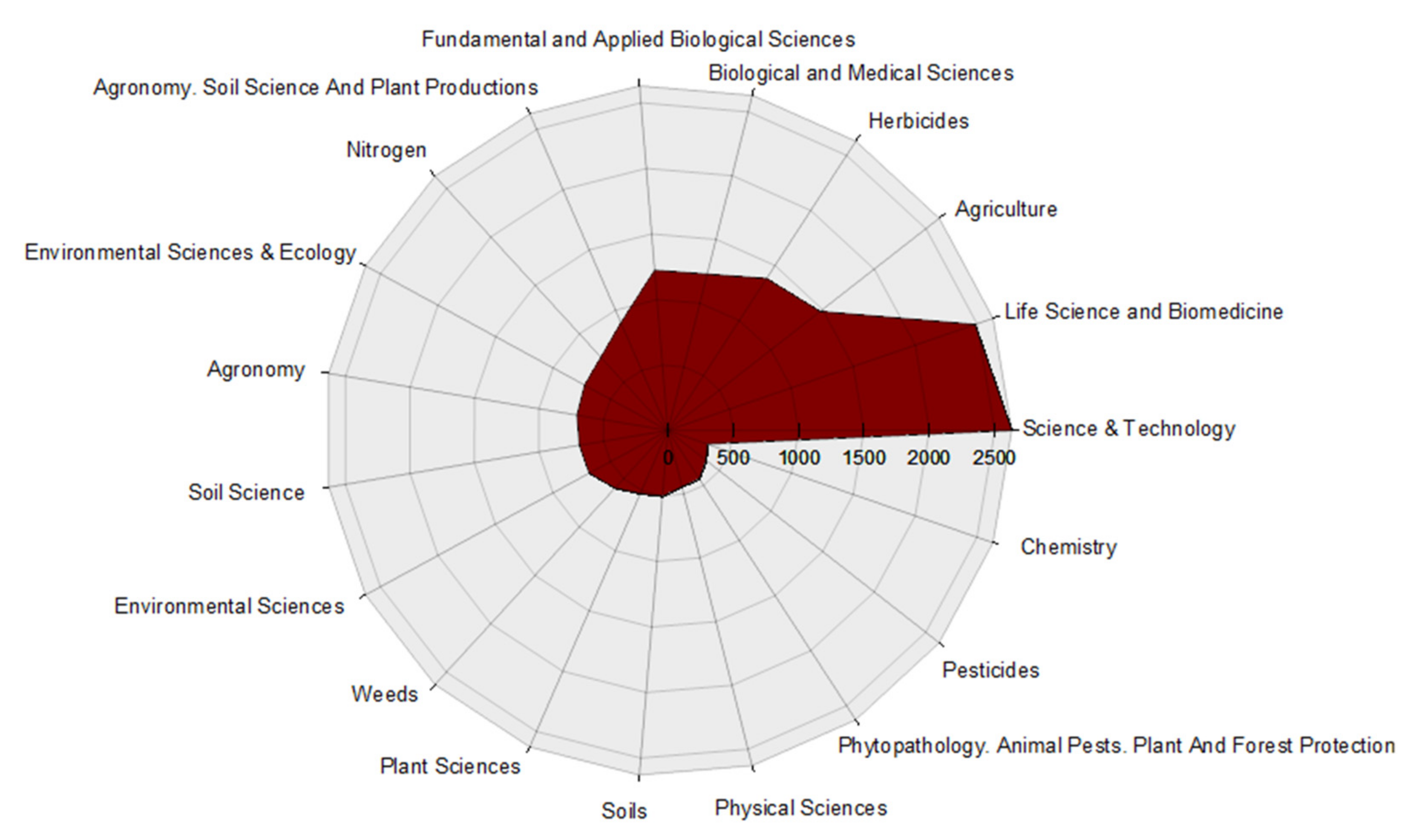
3.3. Distribution of Published Articles by Thematic Areas
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Paniagua-López, M.; Jiménez-Pelayo, C.; Gómez-Fernández, G.O.; Herrera-Cervera, J.A.; López-Gómez, M. Reduction in the Use of Some Herbicides Favors Nitrogen Fixation Efficiency in Phaseolus vulgaris and Medicago Sativa. Plants 2023, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of Agrochemicals on Soil Health. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 161–187. [Google Scholar] [CrossRef]
- Du, P.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Effects of Trifluralin on the Soil Microbial Community and Functional Groups Involved in Nitrogen Cycling. J. Hazard. Mater. 2018, 353, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.A.; Tetteh, W.; Bello, M. Impact of Commonly Used Agrochemicals on Bacterial Diversity in Cultivated Soils. Indian J. Microbiol. 2009, 49, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crouzet, O.; Batisson, I.; Besse-Hoggan, P.; Bonnemoy, F.; Bardot, C.; Poly, F.; Bohatier, J.; Mallet, C. Response of Soil Microbial Communities to the Herbicide Mesotrione: A Dose-Effect Microcosm Approach. Soil Biol. Biochem. 2010, 42, 193–202. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, Y.; Zhu, L.; Zhang, J.; Li, B.; Wang, J.; Wang, J.; Zhang, C.; Cheng, C. Effects of the Herbicide Mesotrione on Soil Enzyme Activity and Microbial Communities. Ecotoxicol. Environ. Saf. 2018, 164, 571–578. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Dai, G.; Cui, K.; Wu, X.; Qin, F.; Xu, J.; Dong, F.; Pan, X.; Zheng, Y. The Long-Acting Herbicide Mesosulfuron-Methyl Inhibits Soil Microbial Community Assembly Mediating Nitrogen Cycling. J. Hazard. Mater. 2023, 443, 130293. [Google Scholar] [CrossRef]
- Brito, M.d.M.P.; Muraoka, T.; Silva, E.C.d. Contribuição Da Fixação Biológica de Nitrogênio, Fertilizante Nitrogenado e Nitrogênio Do Solo No Desenvolvimento de Feijão e Caupi. Bragantia 2011, 70, 206–215. [Google Scholar] [CrossRef]
- Cunha, L.d.S.; Duarte Júnior, J.B.; Lana, M.d.C.; Ribeiro, L.L.O.; Shimada, B.S.; Richart, A.; Costa, A.C.T.d.; Rosa, W.B. Inoculation, Co-Inoculation and Nitrogen Fertilization in Soybean Culture. Concilium 2023, 23, 454–472. [Google Scholar] [CrossRef]
- Kamran, A.; Mushtaq, M.; Arif, M.; Rashid, S. Role of Biostimulants (Ascorbic Acid and Fulvic Acid) to Synergize Rhizobium Activity in Pea (Pisum sativum L. Var. Meteor). Plant Physiol. Biochem. 2023, 196, 668–682. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic Regulation of Biological Nitrogen Fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Bian, Y.; Zhao, Z.; Wang, Z.; Wang, Y.; Mei, Z.; Jiang, X.; Schäffer, A.; et al. Manure- and Straw-Derived Biochars Reduce the Ecological Risk of PBDE and Promote Nitrogen Cycling by Shaping Microbiomes in PBDE-Contaminated Soil. Chemosphere 2023, 312, 137262. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, J.; Zheng, X.; Zhang, Y.; Chen, D.; Ding, H. Divergent Modulation of Land Use-Driven Changes in Soil Properties and Herbicide Acetochlor Application on Soil Nitrogen Cycling. Soil Tillage Res. 2022, 215, 105231. [Google Scholar] [CrossRef]
- Rose, M.T.; Cavagnaro, T.R.; Scanlan, C.A.; Rose, T.J.; Vancov, T.; Kimber, S.; Kennedy, I.R.; Kookana, R.S.; Van Zwieten, L. Impact of Herbicides on Soil Biology and Function. Adv. Agron. 2016, 136, 133–220. [Google Scholar] [CrossRef]
- Carmo, J.B.d.; Andrade, C.A.d.; Cerri, C.C.; Piccolo, M.d.C. Disponibilidade de Nitrogênio e Fluxos de N2O a Partir de Solo Sob Pastagem Após Aplicação de Herbicida. Rev. Bras. Ciência Solo 2005, 29, 735–746. [Google Scholar] [CrossRef]
- Angelini, J.; Silvina, G.; Taurian, T.; Ibáñez, F.; Tonelli, M.L.; Valetti, L.; Anzuay, M.S.; Ludueña, L.; Muñoz, V.; Fabra, A. The Effects of Pesticides on Bacterial Nitrogen Fixers in Peanut-Growing Area. Arch. Microbiol. 2013, 195, 683–692. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohammad, N.; Roychoudhury, N. Soil Pollution: Causes, Effects and Control. Trop. For. Res. Inst. 2015, 3, 20–30. [Google Scholar]
- Cycoń, M.; Wójcik, M.; Borymski, S.; Piotrowska-Seget, Z. Short-Term Effects of the Herbicide Napropamide on the Activity and Structure of the Soil Microbial Community Assessed by the Multi-Approach Analysis. Appl. Soil Ecol. 2013, 66, 8–18. [Google Scholar] [CrossRef]
- Gonzalez, A.; Gonzalez-Murua, C.; Royuela, M. Influence of Imazethapyr on Rhizobium Growth and Its Symbiosis with Pea (Pisum sativum). Weed Sci. 1996, 44, 31–37. [Google Scholar] [CrossRef]
- Kennedy, I. Non-Symbiotic Bacterial Diazotrophs in Crop-Farming Systems: Can Their Potential for Plant Growth Promotion Be Better Exploited? Soil Biol. Biochem. 2004, 36, 1229–1244. [Google Scholar] [CrossRef]
- Bloom, A.J. The Increasing Importance of Distinguishing among Plant Nitrogen Sources. Curr. Opin. Plant Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Geisler, G.; Lea, P.J.; Morot-Gaudry, J.-F. (Eds.) 2001: Plant Nitrogen. J. Agron. Crop Sci. 2003, 189, 197–198. [Google Scholar] [CrossRef]
- Du, P.; He, H.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Mesosulfuron-Methyl Influenced Biodegradability Potential and N Transformation of Soil. J. Hazard. Mater. 2021, 416, 125770. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Mendes, I.C.; Nakatani, A.S.; dos Reis-Junior, F.B.; Morais, J.Z.; de Oliveira, M.C.N.; Fernandes, M.F. Effects of the Glyphosate-Resistance Gene and Herbicides on Soybean: Field Trials Monitoring Biological Nitrogen Fixation and Yield. Field Crops Res. 2014, 158, 43–54. [Google Scholar] [CrossRef]
- Threatt, S.D.; Rees, D.C. Biological Nitrogen Fixation in Theory, Practice, and Reality: A Perspective on the Molybdenum Nitrogenase System. FEBS Lett. 2022, 597, 45–58. [Google Scholar] [CrossRef]
- Barbieri, P.; Starck, T.; Voisin, A.-S.; Nesme, T. Biological Nitrogen Fixation of Legumes Crops under Organic Farming as Driven by Cropping Management: A Review. Agric. Syst. 2023, 205, 103579. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Reddy, K.N. Impact of Glyphosate on the Symbiosis with Glyphosate-Resistant Transgenic Soybean. J. Environ. Qual. 2004, 33, 825. [Google Scholar] [CrossRef]
- Delong, G. Soybean Root Structure with Nodule. Available online: https://pixels.com/featured/soybean-root-structure-with-nodule-garry-delong.html (accessed on 18 May 2023).
- Chalk, P.M. The Contribution of Associative and Symbiotic Nitrogen Fixation to the Nitrogen Nutrition of Non-Legumes. Plant Soil 1991, 132, 29–39. [Google Scholar] [CrossRef]
- Kennedy, I.R.; Tchan, Y.-T. Biological Nitrogen Fixation in Non-Leguminous Field Crops: Recent Advances. Plant Soil 1992, 141, 93–118. [Google Scholar] [CrossRef]
- Chen, W.C.; Yen, J.H.; Chang, C.S.; Wang, Y.S. Effects of Herbicide Butachlor on Soil Microorganisms and on Nitrogen-Fixing Abilities in Paddy Soil. Ecotoxicol. Environ. Saf. 2009, 72, 120–127. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. Denitrification in Soil: Effects of Herbicides. Soil Biol. Biochem. 1985, 17, 447–452. [Google Scholar] [CrossRef]
- Bollag, J.-M.; Henninger, N.M. Influence of Pesticides on Denitrification in Soil and with an Isolated Bacterium. J. Environ. Qual. 1976, 5, 15–18. [Google Scholar] [CrossRef]
- Pell, M.; Stenberg, B.; Stenström, J.; Torstensson, L. Potential Denitrification Activity Assay in Soil—With or without Chloramphenicol? Soil Biol. Biochem. 1996, 28, 393–398. [Google Scholar] [CrossRef]
- Gulhane, P.A.; Ashok, V.G.; Kajal, M.S. Influence of pesticides on nitrogen fixing bacteria. Int. J. Tech. Res. Appl. 2015, 3, 157–160. [Google Scholar]
- Koike, I.; Hattori, A. Growth Yield of a Denitrifying Bacterium, Pseudomonas Denitrificans, under Aerobic and Denitrifying Conditions. J. Gen. Microbiol. 1975, 88, 1–10. [Google Scholar] [CrossRef]
- Grenier, V.; Laur, J.; Gonzalez, E.; Pitre, F.E. Glyphosate Has a Negligible Impact on Bacterial Diversity and Dynamics during Composting. Environ. Microbiol. 2023. early view. [Google Scholar] [CrossRef]
- Wan, X.; Wan, G.; Snozzi, M. Microbiological Denitrification and Denitrifying Activity of Paracoccus Denitrificans. Chin. J. Geochem. 2000, 19, 186–192. [Google Scholar] [CrossRef]
- Su, G.; Chen, B.; Wu, X.; Xu, J.; Yang, K.; Lin, D. nZVI Decreases N2O Emission from Pesticide-Contaminated Paddy Soil. Sci. Total Environ. 2023, 892, 164613. [Google Scholar] [CrossRef]
- Crouzet, O.; Wiszniowski, J.; Donnadieu, F.; Bonnemoy, F.; Bohatier, J.; Mallet, C. Dose-Dependent Effects of the Herbicide Mesotrione on Soil Cyanobacterial Communities. Arch. Environ. Contam. Toxicol. 2012, 64, 23–31. [Google Scholar] [CrossRef]
- Forde, B.G.; Clarkson, D.T. Nitrate and Ammonium Nutrition of Plants: Physiological and Molecular Perspectives. Adv. Bot. Res. 1999, 30, 1–90. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Omar, S.A.; Karanxha, S. The Impact of Pesticides on Arbuscular Mycorrhizal and Nitrogen-Fixing Symbioses in Legumes. Appl. Soil Ecol. 2000, 14, 191–200. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Santarém, E.R. Fisiologia Vegetal, 4th ed.; Artmed: Porto Alegre, Brazil, 2009; Volume 1. [Google Scholar]
- Patnaik, G.K.; Bose, L.K.; Mehta, A.M.; Rajaramamohan Rao, V. Rhizosphere Nitrogenase and Azospirillum sp. Association with Wild, Trisomic and Cultivated Rice. Microbiol. Res. 1994, 149, 42–46. [Google Scholar] [CrossRef]
- Singh, G.; Wright, D. Effects of Herbicides on Nodulation, Symbiotic Nitrogen Fixation, Growth and Yield of Pea (Pisum sativum). J. Agric. Sci. 1999, 133, 21–30. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in Soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef]
- Prata, F.; Lavorenti, A.; Regitano, J.B.; Tornisielo, V.L. Influência Da Matéria Orgânica Na Sorção e Dessorção Do Glifosato Em Solos Com Diferentes Atributos Mineralógicos. Rev. Bras. Ci. Solo 2000, 24, 947–951. [Google Scholar] [CrossRef]
- Takeshita, V.; Mendes, K.F.; Alonso, F.G.; Tornisielo, V.L. Effect of Organic Matter on the Behavior and Control Effectiveness of Herbicides in Soil. Planta Daninha 2019, 37, 019214401. [Google Scholar] [CrossRef]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow Bone Char as a Sorbent to Increase Sorption and Decrease Mobility of Hexazinone, Metribuzin, and Quinclorac in Soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Prata, F.; Lavorenti, A.; Regitano, J.B.; Tornisielo, V.L. Degradação e Sorção de Ametrina Em Dois Solos Com Aplicação de Vinhaça. Pesqui. Agropecuária Bras. 2001, 36, 975–981. [Google Scholar] [CrossRef]
- Li, X.G.; Jia, B.; Lv, J.; Ma, Q.; Kuzyakov, Y.; Li, F. Nitrogen Fertilization Decreases the Decomposition of Soil Organic Matter and Plant Residues in Planted Soils. Soil Biol. Biochem. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Dupont, Y.L.; Strandberg, B.; Damgaard, C. Effects of Herbicide and Nitrogen Fertilizer on Non-Target Plant Reproduction and Indirect Effects on Pollination in Tanacetum vulgare (Asteraceae). Agric. Ecosyst. Environ. 2018, 262, 76–82. [Google Scholar] [CrossRef]
- Langaro, A.C.; Agostinetto, D.; Oliveira, C.; Franco, J.J.; Zandoná, R.R.; Vargas, L. Influence of Nitrogen Fertilization on Herbicide Selectivity in Rice. Planta Daninha 2018, 36, 018180161. [Google Scholar] [CrossRef]
- Hungria, M.; Mendes, I.C. Nitrogen Fixation with Soybean: The Perfect Symbiosis? In Biological Nitrogen Fixation; de Bruijn, F.J., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1009–1024. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; Bender, F.R.; Sanzovo, A.W.S.; Ferreira, E.; Nogueira, M.A.; Hungria, M. Impact of Pesticides in Properties of Bradyrhizobium Spp. and in the Symbiotic Performance with Soybean. World J. Microbiol. Biotechnol. 2020, 36, 172. [Google Scholar] [CrossRef]
- Burul, F.; Barić, K.; Lakić, J.; Milanović-Litre, A. Herbicides Effects on Symbiotic Nitrogen-Fixing Bacteria. J. Cent. Eur. Agric. 2022, 23, 89–102. [Google Scholar] [CrossRef]
- Aliverdi, A.; Ahmadvand, G. Herbicide Toxicity to Soybean–Rhizobium Symbiosis as Affected by Soil pH. Bull. Environ. Contam. Toxicol. 2018, 101, 434–438. [Google Scholar] [CrossRef]
- Nunes, A.L.; Lorenset, J.; Gubiani, J.E.; Santos, F.M. A Multy-Year Study Reveals the Importance of Residual Herbicides on Weed Control in Glyphosate-Resistant Soybean. Planta Daninha 2018, 36, 018176135. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Mushtaq, W.; Anusha Siddiqui, S.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; AL-Taey, D.K.A.; Tampubolon, K. Herbicide Residues in Agroecosystems: Fate, Detection, and Effect on Non-Target Plants. Rev. Agric. Sci. 2021, 9, 157–167. [Google Scholar] [CrossRef]
- Madureira Barroso, G.; dos Santos, J.B.; de Oliveira, I.T.; Rocha Nunes, T.K.M.; Alves Ferreira, E.; Marinho Pereira, I.; Valadão Silva, D.; de Freitas Souza, M. Tolerance of Bradyrhizobium sp. BR 3901 to Herbicides and Their Ability to Use These Pesticides as a Nutritional Source. Ecol. Indic. 2020, 119, 106783. [Google Scholar] [CrossRef]
- Vercellino, M.; Gómez, M.A. Denitrifying Capacity of Rhizobial Strains of Argentine Soils and Herbicide Sensitivity. Ann. Microbiol. 2013, 63, 1563–1570. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Poloni, N.M.; Lazarini, E.; Bettiol, J.V.T.; Fischer Filho, J.A.; Negrisoli, M.M. Development of RR Soybean in Function of Glyphosate Doses and Bradyrhizobium Inoculation. Rev. Bras. Eng. Agrícola Ambient. 2018, 22, 854–858. [Google Scholar] [CrossRef]
- Khan, M.d.S.; Zaidi, A.; Aamil, M. Influence of Herbicides on Chickpea-Mesorhizobium Symbiosis. Agronomie 2004, 24, 123–127. [Google Scholar] [CrossRef]
- Santos, J.B.; Silva, A.A.; Costa, M.D.; Jakelaitis, A.; Vivian, R.; Santos, E.A. Ação de Herbicidas Sobre o Crescimento de Estirpes de Rhizobium Tropici. Planta Daninha 2006, 24, 457–465. [Google Scholar] [CrossRef]
- Melo, C.A.D.; Massenssini, A.M.; Passos, A.B.R.J.; Carvalho, F.P.; Ferreira, L.R.; Silva, A.A.; Costa, M.D. Isolation and Characteristics of Sulfentrazone-Degrading Bacteria. J. Environ. Sci. Health Part B 2016, 52, 115–121. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Y.; Wang, Z.; Li, M.; Yang, F.; Jiang, D.; Jiang, Z. Insight into the Variation of Bacterial Structure in Atrazine-Contaminated Soil Regulating by Potential Phytoremediator: Pennisetum americanum (L.) K. Schum. Front. Microbiol. 2018, 9, 864. [Google Scholar] [CrossRef]
- Mielke, K.C.; Bertuani, R.R.; Laube, A.F.S.; Brochado, M.G.d.S.; Ribeiro, A.C.M.; Paula, D.F.d.; Pires, F.R.; D’Angeri, R. Fitorremediação de Solos Contaminados Com Sulfentrazone Em Função Da Inoculação de Bactérias Simbióticas Em Crotalaria juncea. Rev. Ibero-Am. Ciências Ambient. 2021, 12, 140–151. [Google Scholar] [CrossRef]
- Mielke, K.C.; Bertuani, R.R.; Pires, F.R.; Bueno Cotta, A.J.; Egreja Filho, F.B.; Madalão, J.C. Does Canavalia ensiformis Inoculation with Bradyrhizobium sp. Enhance Phytoremediation of Sulfentrazone-Contaminated Soil? Chemosphere 2020, 255, 127033. [Google Scholar] [CrossRef]
- CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Portal de Periódicos da Capes. Available online: https://www-periodicos-capes-gov-br.ezl.periodicos.capes.gov.br/index.php (accessed on 31 March 2023).
- CLARIVATE Science Citation Index-Expanded. Available online: https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/web-of-science/web-of-science-core-collection/science-citation-index-expanded/#resources (accessed on 23 March 2023).
- ELSEVIER Science Direct. Available online: https://www.sciencedirect.com/browse/journals-and-books (accessed on 23 March 2023).
- PMC National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/pmc/journals/#csvfile (accessed on 23 March 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brochado, M.G.d.S.; Silva, L.B.X.d.; Lima, A.d.C.; Guidi, Y.M.; Mendes, K.F. Herbicides versus Nitrogen Cycle: Assessing the Trade-Offs for Soil Integrity and Crop Yield—An In-Depth Systematic Review. Nitrogen 2023, 4, 296-310. https://doi.org/10.3390/nitrogen4030022
Brochado MGdS, Silva LBXd, Lima AdC, Guidi YM, Mendes KF. Herbicides versus Nitrogen Cycle: Assessing the Trade-Offs for Soil Integrity and Crop Yield—An In-Depth Systematic Review. Nitrogen. 2023; 4(3):296-310. https://doi.org/10.3390/nitrogen4030022
Chicago/Turabian StyleBrochado, Maura Gabriela da Silva, Laryssa Barbosa Xavier da Silva, Alessandro da Costa Lima, Yure Marin Guidi, and Kassio Ferreira Mendes. 2023. "Herbicides versus Nitrogen Cycle: Assessing the Trade-Offs for Soil Integrity and Crop Yield—An In-Depth Systematic Review" Nitrogen 4, no. 3: 296-310. https://doi.org/10.3390/nitrogen4030022
APA StyleBrochado, M. G. d. S., Silva, L. B. X. d., Lima, A. d. C., Guidi, Y. M., & Mendes, K. F. (2023). Herbicides versus Nitrogen Cycle: Assessing the Trade-Offs for Soil Integrity and Crop Yield—An In-Depth Systematic Review. Nitrogen, 4(3), 296-310. https://doi.org/10.3390/nitrogen4030022