Abstract
Although NH4+ fertilization is known to acidify rhizosphere and enhance nutrient uptake, the effects on a nutrient-sufficient acidic soil amended with lime are not demonstrated. Thus, the influence of NH4+ fertilization of an unlimed and limed (3 g calcium carbonate per kg soil) acidic soil on the nutrient uptake and growth of maize was studied in comparison to NH4NO3 fertilization. The pH of limed rhizosphere soil was about two units higher than that of the unlimed soil. The maize plants were grown in pots under greenhouse conditions for about two months. The results showed that the pH of the NH4+-fertilized unlimed and limed soil was 0.54 and 0.15 units lower than the NH4NO3-fertilized soil. Liming negatively affected shoot and root dry matter production, whereas the NH4+-fertilized plants produced higher dry matter than the NH4NO3-fertilized plants, with significant difference of 28% in the limed soil only. Liming decreased Fe concentration in rhizosphere soil from 99 to 69 mg kg−1 and decreased plant-available Mn the most (71%), whereas the NH4+-fertilized unlimed and limed soil had 48% and 21% higher Mn concentration than the respective NH4NO3-fertilized soils. Similarly limed rhizosphere soil had 50% lower plant-available Zn concentration than the unlimed soil, and the NH4+-fertilized soil had an 8% higher Zn concentration than the NH4NO3-fertilized unlimed soil. The liming negatively affected P, K, Mn, and Zn concentrations and contents in maize shoot to a lower degree in the NH4+-fertilized soil, whereas the positive effect of NH4+ on the nutrient concentration and contents was vigorous in the unlimed soil than the limed soil. It is concluded that NH4+ fertilization could be beneficial in enhancing nutrient uptake and growth of maize in both acidic and alkaline soils, despite the higher inherent plant-available concentrations of the nutrient in soil.
1. Introduction
Zinc (Zn) and manganese (Mn) are micronutrients that are inadequately available to plants worldwide [1], and Zn deficiency is the most often in plants of all the micronutrients [2]. In plants, Zn and Mn are transported predominantly as a divalent ion (Zn2+, Mn2+, respectively) or in organically bound [3]. Unlike Fe and Cu, Zn is not involved in redox reactions in plants [4]; instead, it plays a primary role in connection with various enzymes, for example, RNA polymerase, which is crucially involved in protein synthesis, contains two Zn atoms [5], and carbonic anhydrase, which contains a Zn atom, is required in the carbon cycle of C4 plants to convert dissolved CO2 to HCO3− in the mesophyll cells, and allows diffusion of CO2 to the RUBISCO in C3 plants [4,6]. Zn is directly involved in transcription because it is responsible for the finger-like structuring of polypeptide chains of some proteins. These “Zn fingers” enable the binding of DNA or RNA to proteins [7]. In addition, Zn is central to the structure of ribosomes and also suppresses RNAse [4]. The Zn-containing CuZnSOD protects plant cells from oxidative stress, which is common under abiotic stresses. The resulting H2O2 is subsequently degraded to H2O by catalases, the function being dependent on an adequate Zn supply [4].
The transport of Zn and Mn in the soil to the plant roots is mainly by diffusion from the rhizosphere [8], and their uptake, predominantly as divalent cations (Mn2+ and Zn2+) in acidic environments and MnOH+/ZnOH+ under alkaline conditions [4], occurs via ion channels. In addition, the uptake of their sulfate salts and chelates is also possible [9]. The availability and dynamics of micronutrients in soil depend on several factors such as the total contents and characteristics of soils. The total amount of Zn or Mn in soils is generally composed of five fractions; however, only exchangeable and dissolved fractions are directly available for plant uptake. The exchangeable fraction is reversibly adsorbed to organic or oxidic soil particles and is in exchange with the dissolved fraction [10].
A decisive factor for the solubility and thus the availability of Zn and Mn to plants is the soil pH: the slightly acidic to acidic range is optimal for the availability and uptake of most micronutrients [11]. Soil pH not only directly affects the form of the metals but also the CEC of the soil. Thus, Zn and Mn availabilities are also indirectly affected by pH: high pH values cause lower occupancies of the exchangers, which increase the adsorption (reversible) of these metallic ions onto soil particles [12]. The fixation of the metals to clay minerals and carbonates is also higher with an increase in pH [13]. Carbonate-rich or alkaline soils, such as those used for agriculture in arid regions of the world, are, therefore, particularly affected by the deficiency of these nutrients [1].
Soil pH, in turn, is governed by various factors. During pedogenesis, from carbonate-bearing rocks receiving little precipitation, neutral to alkaline soils with high CEC values tend to form. Silicate-rich parent rocks receiving high precipitation, on the other hand, create acidic soils [12]. Plants also influence the pH of the soil near the roots. For example, depending on plant species, nutrient supply, and other factors, the difference in pH between the rhizosphere and the soil independent of plants can be as much as two pH units [13].
In addition to these natural factors, agronomic practices affect soil pH: the liming of agricultural acidic soils increases soil pH, whereas the application of acidic mineral fertilizers containing SO42− can lead to a lowering of the soil pH [3]. The application of nitrogenous (N) fertilizers, depending upon whether the N form contained in the fertilizer is ammonium (NH4+) or nitrate (NO3−), has also been reported to have physiological and/or chemical effects on soil pH, and, in turn, on the availability of Mn [14]. During NH4+ uptake, plant roots release protons (H+) to maintain a constant intracellular charge balance. This process leads to a decrease in rhizospheric pH [15], which is also called physiological acidification. Fertilization with NH4+ also has a chemical acidifying effect beyond the rhizosphere through the process of nitrification, which is independent of plant roots [16]. Protons are released in this process, which acidify the soil [17].
Nitrate uptake has the effect opposite to NH4+ nutrition, whereby plants absorb H+ by co-transportation with nitrate across the plasma membrane of root cells, resulting in higher pH in the root apoplast and rhizosphere [18]. In addition, when NO3− is taken up, OH− is released, which also raises the soil pH in the rhizosphere [14]. The application of ammonium nitrate (NH4NO3) has a “mixed” effect on pH; however, NH4+ is preferentially taken up in the rhizosphere at equal concentrations of the two N-forms [19], leading to a decrease in pH, despite NO3− supply. Although NH4+ fertilization is known to acidify rhizosphere and enhance nutrient uptake, these effects on a nutrient-sufficient acidic soil amended with lime have not been demonstrated. To investigate the interactions between the form of applied N fertilizer and a liming treatment of neutral soil, a pot experiment was conducted on maize (Zea mays L.). It was hypothesized that NH4+ uptake would lead to a lowering of soil pH in both limed and unlimed soil, which, in turn, would increase the solubility, availability, and uptake of micronutrients such as Mn and Zn. Furthermore, as limed soil would have stronger pH buffering, the effect of NH4+ fertilization on the uptake of Mn or Zn would be weaker in the limed soil than in unlimed soil.
2. Materials and Methods
2.1. Collection, Preparation and Characterization of Soil
The experiment was conducted in the greenhouse of the Institute of Plant Nutrition and Soil Science at Kiel University (54.35° N, 10.11° E), Germany under semi-controlled conditions. The mean air temperature in the greenhouse during the experimental period was 21 °C, and humidity was adjusted between 50–60% with an automatic humidifier. When the sunlight was intense, sunshades were automatically lowered. The lighting duration was 13 h during the day (7–20 h). The experimental soil was collected from an arable field in Ostholstein, Schleswig-Holstein, Germany. The soil was first air dried and then sieved to remove stones and plant residues. The soil was analyzed for pH and plant-available nutrients using the procedure described in Section 2.4. As plant-available Zn in the original soil was 6.7 mg kg−1 (Table 1), which corresponded to a sufficient level for plant growth, no additional Zn was fertilized during the entire course of the experiment. The soil, approximately 120 kg, was fertilized using a soil mixer with the following fertilizers as a basal dose (mg per kg of soil): 348 KH2PO4, 743 K2SO4, 306 CaCl2∙2H2O, 563 MgSO4∙7H2O, and 19.8 Fe-EDTA, 6.5 CuSO4∙5H2O, 15 MnSO4∙H2O, 9.5 H3BO3, and 0.67 (NH4)6Mo7O24∙4 H2O.

Table 1.
Salient characteristics of the experimental soils.
2.2. Treatment Application and Sowing of Maize
Half of the nutrient-added soil was kept unlimed (control), whereas the other half was limed. For this purpose, CaCO3 was added at the rate of 3 g kg−1 soil and mixed well. Adding this amount of CaCO3 raised the soil pH to about 7, which was originally 5.6. After liming and application of the basic nutrients, the unlimed soil (C) and limed soil (CC) was filled in pots (L 20 cm, diameter 20 cm) at the rate of 6 kg soil pot−1 (4.71 dm3) to achieve a bulk density of 1.27 kg dm−3. Four maize kernels (cv KWS Keops, KWS SAAT SE & Co. KGaA, Einbeck, Germany) were sown in each pot to a depth of about 2.5 cm. Keops is an early-maturing silage maize variety (S210) and is suitable for biogas production and cattle feeding. After sowing, both the soils were fertilized with 1g N kg−1 soil using aqueous solutions of either ammonium nitrate or ammonium sulfate fertilizers. This constituted, overall, four treatments (two N source under each of the soil), each having four independent pot replicates. Each pot was applied with 400 mL of respective N fertilizer solution, which moistened the soil to 60% of water holding capacity (WHC).
The soil was kept continuously moist to approximately 60–70% WHC using deionized water. Seven to ten days after sowing, all maize plants had emerged. Only two plants were retained in each pot for final growth. Two further fertilizations of phosphorus (P, 40 mg kg−1 soil) and potassium (K, 50 mg K kg−1) were carried out 35 and 50 days after sowing. Each re-fertilization corresponded to about half the rate of basal fertilization. Similarly, fertilization of Ca, Mg, S, and micronutrients, each equal to the basal fertilizer, was also carried out 50 days after sowing.
2.3. Harvesting, Sample Collection and Preparation
Maize plants were harvested 62 days after sowing. The shoot was cut off near the ground with a scalpel, coarsely chopped, placed in paper bags, and dried in an oven at 65 °C to a constant weight. The post-harvest soil was removed from the pots, and roots were separated as best as possible. The roots were washed and dried in an oven. The dry matters of roots and shoots were recorded on a weighing balance. The dried shoot samples were ground using a mill (Retsch SM300, Retsch GmbH, Haan, Germany) adjusted to 1200 rpm and fitted with 0.5 mm sieve. The root samples were cut into small pieces with scissors and then ground in a fine mill (Foss Cyclotec 1093, Foss GmbH, Hamburg, Germany). The ground root and shoot samples were further ground to fine powder in a vibrating mill (Retsch MM400, Retsch GmbH, Haan, Germany). The root to shoot ratio (WSV) was calculated.
After removing the roots from the pots, the adhering soil was separated from them by gently tapping against a hard surface. The collected soil samples were dried in air, sieved (0.63 mm), and stored in plastic bags for further analysis.
2.4. Chemical Analysis of the Soil
The pH of the original soil samples of both unlimed and limed soils were measured. For this purpose, 20 mL of deionized water was added to a 20 g soil sample in a 50 mL Falcon tube and shaken using an orbital motion shaker (Gerhardt RO5, C. Gerhardt & GmbH, Königswinter, Germany). The suspensions were centrifuged for 7 min at 2000 rpm (Heraeus Multifuge X3R, Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, the pH of the solution was measured using a pH meter (WTW inoLab pH Level 1, Xylem Analytics GmbH, Ingolstadt, Germany).
The concentration of plant-available P in the soil was measured following the method of Olsen et al. [20]. In brief, 2.5 g of each soil sample was extracted with 50 mL of 0.5 M NaHCO3 solution (pH 8.5, adjusted with NaOH) for 30 min at 150 rpm for 30 min. The extracts were filtered (Whatman 5 filter paper), and P concentrations in them were measured using an autoanalyzer (SAN++ continuous flow analyzer, Skalar, Netherlands) pre-calibrated with a series of P standards (1–5 mg P L−1).
The concentrations of micronutrients viz. Zn, Mn, Cu, and Fe in the original soil and the rhizosphere soil samples were determined according to Lindsay and Norvell [21]. In brief, 15 g of each soil sample was extracted with 30 mL of 0.005 M DTPA solution (made in 1.0 M CaCl2 and adjusted to pH 7.3 with 1:1 HCl) for 2 h using the above said orbital shaker at 120 rpm min−1. The suspensions were centrifuged for 10 min at 2000 rpm and filtered using Whatman 42 filter paper. Subsequently, micronutrient concentrations were measured by atomic absorption spectrometer (SOLAAR S Series AA, Thermo Fisher Scientific, Waltham, MA, USA) (Table 1).
2.5. Plant Analysis
To determine the nutrient concentrations (P, K, Mg, Ca, Fe, Cu, Mn, and Zn) in the plant tissues, the samples were digested in nitric acid (HNO3). Approximately 200 mg of each plant sample was weighed in the digestion tube, and 10 mL of HNO3 (69% Suprapur®) was added. The tubes were then placed in the microwave (MARS 6 Xpress, CEM Corporation, Matthews, NC, USA), where digestion was performed (“Classic” program: 20 min at 190 °C). The digests were cooled to room temperature and diluted to 100 mL with ultrapure water. The obtained aliquots were further diluted 20 times with ultrapure water, and measurements of nutrient concentrations were performed by an inductively coupled plasma mass spectrometer (ICP-MS; Agilent 7700, Agilent Technologies Inc., Santa Clara, CA, USA). The nutrient contents in roots and shoots were calculated by multiplying the concentrations (mg g−1) with dry matters (g pot−1).
2.6. Statistical Analysis
The experimental pots were arranged in a completely randomized design. The analysis of variance (ANOVA) was performed following a two-factor factorial design, whereas the significant differences between the individual treatments were determined by Tukey’s Honestly Significant Difference test (p ≥ 0.5). For this purpose, the programming language “R” (R 4.1.1) was used with the user interface “RStudio” (2021.09.0, © 2009–2021 RStudio, PBC) and the function “gls” therein.
3. Result
3.1. Plant Growth and Dry Matter Production
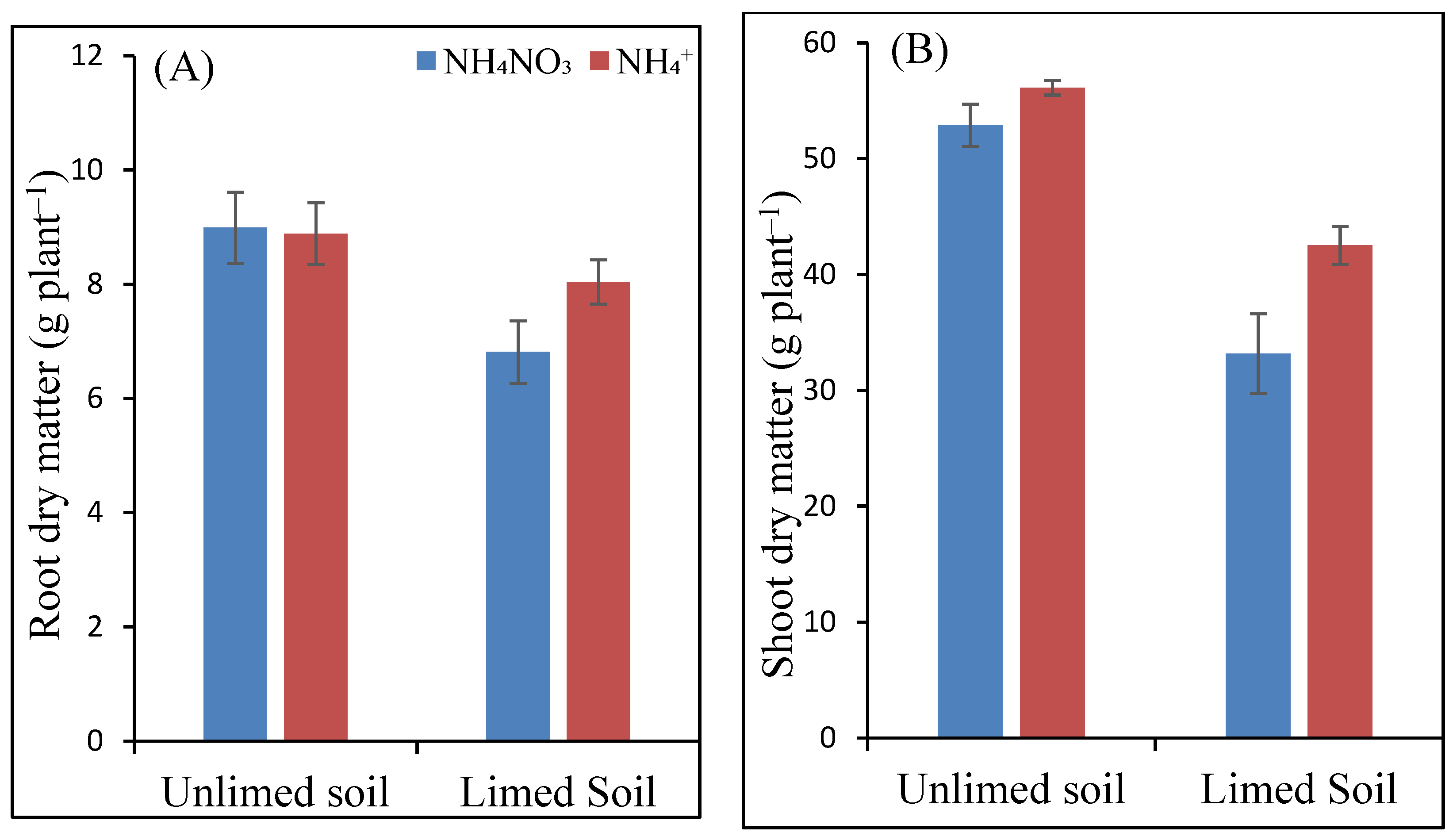
Until harvest, no nutrient deficiency symptoms could be observed on the maize leaves. At the same time, treatment-related differences in growth were visually apparent four weeks before harvest. Thus, the plants grown in the unlimed soil were larger and more vigorous than those grown in the limed soil. Accordingly, root dry matters (DM) of maize plants were significantly affected by liming: liming resulted in lower root DM under both the N forms than the control treatment (Figure 1). The difference was higher in the AN-fertilized plants, with an average of 9 g DM without liming compared to just 7 g DM in the limed soil. However, the differences between the N forms and their interactions with soil liming were not significant.
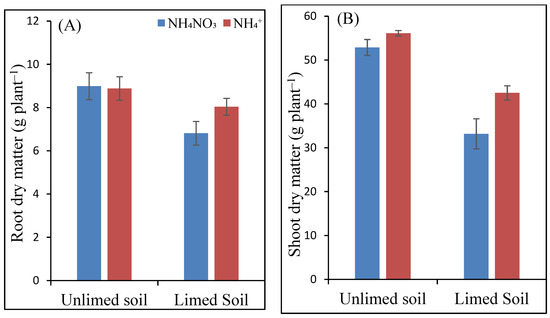
Figure 1.
Dry matter (g plant−1) of maize roots (A) and shoots (B) as a function of soil liming and N fertilizers. Different letters indicate significantly different values in each case (p < 0.05).
The effect of liming and applied N forms on shoot DM was significant, as well as the interaction between the two factors. Under both the N forms, shoot dry matter in unlimed soil was higher than the limed soil (Figure 1). The difference between unlimed and limed soils was higher for the NH4NO3-fertilized plants. Although NH4+ form yielded a higher shoot DM than the NH4NO3 formed in both unlimed and limed soil, the effect was only significant in the limed soil, with NH4+ producing a 28% higher SDM yield.
3.2. pH of the Rhizosphere Soil
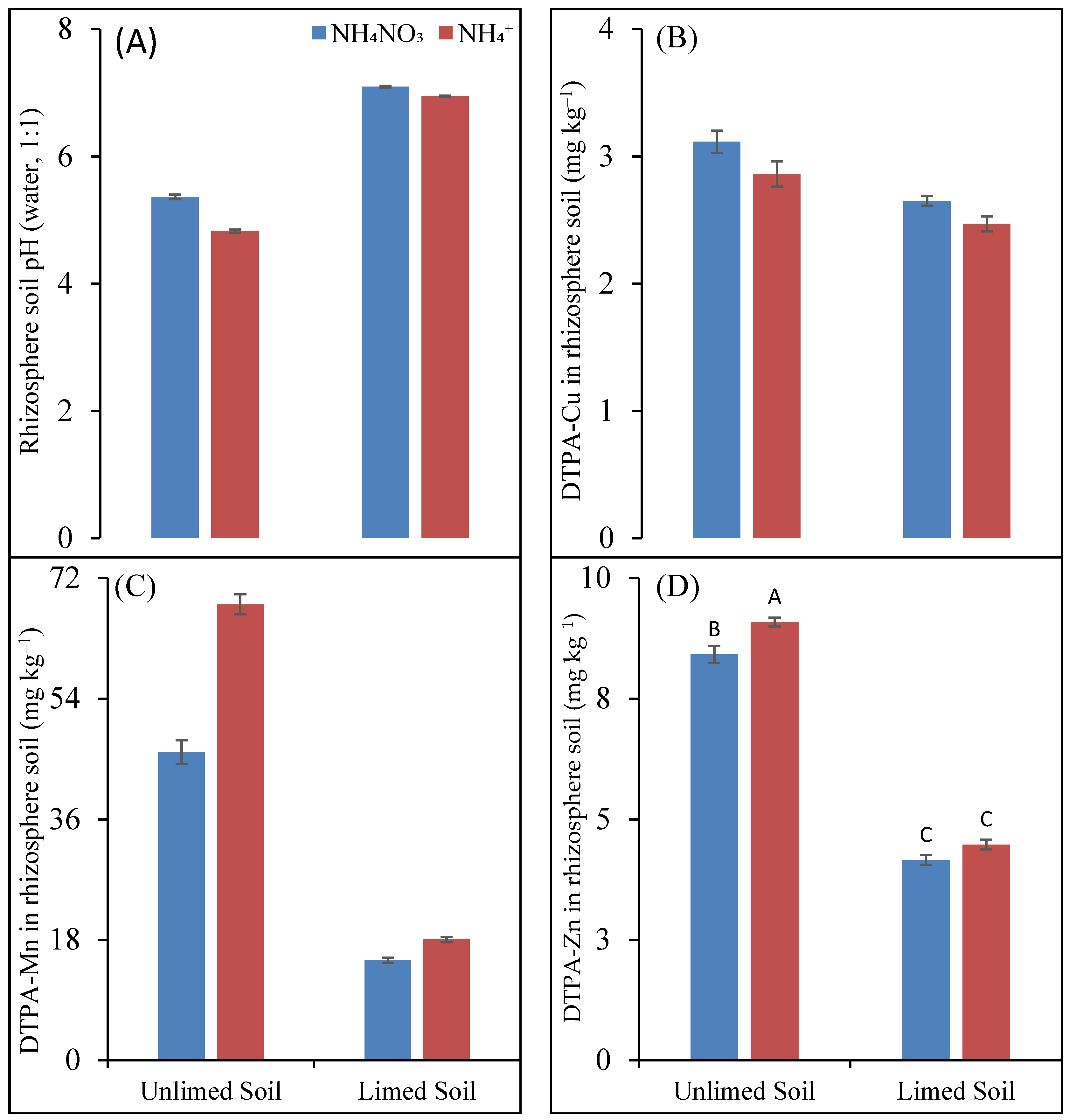
The pH of limed rhizosphere soil was significantly higher, about two units more, than that of unlimed rhizosphere soil (Figure 2A). There were also significant differences in the pH values of the rhizosphere soils fertilized with NH4NO3 and NH4+ fertilizers. In the unlimed rhizosphere soil, the pH of the NH4+-fertilized soil was 0.54 units less than the NH4NO3-fertilized soil, whereas the corresponding difference in the limed soil was only 0.15 units.
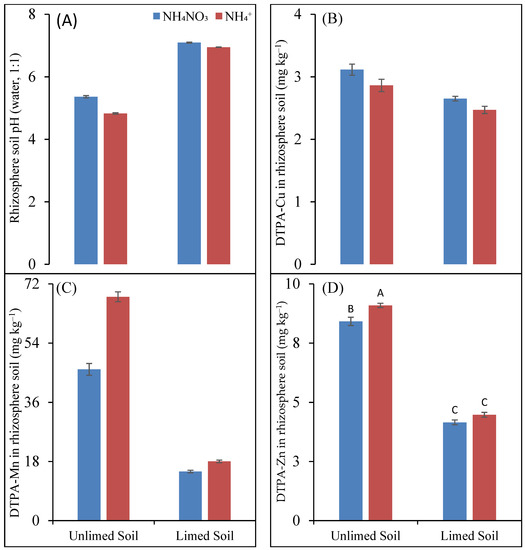
Figure 2.
pH (A) and concentration (mg kg−1) of plant-available (DTPA-extractable) Cu (B), Mn (C), and Zn (D) in maize rhizosphere soil as a function of soil liming treatment and N fertilizers. Different letters denote significantly different values (p < 0.05).
3.3. Plant-Available Nutrients in Rhizosphere Soil
No significant differences could be found between the different treatments for Olsen P concentrations in the rhizosphere soil.
The concentration of plant-available (DTPA-extractable) Cu in the rhizosphere soil was affected both by liming treatments and N forms. The liming treatments decreased plant-available Cu concentration in the rhizosphere soil by about 14%, and the effect of liming was independent of N forms (Figure 2B). Interestingly, the Cu concentrations in the NH4+-fertilized unlimed and limed rhizosphere soils were 8% and 6.4% lesser than the NH4NO3-fertilized soil.
On average, liming decreased plant-available Fe concentration in rhizosphere soil from 99 to 69 mg kg−1, corresponding to a decrease of 30%. However, plant-available Fe concentrations did not differ between NH4NO3 and NH4+ forms, with mean values of 85 and 84 mg kg−1 soil.
Among the studied micronutrients, the plant-available Mn concentrations in the rhizosphere soil had the strongest effects on the liming treatment and N forms (Figure 2C). Liming decreased the plant-available Mn concentrations in the rhizosphere soil by 68% and 74%, respectively, in the NH4NO3- and NH4+-fertilized soil. On the other hand, the plant-available Mn concentrations in the NH4+-fertilized unlimed and limed soils were 48% and 21% higher than the NH4NO3-fertilized soils, respectively. In the case of the limed soil, the difference in the plant-available Mn concentration between NH4NO3 and NH4+ was non-significant.
The effect of liming treatments on the Zn concentration in the rhizosphere soil was independent of N forms, but that of N forms was only significant in the unlimed soil (Figure 2D). On average, the limed rhizosphere soil had a 50% lower plant-available Zn concentration than the unlimed soil, and the NH4+-fertilized soil had an 8% higher Zn concentration than the NH4NO3-fertilized unlimed soil.
3.4. Macronutrients Concentration and Content in Maize Shoots
Shoot P concentrations were determined by liming, N-forms, and the interactions between these factors. Compared to control, soil liming reduced P concentration in shoots by more than half (55%) in the NH4NO3-fertilized soil and only 18% in the NH4+-fertilized soil (Table 2). Conversely, plants fertilized with NH4+ had 27% and 130% higher shoot P concentrations than those fertilized with NH4NO3 in the unlimed and limed soil, respectively. Similar to P concentrations, P contents in maize shoots were negatively affected by liming to a higher degree in the NH4NO3-fertilized soil (72%) than in the NH4+-fertilized soil (38%), and NH4+ had a lower enhancing effect on the maize shoot P contents in the limed soil (35%) than the unlimed soil (198%) (Table 3).

Table 2.
Macronutrient concentrations (mg g−1) in maize shoots as a function of soil liming (C, unlimed; CC, limed) and N forms (AN, ammonium nitrate; A, ammonium).

Table 3.
Macronutrient contents (mg plant−1) in maize shoots as a function of soil liming (C, unlimed; CC, limed) and N forms (AN, ammonium nitrate; A, ammonium).
Soil liming negatively affected shoot K concentration under both the N source and decreased it by 32% and 10% in the NH4NO3- and NH4+-fertilized soil, respectively (Table 2). This was reflected in significantly lower shoot K contents in the limed soil, being 55% and 32% lower in the NH4NO3 and NH4+-fertilized plants, respectively (Table 3). The shoot K concentrations of the NH4+-fertilized plants were 9% and 45% higher than the NH4NO3-fertilized plants in the unlimed and limed soil, respectively (Table 2). Accordingly, the shoot K contents of the NH4+-fertilized plants were 26% and 89% higher in the limed and unlimed soil.
Shoot Ca concentration was improved by liming; however, the effect was only significant (+65%) for the NH4NO3-fertilized plants (Table 2). On the other hand, the NH4+-fertilized plants had lower Ca concentrations in shoots, with a significant difference (−27%) only in the limed soil. The interaction of liming and N formation on shoot Ca concentration was converse to that found for Ca content (Table 3). The calcium content of the maize shoot was increased (14%) by liming only in NH4+-fertilized plants, whereas NH4+ had a lower Ca content (15%) only in the unlimed soil.
Liming did not affect Mg concentration in shoots, whereas the NH4+-fertilized plants had 15% and 25% lower Mg concentrations in shoots than in the NH4NO3-fertilized plants in the unlimed and limed soil, respectively (Table 2). On the other hand, liming decreased shoot Mg content by 36% and 30% in the NH4NO3-fertilized and NH4+-fertilized plants, respectively (Table 3). NH4+ negatively affected shoot Mg content (7%) only in the unlimed soil.
3.5. Micronutrients Concentration and Content in Maize Shoots
Liming had an enhancing effect on shoot Cu concentration, but the magnitude of effect was non-significant (Table 4). On the other hand, mean shoot Cu contents decreased by liming from 133 to 81 µg plant−1 (Table 5). NH4+ decreased shoot Cu concentration only in the limed soil (44%), whereas it decreased shoot Cu content in both the unlimed and limed soil (Table 5).

Table 4.
Micronutrient concentrations (µg g−1) in maize shoots as a function of soil liming (C, unlimed; CC, limed) and N forms (AN, ammonium nitrate; A, ammonium).

Table 5.
Micronutrient contents (µg plant−1) in maize shoots as a function of soil liming (C, unlimed; CC, limed) and N forms (AN, ammonium nitrate; A, ammonium).
Liming decreased shoot Fe concentration by 7.4% and shoot Fe content by 35%, whereas the effect of N forms and their interactions with liming on these attributes was non-significant (Table 4 and Table 5).
Among the studied micronutrients, the strongest positive effect of NH4+, as compared to NH4NO3, was recorded on Mn concentration and content (Table 4 and Table 5). The NH4+-fertilized plants had double shoot Mn concentrations and contents compared to the NH4NO3-fertilized plants in the unlimed soil (Table 4 and Table 5). In the limed soil, shoot Mn concentration in the NH4+-fertilized plants was also double of what was recorded for the NH4NO3-fertilized plants, whereas the corresponding difference for shoot Mn content was about three-fold (Table 4 and Table 5).
In the unlimed soil, the NH4+-fertilized plants had significantly higher (23%) shoot Zn concentrations than the NH4NO3-fertilized plants, whereas the two N forms did not differ for shoot Zn concentrations in the limed soil (Table 4). Similarly, the NH4+-fertilized plants had significantly higher Zn contents (31%) than the NH4NO3-fertilized plants only in the unlimed soil (Table 5). The liming almost halved the shoot Zn content of what was recorded without liming.
4. Discussion
The purpose of this experiment was to investigate the influence of NH4NO3 and NH4+ forms on the uptake of various nutrients, especially the micronutrients Mn and Zn, and maize growth, with particular reference to liming of an acidic soil. Chemical, biological, and physical conditions in the rhizosphere can differ greatly from the rest of the soil due to the exudation of organic compounds, ions, or water through the roots. The pH of the rhizosphere soil is among the most significantly affected soil properties. Different plant species alter pH differently, and the initial soil pH also determines whether the rhizosphere pH is raised or lowered by root influence.
The application of lime (CaCO3) by itself has the opposite effects to that of soil NH4+ fertilization. In this experiment, liming was carried out to raise the soil pH to simulate the widespread alkaline Zn deficient soils, which are common in intensive arable farming system of semiarid and arid regions. After the application of lime, the protons present in the soil are buffered until saturation, i.e., until all lime is used up [12,22]. The resultant increase in pH either has a positive or negative effect on plant growth, depending upon the initial soil pH and the plant species. An excessive increase in pH can have negative effects on plant growth. In particular, P dynamics along with the availability and uptake of micronutrients have an optimal soil pH range that depends on the plant species.
4.1. Plant Growth and Dry Matter Production
In general, mixed fertilization with NH4NO3 is considered optimal for many crop species such as wheat, as this allows the benefits of both N forms to be exploited under practical conditions [23]. Pedersen et al. [24] showed that a mixed application of NH4+ and NO3− produced the highest DM yields, whereas pure NH4+ fertilization resulted in the reduced growth of young maize. However, at neutral to slightly acid soil pH values, plant growth was improved by NH4+ compared to NH4NO3 [25]. This was consistent with the observations from this study (Figure 1). It is possible that some of the NH4+ here was nitrified during the experimental period, so that the plants ultimately took up NO3−, and the NH4+ concentration remained below the phytotoxic level. In the present study, most of the observed pH effects of the NH4+ fertilization could have been due to nitrification-induced acidification. However, acidification in the rhizosphere soil is further stimulated by the ammonium uptake of maize.
4.2. The Uptake of NH4+ Leads to a Lowering of the Soil pH in Limed and Unlimed Soil
The expected lowering of pH by NH4+ compared to NH4NO3 fertilization was observed in this study (Figure 2A). The mechanism of the uptake of NH4+ by plants having an acidifying effect on rhizosphere soil has already been shown by other researchers. For example, Marschner and Römheld [15] observed a strong acidification of the rhizosphere for various plant species when NH4+ was in the form of N applied. Acidification of rhizosphere soil by NH4+ fertilization is associated with both the release of H+ by plant roots, due to the stimulation of H+-ATPase, to counterbalance the charge resulting from the NH4+ absorption by the roots and the nitrification of the NH4+ itself [16,26]. However, the magnitude of the effect is very high in the former case, which is further enhanced by the addition of nitrification inhibitors [16]. Consistent with this, Taylor and Bloom [27] observed a significant decrease in pH on the rhizosphere of maize plants when NH4+ was the only N source.
The lowering of the pH due to the NH4+ fertilization was recorded in both the limed and unlimed rhizosphere soils; however, the unlimed rhizosphere soil experienced a higher degree of decline (0.54 units) than the limed soil (0.15 units) (Figure 2A). Only a slight decline in the pH of the limed rhizosphere soil was explained by the buffering effect of the lime, which greatly attenuated the NH4+-induced acidification by neutralizing the H+ [12]. However, it is worth considering that the effect of NH4+ fertilization was significant despite the pH buffering; thus, the rhizosphere pH of the NH4+-fertilized soil was lower than the NH4NO3-fertilized soil.
The combined fertilization of the NH4+ and NO3− as NH4NO3 resulted in only a slight lowering of the rhizosphere soil pH compared to the pH of the original soil. The small effect of the NH4NO3 fertilization on the pH was explained by the offsetting of the acidifying effect of the ammonium by the H+/NO3– cotransport that alkalized the rhizosphere thereafter [23]. Even a low concentration of NO3− could partially offset the negative effects of the NH4+ fertilization [28]. However, due to the lower energy required for uptake and assimilation, plants preferentially took up NH4+ when both N forms were present in equal concentrations in the rhizosphere [23]. This explained the slightly lower rhizosphere pH of the NH4NO3-fertilized soil compared to original soil. When maize was fertilized only with NH4+, it also led to a decrease in rhizospheric soil pH in direct proportion with the quantity of NH4+ uptake [24].
4.3. Influence of Liming and N-Form on Soil and Plant Macronutrients
Although soil phosphorus concentration was neither affected by N forms, nor was the difference between liming treatments significant, a significant difference in shoot P concentration was recorded within liming treatments as well as the N forms (Table 2). This discrepancy may have been due to the test procedure used for analyzing soil P: extraction with NaHCO3 according to Olsen et al. [20] mainly detected the potentially available organic P compounds as well as the P bound to the Fe-Al oxides [10]. These the main fixed forms at the soil pH values < 6.5 [29]. However, the P fixed to Ca tends to be underestimated by the test method used, which may have been why inaccuracies occurred in the pH range tested here. Furthermore, a reliable statement about the P availability for the plants could only be derived in the case of poor P supply level, which, however, was not present here: the soil used was sufficient in plant-available P at the start of the experiment [10] (Table 1).
Thus, although soil P concentration was independent of liming, there was a negative, significant effect of liming on the plant P concentration and content (Table 2). In addition, the plants with NH4+ fertilization had significantly higher P contents in shoots than the NH4NO3-fertilized plants. In the shoots, there were interactions between the N-forms and liming levels, in that the effect was enhanced by liming (Table 2). Riley and Barber [30] showed that NH4+ fertilization in soybeans (Glycine max L.) resulted in lower rhizosphere pH levels, which ultimately increased P concentrations in the plants. These observations may be explained by the enhanced solubilization of Ca phosphates by the lowering of pH, which otherwise decreases as a result of liming and the associated higher pH and Ca activity [10,24].
Potassium concentrations and contents in maize shoots were significantly decreased by liming and increased by NH4+ formation (Table 2 and Table 3); however, the decline in soil pH seemed to be the governing factor. At lower pH levels, H+ had stronger competition with the exchangeable K+ for the soil exchange colloid, thereby moving it into the soil solution and vice versa. Consequently, the NH4+-uptake-induced acidification of the rhizosphere soil increased both K+ concentrations and contents in maize shoots, whereas the reverse response was observed with liming. The NH4+-uptake-induced lowering of the pH might have overwhelmed the frequently described competitive negative effect of the NH4+ fertilization on the K+ uptake [19], and the lime-induced alkalization of the soil had acted the same against the enhancing effect of Ca2+ on the K+ uptake.
Liming increased Ca concentration and content in shoots, whereas NH4+ decreased these attributes compared to NH4NO3 (Table 2 and Table 3). The increase in shoot Ca concentrations in the limed soil was well-expected, whereas the negative effect of the NH4+ on the Ca2+ uptake was very well known [31]. According to Weil et al. [32], NH4+ limits the uptake and accumulation of cations such as Ca2+. Similarly, Na et al. [33] observed lower concentrations of Ca2+ in the shoot with a higher NH4+ application rate.
Mg concentration was higher with NH4NO3 compared to NH4+ fertilizer, which could be justified by the competitive uptake between the cations Mg2+ and NH4+ [10]. According to Senbayram et al. [34], low pH levels can increase the availability of Mg in the soil, and its uptake is limited due to higher H+ concentrations. In our study, shoot Mg content was significantly decreased by liming (Table 3) because elevated concentrations of Ca due to liming may have also had an antagonistic effect on Mg2+ uptake [35].
4.4. NH4+ Uptake-Induced pH Lowering Increases Availability and Uptake of Micronutrients
The solubility, availability, and uptake of all micronutrients did not clearly depend on different N forms. Liming, on the other hand, affected all the studied micronutrients, as expected. The concentration of available Cu in the rhizosphere substrate was negatively affected by liming (Figure 2B). On the other hand, despite a lowering of rhizosphere soil, NH4+ fertilization tended to decrease DTPA-extractable Cu in rhizosphere soil. Thus, the two factors differing in their effect on soil pH had the same effect on available Cu in the soil. This was in line with the findings of Jahiruddin et al. [36], who found no clear influence of soil pH on the DTPA extractability of Cu. On the other hand, however, consistent with this study, according to Fageria et al. [37], Cu2+ dissolved in soil is more strongly adsorbed onto clay minerals and organic matter at higher pH values. They explain this by the hydrolysis of hydrated Cu, which occurs at pH values above six. Thus, while availability was pH dependent, Cu uptake was, at the same time, hardly limited by the different pH-dependent Cu binding forms: many of these binding forms, e.g., organometallic complexes (Cuorg), can be taken up by plants [10]. Therefore, Cu availability is generally assumed to be less pH dependent than is the case for Fe or Mn [37]. Although availability of a nutrient in the soil is critical for plant uptake, the Cu concentrations in shoots showed a different pattern than the available concentrations in the soils: liming did not affect Cu concentrations in shoots, whereas it was higher when fertilization with NH4NO3 was carried out. The lower Cu concentration observed here in the NH4+-fertilized plants could not be explained by a dilution effect since the Cu content between the NH4NO3- and NH4+-fertilized plants also followed the same trend.
Although Fe is present in sufficient quantities in almost all soils, its availability is a problem on alkaline sites worldwide, mainly due to their high pH levels [22]. Accordingly, in this experiment, available Fe concentration in soil was reduced from a mean value of about 100 mg kg−1 soil without liming to 68 mg kg−1 soil with liming. The Fe concentration in the maize shoot was influenced by liming in this trial, such as the concentration in the soil (Table 5). However, this did not manifest itself as so-called lime chloroses, probably due to the reason that the plant-available Fe concentration in the limed soil was still enough to provide the required quantity of Fe to plants. Despite the difference in the rhizosphere pH between the N forms, similar to Cu, no positive effect of the NH4+ fertilization on the concentration of the available Fe in soil and in shoots was observed in this experiment. This contradicted results from other studies, where NH4+ feeding significantly increased Fe uptake and thus Fe concentrations in the shoot [33,38]. This discrepancy could have been explained by the fact that the NH4+-induced change in the pH was not sufficient to solubilize the Fe in the soil, the concentration of which was already sufficient for plant growth.
Among the studied nutrients, the solubility and availability of Mn was most affected by N forms both in the unlimed and limed soil. As expected, soil Mn concentration was higher with NH4+ fertilization than recorded with NH4NO3 fertilization (Table 4 and Table 5). The high pH dependence of Mn availability was also evident in the soil: NH4+ fertilization caused a rhizosphere pH of below five, highly improved the available Mn concentration in the soil (Figure 2C), and almost doubled shoot Mn concentration in both the unlimed and limed soils. The concentration of plant-available Mn2+ was found to increase 100-fold when the soil pH decreased by one unit [10]. The sharp increase in soil Mn2+ concentration in an acidic soil (pH < 5.5) could be explained by the dissolution of MnO2 [39]. These observations were in agreement with that of Sabir et al. [40] for maize. It was noteworthy that NH4+ fertilization brought an additional, positive effect in terms of availability and uptake despite the very good supply of Mn in the soil in this experiment (Table 1). According to Rengel [41], this was due to the strong pH dependence of Mn, which has a stronger effect on the availability of this nutrient than the total content in the soil.
Zinc availability is also dependent on soil pH; therefore, a lower soil Zn concentration in the rhizosphere of the limed soil compared to the unlimed soil and a reverse effect were found to be true in the NH4+- compared to the NH4NO3-fertilized unlimed soil only. Accordingly, the increased availability of Zn in the unlimed soil, in turn, reflected in the improved Zn concentrations and uptakes, leading to a conclusion that plant Zn concentration could also be increased using NH4+ fertilization [16,39,41]. According to Ma et al. [38], for example, underfoot fertilization with NH4+ increased shoot Zn concentration and yield of maize. On the one hand, they explained this with altered root growth. Thus, traits such as root length and density were increased due to NH4+ application. On the other hand, they also observed an acidification of the rhizosphere and indicated this as the cause for the increased Zn concentration in the shoot. Acidification of the rhizosphere was also observed in this experiment. Interestingly, Zn availability was further increased due to pH reduction, even though the soil had an already sufficient concentration of plant-available Zn (Table 1).
5. Conclusions
The results of this study suggested that NH4+-based N fertilizer improved nutrient availability, nutrient uptake, and growth of maize in acidic and limed acidic (alkaline) soils despite sufficient plant-available concentrations of nutrients. The improved nutrient uptake would translate into higher nutrient concentrations in the edible parts of plants, leading to the biofortification of micronutrients, especially zinc and manganese.
Author Contributions
Conceptualization, K.H.M.; methodology, software, P.D.; writing—first draft, A.N. and P.D.; Reviewing and editing, A.N. and K.H.M.; supervision, K.H.M.; funding acquisition, K.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alexander von Humboldt Foundation through grant of George Forster Post-Doctorate Fellowship.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions.
Acknowledgments
Asif Naeem is thankful to the Alexander von Humboldt Foundation for the grant of the George Forster Post-Doctorate Fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sillanpää, M. Micronutrients and the Nutrient Status of Soils: A Global Study; FAO Soils Bulletin No. 48; Food and Agriculture Organization of the United Nations: Rome, Italy, 1982. [Google Scholar]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Alloway, B.J. Fundamental Aspects. In Zinc in Soils and Crop Nutrition, 2nd ed.; Alloway, B.J., Ed.; IZA: Brüssel, Belgien; IFA: Paris, Frankreich, 2008; pp. 14–58. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: London, UK, 2012; pp. 313–369. [Google Scholar]
- Falchuk, K.H.; Ulpino, L.; Mazus, B.; Vallee, B.L. Euglena gracilis RNA polymerase I: A zinc metalloenzyme. Biochem. Biophys. Res. Commun. 1977, 74, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Price, G.D. The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Papworth, M.; Kolasinska, P.; Minczuk, M. Designer zinc-finger proteins and their applications. Gene 2006, 366, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Zinc uptake from Soils. In Zinc in Soils and Plants; Robson, A.D., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1993; Volume 55, pp. 59–77. [Google Scholar]
- Marschner, P.; Rengel, Z. Nutrient Availability in Soils. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: London, UK, 2012; pp. 483–505. [Google Scholar]
- Brümmer, G.W. Böden als Pflanzenstandorte. In Scheffer/Schachtschabel Lehrbuch der Bodenkunde; Blume, H.-P., Brümmer, G.W., Horn, R., Kandeler, E., Kögel-Knabner, I., Kretzschmar, R., Stahr, K., Wilke, B.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 379–448. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, R. Chemische Eigenschaften und Prozesse. In Scheffer/Schachtschabel Lehrbuch der Bodenkunde. 16; Blume, H.-P., Brümmer, G.W., Horn, R., Kandeler, E., Kögel-Knabner, I., Kretzschmar, R., Stahr, K., Wilke, B.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 121–170. [Google Scholar]
- Buekers, J.; Degryse, F.; Maes, A.; Smolders, E. Modelling the effects of ageing on Cd, Zn, Ni and Cu solubility in soils using an assemblage model. Eur. J. Soil Sci. 2008, 59, 1160–1170. [Google Scholar] [CrossRef]
- Dreyer, M.; Wichmann, M.; Rischen, M.; Görlach, B.M.; Ehmke, A.; Pitann, B.; Mühling, K.H. Ammonium-driven nitrification plays a key role in increasing Mn availability in calcareous soils. J. Plant Nutr. Soil Sci. 2020, 183, 389–396. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V. In vivo measurement of root-induced pH changes at the soil-root interface: Effect of plant species and nitrogen source. Z. Für Pflanzenphysiol. 1983, 111, 241–251. [Google Scholar] [CrossRef]
- Thomson, C.J.; Marschner, H.; Römheld, V. Effect of nitrogen fertilizer form on pH of the bulk soil and rhizosphere, and on the growth, phosphorus, and micronutrient uptake of bean. J. Plant Nutr. 1983, 16, 493–506. [Google Scholar] [CrossRef]
- Hofman, G.; Van Cleemput, O. Soil and Plant Nitrogen; International Fertilizer Association: Paris, France, 2004. [Google Scholar]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: London, UK, 2012; pp. 7–47. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular 939; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Shenker, M.; Chen, Y. Increasing iron availability to crops: Fertilizers, organo-fertilizers, and biological approaches. Soil Sci. Plant Nutr. 2005, 51, 1–17. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: London, UK, 2012; pp. 135–189. [Google Scholar]
- Pedersen, I.F.; Sørensen, P.; Rasmussen, J.; Withers, P.J.A.; Rubæk, G.H. Fertilizer ammonium: Nitrate ratios determine phosphorus uptake by young maize plants. J. Plant Nutr. Soil Sci. 2019, 182, 541–551. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, X.Q.; Chen, Y.L.; Zhang, L.Y.; Shen, R.F. Case of a stronger capability of maize seedlings to use ammonium being responsible for the higher 15N recovery efficiency of ammonium compared with nitrate. Plant Soil 2019, 440, 293–309. [Google Scholar] [CrossRef]
- Murányi, A.; Seeling, B.; Ladewig, E.; Jungk, A. Acidification in the rhizosphere of rape seedlings and in bulk soil by nitrification and ammonium uptake. Z. Für Pflanz. Bodenkd. 1994, 157, 61–65. [Google Scholar] [CrossRef]
- Taylor, A.R.; Bloom, A.J. Ammonium, nitrate, and proton fluxes along the maize root. Plant Cell Environ. 1998, 21, 1255–1263. [Google Scholar] [CrossRef]
- Garnica, M.; Houdusse, F.; Zamarreño, A.M.; Garcia-Mina, J.M. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. J. Plant Physiol. 2010, 167, 1264–1272. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Riley, D.; Barber, S.A. Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-induced pH changes at the root-soil interface. Soil Sci. Soc. Am. J. 1971, 35, 301–306. [Google Scholar] [CrossRef]
- Bonomelli, C.; de Freitas, S.T.; Aguilera, C.; Palma, C.; Garay, R.; Dides, M.; Brossard, N.; O’Brien, J.A. Ammonium Excess Leads to Ca Restrictions, Morphological Changes, and Nutritional Imbalances in Tomato Plants, Which Can Be Monitored by the N/Ca Ratio. Agronomy 2021, 11, 1437. [Google Scholar] [CrossRef]
- Weil, S.; Barker, A.V.; Zandvakili, O.R.; Etemadi, F. Plant growth and calcium and potassium accumulation in lettuce under different nitrogen regimes of ammonium and nitrate nutrition. J. Plant Nutr. 2021, 44, 270–281. [Google Scholar] [CrossRef]
- Na, L.; Li, Z.; Xiangxiang, M.; Ara, N.; Jinghua, Y.; Mingfang, Z. Effect of nitrate/ammonium ratios on growth, root morphology and nutrient elements uptake of watermelon (Citrullus Lanatus) seedlings. J. Plant Nutr. 2014, 37, 1859–1872. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Jahiruddin, M.; Chambers, B.J.; Livesey, N.T.; Cresser, M.S. Effect of liming on extractable Zn, Cu, Fe and Mn in selected Scottish soils. Eur. J. Soil Sci. 1986, 37, 603–615. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Ma, Q.; Wang, X.; Li, H.; Li, H.; Cheng, L.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of NH4+-N plus P enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. Field Crops Res. 2014, 164, 107–116. [Google Scholar] [CrossRef]
- Sarkar, A.N.; Wynjones, R.G. Effect of rhizosphere pH on the availability and uptake of Fe, Mn and Zn. Plant Soil 1982, 66, 361–372. [Google Scholar] [CrossRef]
- Sabir, M.; Hanafi, M.M.; Malik, M.T.; Aziz, T.; Rehman, M.Z.; Ahmad, H.R.; Hakeem, K.R.; Shahid, M. Differential effect of nitrogen forms on physiological parameters and micronutrient concentration in maize (Zea mays L.). Aust. J. Crop Sci. 2013, 7, 1835–2693. [Google Scholar]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).