Ecological Risks from Atmospheric Deposition of Nitrogen and Sulphur in Jack Pine forests of Northwestern Canada
Abstract
1. Introduction
2. Materials and Methods
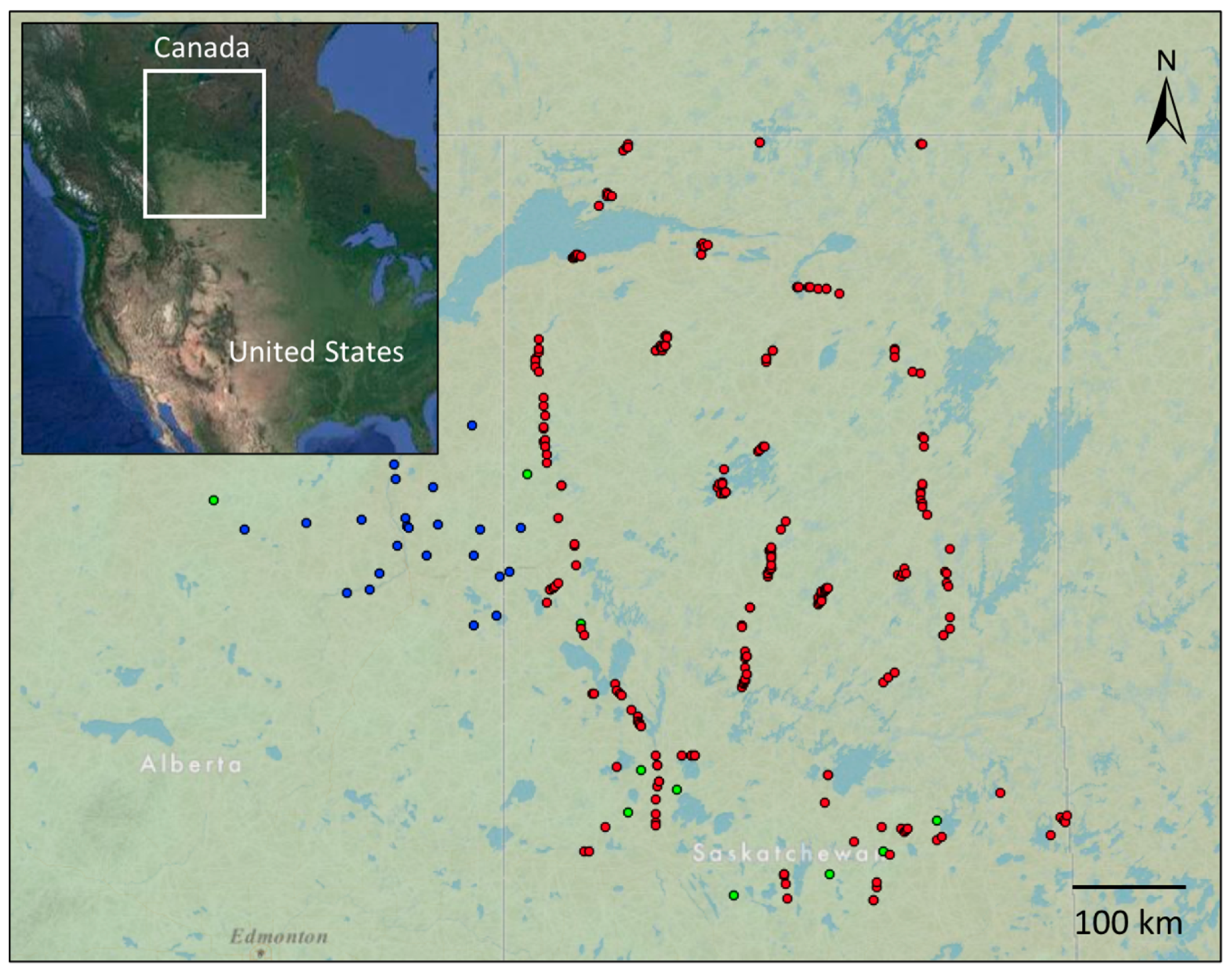
2.1. Study Area
2.2. Data Sources
2.3. Environmental Data
2.4. Statistical Analysis
2.5. GradientForest Output
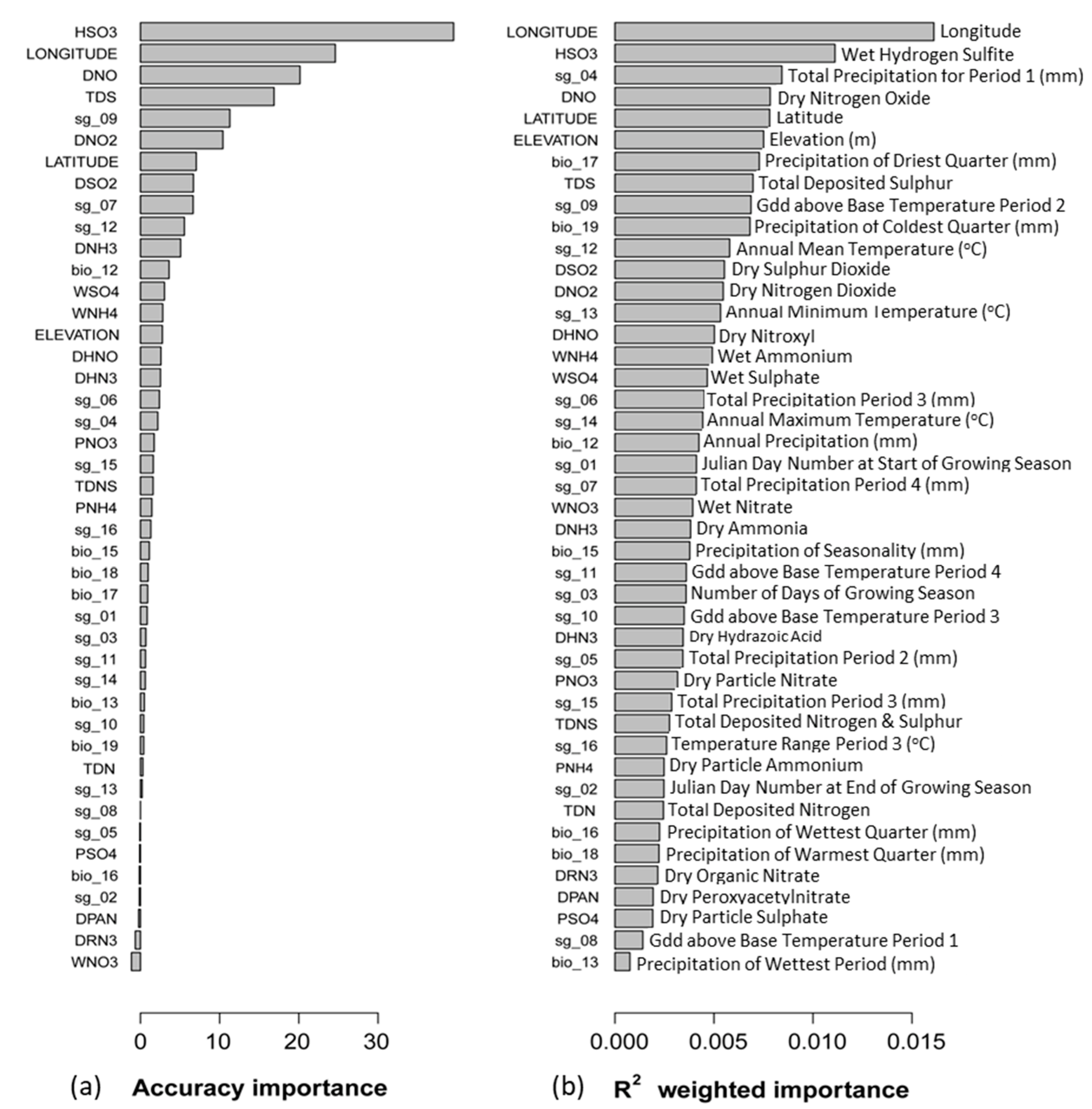
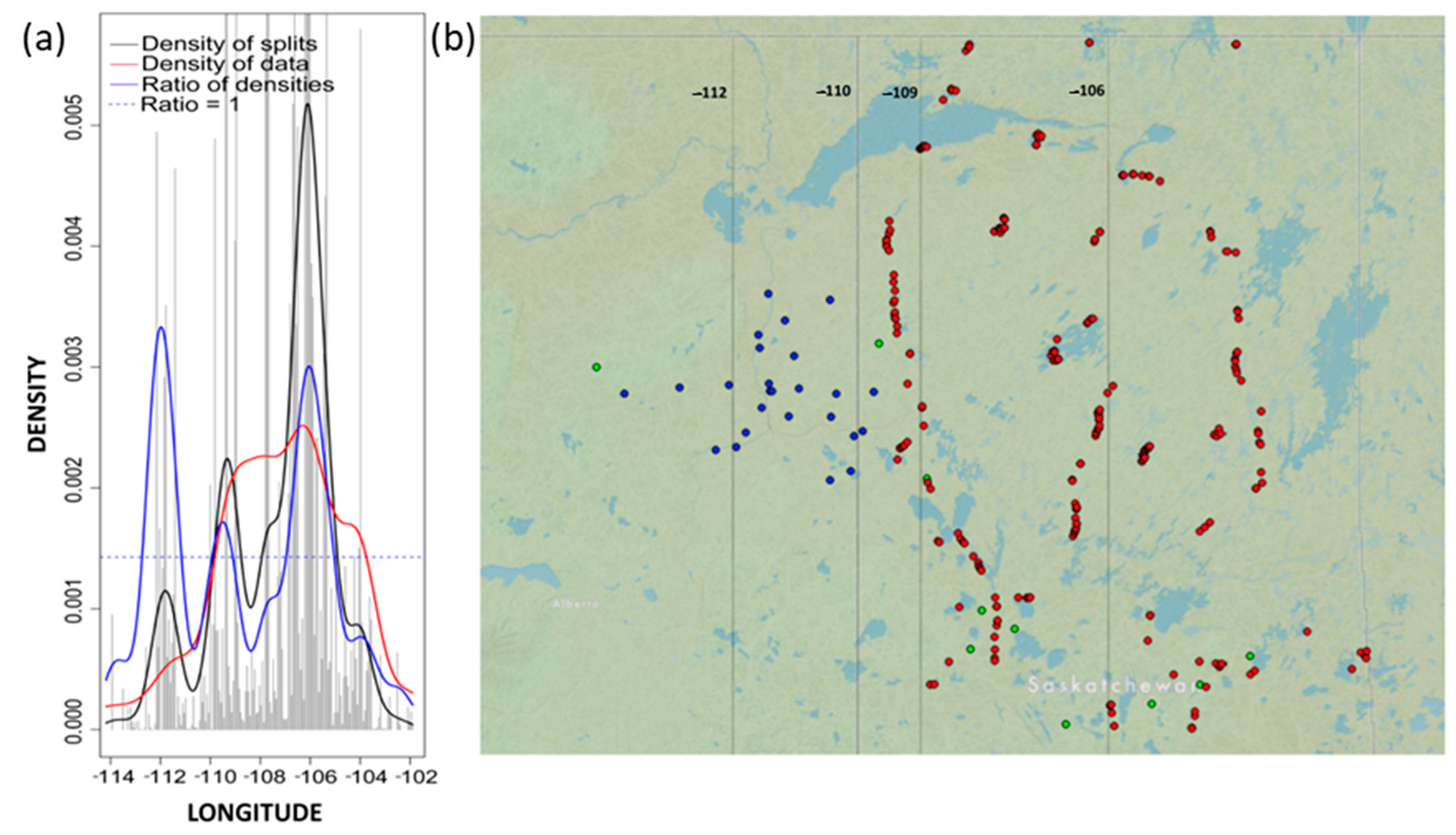
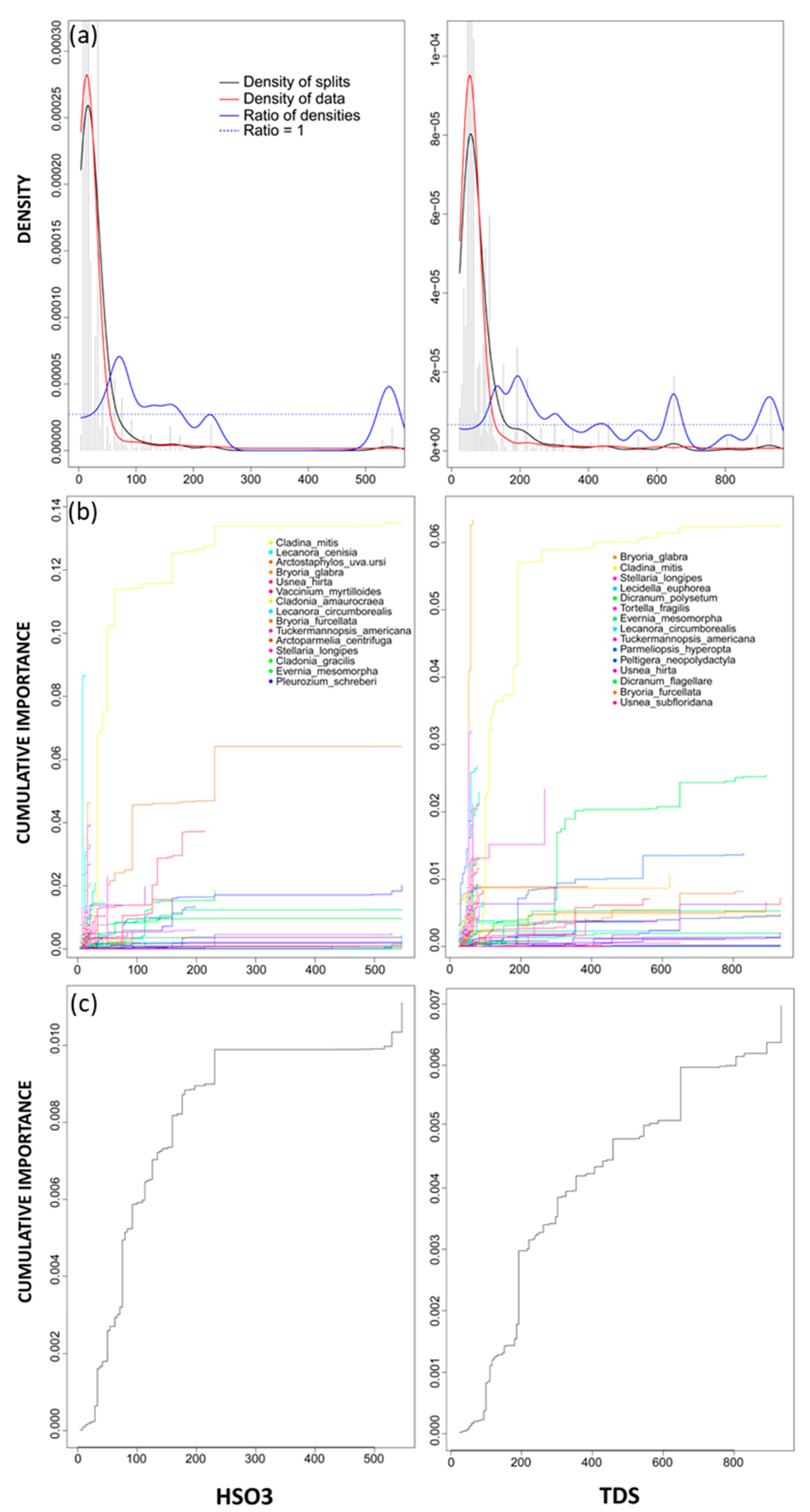
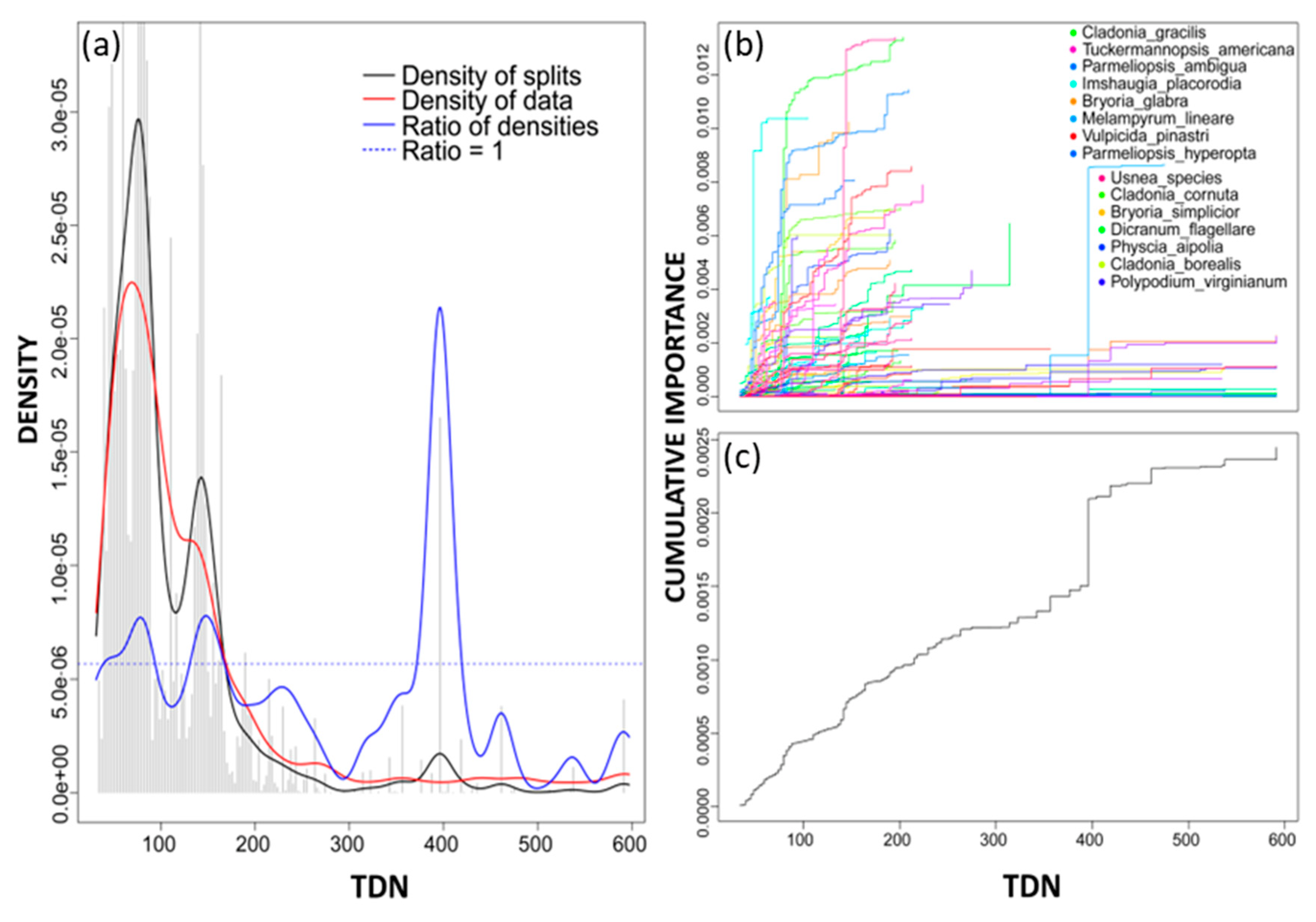
3. Results
4. Discussion
4.1. Drivers of Community Thresholds
4.2. Species & Community Level Response
4.3. Uncertainties & Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Fenn, M.E.; Haeuber, R.; Tonnesen, G.S.; Baron, J.S.; Grossman-Clarke, S.; Hope, D.; Jaffe, D.A.; Copeland, S.; Geiser, L.; Rueth, H.M.; et al. Nitrogen emissions, deposition and monitoring in the Western United States. Bioscience 2003, 53, 391–403. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Canadian Environmental Sustainability Indicators: Air Pollutant Emissions. 2019. Available online: www.canada.ca/en/environment-climate-change/services/environmental-indicators/air-pollutant-emissions.html (accessed on 19 June 2019).
- Aherne, J.; Shaw, P. Impacts of sulphur and nitrogen deposition in western Canada. J. Limnol. 2010, 69, 1–3. [Google Scholar] [CrossRef]
- Bobbink, R.; Hornung, M.; Roelofs, J.G.M. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 1998, 86, 717–738. [Google Scholar] [CrossRef]
- Dise, N.B.; Ashmore, M.; Belyazid, S.; Bleeker, A.; Bobbink, R.; de Vries, W.; Erisman, J.W.; Spranger, T.; Stevens, C.J.; van den Berg, L. Nitrogen as a threat to European terrestrial biodiversity. In The European Nitrogen Assessment; Sutton, M.A., Howard, C.M., Erisman, J.W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: Cambridge, UK; European Union: Brussels, Belgium, 2011; Chapter 20. [Google Scholar]
- Nordin, A.; Sheppard, L.; Strengbom, J.; Gunnarsson, U.; Hicks, K.; Sutton, M.A. Understanding of nitrogen deposition impacts: Topic 3. In Proceedings of the Background Document for the COST 729 Workshop on ‘Nitrogen Deposition and Natura 2000: Science and Practice in Determining Environmental Impacts’, Brussel, Belgium, 18–20 May 2009. [Google Scholar]
- Bobbink, R.; Ashmore, M.; Braun, S.; Flückiger, W.; Van den Wyngaert, I.J.J. Empirical nitrogen loads for natural and semi-natural ecosystems: 2002 update. In Empirical Critical Loads for Nitrogen, Environmental Documentation No. 164 Air; Achermann, B., Bobbink, R., Eds.; Swiss Agency for Environment, Forest and Landscape SAEFL: Berne, Switzerland, 2003. [Google Scholar]
- Bobbink, R.; Hicks, W.K. Factors affecting nitrogen deposition impacts on biodiversity: An overview. In Nitrogen Deposition, Critical Loads and Biodiversity; Sutton, M.A., Mason, K.E., Shepphard, L.H., Sverdrup, H., Haueber, R., Hicks, W.K., Eds.; Springer: Dordrecht, The Netherlands, 2014; Chapter 14; pp. 127–138. [Google Scholar]
- Duprè, C.; Stevens, C.J.; Ranke, T.; Bleekers, A.; Peppler-Lisbach, C.; Gowing, D.J.G.; Dise, N.B.; Dorland, E.; Bobbink, R.; Diekmann, M. Changes in species richness and composition in European acidic grasslands over the past 70 years: The contribution of cumulative atmospheric nitrogen deposition. Glob. Chang. Biol. 2010, 16, 344–357. [Google Scholar] [CrossRef]
- Köchy, M.; Wilson, S.D. Variation in nitrogen deposition and available soil nitrogen in a forest-grassland ecotone in Canada. Landsc. Ecol. 2004, 20, 191–202. [Google Scholar] [CrossRef][Green Version]
- Beaudoin, A.; Bernier, P.Y.; Villemaire, P.; Guindon, L.; Guo, X.J. Species Composition, Forest Properties and Land Cover Types across Canada’s Forests at 250m Resolution for 2001 and 2011; Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 2017. [Google Scholar]
- ESRD: Environment and Sustainable Resource Development. Appendix I: Vegetation, wetlands and biodiversity. In Environmental Assessment—Cenovus TL ULC Telephone Lake In-Situ Project—EIA Report and Application for Approval; Volume 3 EIA–Appendices; Cenovus TL ULC & EnCana: Calgary, AB, Canada, 2011; Available online: https://open.alberta.ca/dataset/4871f71a-c541-47f5-9d79-58ef4a80851a/resource/c8f47757-8140-4e0e-9236-904eaae15f5d/download/v3appi.pdf (accessed on 15 February 2023).
- Allen, E.; Bayley, S.E. Effects of Nitrogen Deposition on Forests and Peatlands: A Literature Review and Discussion of the Potential Impacts of Nitrogen Deposition in the Alberta Oil Sands Region; Wood Buffalo Environmental Association: Fort McMurray, AB, Canada, 2007. [Google Scholar]
- Nilsson, J.; Grennfelt, P. Critical Loads for Sulphur and Nitrogen: Report from a Workshop Held at Skokloster, Sweden; UNECE and the Nordic Council of Ministers: Stockholm, Sweden, 1988. [Google Scholar]
- de Vries, W.; Hettelingh, J.-P.; Posch, M. Critical Loads and Dynamic Risk Assessments: Nitrogen, Acidity and Metals in Terrestrial and Aquatic Ecosystems; Environmental Pollution Series; Springer: Netherlands, Dordrecht, the Netherlands, 2015; Volume 25. [Google Scholar]
- Aherne, J.; Wilkins, K.; Cathcart, H. Nitrogen-Sulfur Critical Loads: Assessment of Impacts of Air Pollution on Habitats; EPA Research Report (2016-CCRP-MS.43); Environmental Protection Agency, Co.: Wexford, Ireland, 2021; 45p. [Google Scholar]
- Wilkins, K.; Clark, C.; Aherne, J. Ecological thresholds under atmospheric nitrogen deposition for 1200 herbaceous species and 24 communities across the United States. Glob. Chang. Biol. 2022, 28, 2381–2395. [Google Scholar] [CrossRef]
- Pardo, L.H.; Fenn, M.E.; Goodale, C.L.; Geiser, L.H.; Driscoll, C.T.; Allen, E.B.; Baron, J.S.; Bobbink, R.; Bowman, W.D.; Clark, C.M.; et al. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl. 2011, 21, 3049–3082. [Google Scholar] [CrossRef]
- Porter, E.; Blett, T.; Potter, D.U.; Huber, C. Protecting resources on federal lands: Implications of critical loads for atmospheric deposition of nitrogen and sulfur. BioScience 2005, 55, 603–612. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; da Cunha Bustamante, M.M.; Cinderby, S.; Davidson, E.A.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Bobbink, R.; Hettelingh, J. Review and Revision of Empirical Critical Loads and Dose-Response Relationships: Proceedings of an Expert Workshop, Noordwijkerhout, 23–25 June 2010; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2011. [Google Scholar]
- Bobbink, R.; Loran, C.; Tomassen, H. Review and Revision of Empirical Critical Loads of Nitrogen for Europe; Texte 110/2022; German Environment Agency: Dessau-Roßlau, Germany, 2022; p. 358. [Google Scholar]
- Bobbink, R. Effects of Nutrient Enrichment in Dutch Chalk Grassland. J. Appl. Ecol. 1991, 28, 28–41. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Pauli, D.; Peintinger, M.; Schmid, B. Nutrient enrichment in calcareous fens: Effects on plants species and community structure. Basic Appl. Ecol. 2002, 3, 255–266. [Google Scholar] [CrossRef]
- Henrys, P.A.; Stevens, C.J.; Smart, S.M.; Maskell, L.C.; Walker, K.J.; Preston, C.D.; Crowe, A.; Rowe, E.C.; Gowing, D.J.; Emmett, B.A. Impacts of nitrogen deposition on vascular plants in Britain: An analysis of two national observation networks. Biogeosciences 2011, 8, 3501–3518. [Google Scholar] [CrossRef]
- Roth, T.; Kohli, L.; Rihm, B.; Achermann, B. Nitrogen deposition is negatively related to species richness and species composition of vascular plants and bryophytes in Swiss mountain grassland. Agric. Ecosyst. Environ. 2013, 178, 121–126. [Google Scholar] [CrossRef]
- Maskell, L.C.; Smart, S.M.; Bullock, J.M.; Thompson, K.; Stevens, C.J. Nitrogen deposition causes widespread loss of species richness in British habitats. Glob. Chang. Biol. 2010, 16, 671–679. [Google Scholar] [CrossRef]
- Reinds, G.J.; Mol-Dijkstra, J.; Bonten, L.; Wamelink, W.; Hennekens, S.; Goedhart, P.; Posch, M. Probability of Plant Species (PROPS) Model: Latest Developments. In Modelling and Mapping the Impacts of Atmospheric Deposition of Nitrogen and Sulphur; Slootweg, J., Posch, M., Hettelingh, J.-P., Eds.; CCE Status Report 2015, RIVM Report 2015-0193; National Institute for Public Health and the Environment: Bilthoven, Netherlands, 2015; pp. 55–62. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/4038/dokumente/2_cce_sr2015.pdf (accessed on 15 February 2023).
- Payne, R.J.; Dise, N.B.; Stevens, C.J.; Gowin, D.J.; BEGIN Partners. Impact of nitrogen deposition at the species level. Proc. Natl. Acad. Sci. USA 2013, 110, 984–987. [Google Scholar] [CrossRef]
- Wilkins, K.; Aherne, J.; Bleasdale, A. Vegetation community change points suggest that critical loads of nutrient nitrogen may be too high. Atmos. Environ. 2016, 146, 324–331. [Google Scholar] [CrossRef]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Pitcher, C.R.; Lawton, P.; Ellis, N.; Smith, S.J.; Incze, L.S.; Wei, C.-L.; Greenlaw, M.E.; Wolff, N.H.; Sameoto, J.A.; Snelgrove, P.V.R. Exploring the role of environmental variables in shaping patterns of seabed biodiversity composition in regional-scale ecosystems. J. Appl. Ecol. 2012, 49, 670–679. [Google Scholar] [CrossRef]
- Pitcher, C.R.; Ellis, N.; Smith, S.J. Example Analysis of Biodiversity Survey Data with R Package gradientForest. R Vignette. 2011. Available online: http://gradientforest.r-forge.r-project.org/biodiversity-survey.pdf (accessed on 15 February 2023).
- Roubeix, V.; Danis, P.-A.; Feret, T.; Baudoin, J.-M. Identification of ecological thresholds from variations in phytoplankton communities among lakes: Contribution to the definition of environmental standards. Environ. Monit. Assess. 2016, 188, 246. [Google Scholar] [CrossRef]
- ESWG: Ecological Stratification Working Group. A National Ecological Framework for Canada; Agriculture and Agri-Food Canada, Research Branch, Centre for Land and Biological Resources Research and Environment Canada, State of the Environment Directorate, Ecozone Analysis Branch: Ottawa/Hull, ON, Canada, 1995; p. 125. [Google Scholar]
- Wiken, E.B. Terrestrial ecozones of Canada. In Ecological Land Classification No. 19; Lands Directorate: Ottawa, ON, Canada, 1986; p. 26. [Google Scholar]
- Clair, T.A.; Percy, K.E. Assessing Forest Health in the Athabasca Oil Sands Region; WBEA Technical Report 2015-05-25; Wood Buffalo Environmental Association: Fort McMurray, AB, Canada, 2015; p. 180, + Appendices. [Google Scholar]
- McLaughlan, M.S.; Wright, R.A.; Jiricka, R.D. Field Guide to the Ecosites of Saskatchewan’s Provincial Forests; Saskatchewan Ministry of Environment, Forest Service: Prince Albert, SK, Canada, 2010; p. 343. [Google Scholar]
- Beaudoin, A.; Bernier, P.Y.; Villemaire, P.; Guindon, L.; Guo, X.J.; Bergeron, T.; Magnussen, S.; Hall, R.J. Mapping Attributes of Canada’s Forests at Moderate Resolution through kNN and MODIS Imagery. Can. J. For. Res. 2014, 44, 521–532. [Google Scholar] [CrossRef]
- CFIC: Canadian Forest Inventory Committee. Canada’s National Forest Inventory: Ground Sampling Guidelines; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004. [Google Scholar]
- Makar, P.A.; Akingunola, A.; Aherne, J.; Cole, A.S.; Aklilu, Y.-A.; Zhang, J.; Wong, I.; Hayden, K.; Li, S.-M.; Kirk, J.; et al. Estimates of exceedances of critical loads for acidifying deposition in Alberta and Saskatchewan. Atmos. Chem. Phys. 2018, 18, 9897–9927. [Google Scholar] [CrossRef]
- McKenney, D.; Papadopol, P.; Campbell, K.; Lawrence, K.; Hutchinson, M. Spatial models of Canada- and North America-wide 1971–2000 minimum and maximum temperature, total precipitation and derived bioclimatic variables. In Frontline Forest Research Applications; Catalogue No. Fol23-1/106E-PDF; Minister of Supply and Services Canada: Sault Ste. Marie, ON, Canada, 2006. [Google Scholar]
- Compton, T.J.; Bowden, D.A.; Pitcher, C.R.; Hewitt, J.E.; Ellis, N. Biophysical patterns in benthic assemblage composition across contrasting continental margins off New Zealand. J. Biogeogr. 2012, 40, 75–89. [Google Scholar] [CrossRef]
- Large, S.I.; Fay, G.; Friedland, K.D.; Link, J.S. Quantifying patterns of change in marine ecosystem response to multiple pressures. PLoS ONE 2015, 10, e0119922. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional variable importance for random forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef]
- Roubeix, V.; Daufresne, M.; Argillier, C.; Dublon, J.; Maire, A.; Nicolas, D.; Raymond, J.-C.; Danis, P.-A. Physico-chemical thresholds in the distribution of fish species among French lakes. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 41. [Google Scholar] [CrossRef]
- Field, R.; Hawkins, B.A.; Cornell, H.V.; Currie, D.J.; Diniz-Filho, A.F.; Guegan, J.-F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; et al. Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 2009, 36, 132–147. [Google Scholar] [CrossRef]
- Stevens, C.; Duprè, C.; Gaudnik, C.; Dorland, E.; Dise, N.; Gowing, D.; Bleeker, A.; Alard, D.; Bobbink, R.; Fowler, D.; et al. Changes in species composition of European acid grasslands observed along a gradient of nitrogen deposition. J. Veg. Sci. 2011, 22, 207–215. [Google Scholar] [CrossRef]
- Schleyer, M.; Porter, S. Drivers of soft and stony coral community distribution on the high-latitude coral reefs of South Africa. Adv. Mar. Biol. 2018, 80, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Phelan, J.; Doraiswamy, P.; Buckley, J.; Cajka, J.C.; Dennis, R.L.; Lynch, J.; Nolte, C.G.; Spero, T.L. Atmospheric deposition and exceedances of critical loads from 1800–2025 for the conterminous United States. Ecol. Appl. 2018, 28, 978–1002. [Google Scholar] [CrossRef]
- van Dobben, H.; de Vries, W. Relationship between forest vegetation, atmospheric deposition and site conditions at regional and European scales. Environ. Pollut. 2010, 158, 921–933. [Google Scholar] [CrossRef]
- Roth, T.; Kohli, L.; Rihm, B.; Amrhein, V.; Achermann, B. Nitrogen deposition and multi-dimensional plant diversity at the landscape scale. R. Soc. Open Sci. 2015, 2, 150017. [Google Scholar] [CrossRef]
- Dahlman, L.; Persson, J.; Palmqvist, K.; Näsholm, T. Organic and inorganic nitrogen uptake in lichens. Planta 2004, 219, 459–467. [Google Scholar] [CrossRef]
- Hogan, E.J.; Minnullina, G.; Sheppard, L.J.; Leith, I.D.; Crittenden, P.D. Response of phosphomonesterase activity in the lichen Cladonia portentosa to nitrogen and phosphorus enrichment in a field manipulation experiment. New Phytol. 2010, 186, 926–933. [Google Scholar] [CrossRef]
- Southon, G.E.; Field, C.; Caporn, S.J.M.; Britton, A.J.; Power, S.A. Nitrogen deposition reduces plant diversity and alters ecosystem functioning: Field-scale evidence from a nationwide survey of UK heathlands. PloS ONE 2013, 8, e59031. [Google Scholar] [CrossRef]
- Nordin, A.; Strengbom, J.; Ericson, L. Responses to ammonium and nitrate additions by boreal plants and their natural enemies. Environ. Pollut. 2006, 141, 167–174. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, L.J.L.; Laurence, J.; Sheppard, L.J.; Smart, S.M.; Bobbink, R.; Dise, N.B.; Ashmore, M.R. Evidence for differential effects of reduced and oxidised nitrogen deposition on vegetation independent of nitrogen load. Environ. Pollut. 2016, 208, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Kharol, S.K.; Shephard, M.W.; McLinden, C.A.; Zhang, L.; Sioris, C.E.; O’Brien, J.M.; Vet, R.; Cady-Pereira, K.E.; Hare, E.; Siemons, J.; et al. Dry deposition of reactive nitrogen from satellite observations of ammonia and nitrogen dioxide over North America. Geophys. Res. Lett. 2018, 45, 1157–1166. [Google Scholar] [CrossRef]
- Tulloss, E.M.; Cadenasso, M.L. The effect of nitrogen deposition on plant performance and community structure: Is it life stage specific? PLoS ONE 2015, 11, e0156685. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J. Impact of nitrogen deposition on the species richness of grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef]
- van den Berg, L.J.L.; Vergeer, P.; Rich, T.C.G.; Smart, S.M.; Guest, D.; Ashmore, M.R. Direct and indirect effects of nitrogen deposition on species composition change in calcareous grasslands. Glob. Chang. Biol. 2010, 17, 1871–1883. [Google Scholar] [CrossRef]
- Geiser, L.H.; Nelson, P.R.; Jovan, S.E.; Root, H.T.; Clark, C.M. Assessing ecological risks from atmospheric deposition of nitrogen and sulfur to US forests using epiphytic macrolichens. Diversity 2019, 11, 87. [Google Scholar] [CrossRef]
- Králikova, I.; Goga, M.; Bil’ová, I.; Bačkorová, M.; Bačkor, M. Response of lichens Cladonia arbuscula subsp. Mitis Cladonia Furcate Nitrogen Excess. Biol. 2016, 71, 632–638. [Google Scholar] [CrossRef]
- Thormann, M. Lichens as indicators of forest health in Canada. For. Chron. 2006, 82, 335–343. [Google Scholar] [CrossRef]
- Eriksson, O.; Raunistola, T. Impact of forest fertilizers on winter pastured of semi-domesticated reindeer. Rangifer 1991, 13, 203–214. [Google Scholar] [CrossRef]
- Kellner, O.; Marshagen, M. Effects of irrigation and fertilization on the ground vegetation of 130-year-old stand of Scots pine. Can. J. For. Res. 1991, 21, 733–738. [Google Scholar] [CrossRef]
- Fenn, M.E.; Bytnerowics, A.; Schilling, S.L.; Ross, C.S. Atmospheric deposition of nitrogen, sulfur and base cations in Jack pine in the Athabasca Oil Sands Region, Alberta, Canada. Environ. Pollut. 2015, 196, 497–510. [Google Scholar] [CrossRef]
- Laxton, D.L.; Watmough, S.A.; Aherne, J.; Straker, J. An assessment of nitrogen saturation in Pinus banksiana plots in the Athabasca Oil sands Region, Alberta. J. Limnol. 2010, 69, 171–180. [Google Scholar] [CrossRef]
- Carter, T.S.; Clark, C.M.; Fenn, M.E.; Jovan, S.; Perakis, S.S.; Riddell, J.; Schaberg, P.G.; Greaver, T.L.; Hastings, M.G. Mechanisms of nitrogen deposition effects on temperate forest lichens and trees. Ecosphere 2017, 8, e01717. [Google Scholar] [CrossRef]
- Hauck, M. Ammonium and nitrate tolerance in lichens. Environ. Pollut. 2010, 158, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Neixner, T.; Johnson, D.W.; et al. Ecological effects of nitrogen deposition in the Western United States. Bioscience 2004, 53, 404–420. [Google Scholar] [CrossRef]
- Payne, R.J.; Dise, N.B.; Field, C.D.; Dore, A.J.; Caporn, S.J.M.; Stevens, C.J. Nitrogen deposition and plant biodiversity: Past, present, and future. Front. Ecol. Environ. 2017, 15, 431–436. [Google Scholar] [CrossRef]
- Prescott, C.E.; Kumi, J.W.; Weetman, G.F. Long-term effects of repeated N fertilization and straw application in a Jack pine forest. 2. Changes in the ericaceous ground vegetation. Can. J. For. Res. 1995, 25, 1984–1990. [Google Scholar] [CrossRef]
- Nordin, A.; Strengbom, J.; Witzell, J.; Näsholm, T.; Ericson, L. Nitrogen deposition and the biodiversity of boreal forests: Implications for the nitrogen critical load. Ambio A J. Hum. Environ. 2005, 34, 20–24. [Google Scholar] [CrossRef]
- Strengbom, J.; Walheim, M.; Näsholm, T.; Ericson, L. Regional differences in occurrence of understorey forest species reflects differences in N deposition in Swedish forests. Ambio 2003, 32, 91–97. [Google Scholar] [CrossRef]
- Turkington, R.; John, E.; Watson, S.; Seccombe-Hett, P. The effects of fertilization and herbivory on the herbaceous vegetation of the boreal forest in northwestern Canada: A 10-year study. J. Ecol. 2002, 90, 325–337. [Google Scholar] [CrossRef]


| Code | Environmental Variable (eq ha−1 yr−1) | Range * | Median | Mean | Standard Deviation |
|---|---|---|---|---|---|
| TDN | Total Deposited Nitrogen | 31.18–597.01 | 85.27 | 105.91 | 70.88 |
| DHN3 | Dry Hydrazoic Acid | 2.07–83.02 | 17.99 | 21.55 | 15.31 |
| DNH3 | Dry Ammonia | 0.47–114.08 | 3.63 | 6.56 | 11.26 |
| DNO2 | Dry Nitrogen Dioxide | 0.58–309.59 | 3.62 | 11.12 | 31.67 |
| DNO | Dry Nitrogen Oxide | 0.0074–21.52 | 0.032 | 0.35 | 1.91 |
| DPAN | Dry Peroxyacetylnitrate | 0.93–17.24 | 6.62 | 6.65 | 3.28 |
| DHNO | Dry Nitroxyl | 0.011–1.77 | 0.040 | 0.099 | 0.20 |
| DRN3 | Dry Organic Nitrate | 0.46–6.33 | 2.66 | 2.71 | 1.30 |
| PNO3 | Dry Particle Nitrate | 0.32–6.16 | 1.02 | 1.40 | 0.99 |
| PNH4 | Dry Particle Ammonium | 2.09–18.52 | 6.03 | 6.74 | 2.97 |
| WNO3 | Wet Nitrate | 5.79–36.85 | 14.07 | 15.45 | 5.65 |
| WNH4 | Wet Ammonium | 13.93–87.23 | 31.31 | 33.27 | 12.32 |
| TDS | Total Deposited Sulphur | 23.82–1182.20 | 53.55 | 72.75 | 95.21 |
| DSO2 | Dry Sulphur Dioxide | 1.71–391.85 | 7.75 | 18.41 | 42.12 |
| PSO4 | Dry Particle Sulphate | 2.32–15.34 | 6.23 | 6.58 | 2.31 |
| HSO3 | Wet Hydrogen Sulfite | 3.94–844.44 | 13.62 | 22.42 | 54.84 |
| WSO4 | Wet Sulphate | 11.41–57.67 | 24.79 | 25.34 | 6.58 |
| Code | Environmental Variable | Range * | Median | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Latitude | Latitude (°) | 54.01–59.95 | 56.86 | 56.97 | 1.51 |
| Longitude | Longitude (°) | −114.18–101.90 | −106.86 | −107.06 | 2.27 |
| Elevation | Elevation (m asl) | 209.95–733.59 | 454.20 | 439.53 | 92.11 |
| bio_12 | Annual Precipitation (mm) | 349–561 | 460 | 458.25 | 50.41 |
| bio_13 | Precipitation of Wettest Period (mm) | 13–24 | 20 | 19.59 | 2.61 |
| bio_15 | Precipitation of Seasonality (mm) | 39–63 | 51 | 51.22 | 4.39 |
| bio_16 | Precipitation of Wettest Quarter (mm) | 137–244 | 207 | 202.05 | 23.46 |
| bio_17 | Precipitation of Driest Quarter (mm) | 40–81 | 61 | 60.92 | 8.02 |
| bio_18 | Precipitation of Warmest Quarter (mm) | 134–243 | 206 | 200.40 | 23.72 |
| bio_19 | Precipitation of Coldest Quarter (mm) | 44–82 | 61 | 62.49 | 7.44 |
| sg_01 | Julian day number at start of growing season | 115–151 | 134 | 131.34 | 6.80 |
| sg_02 | Julian day number at end of growing season | 272–291 | 284 | 284.17 | 3.64 |
| sg_03 | Number of days of growing Season | 122–176 | 154 | 153.83 | 10.04 |
| sg_04 | Total Precipitation for period 1 (mm) | 49.4–96 | 68.2 | 69.74 | 10.37 |
| sg_05 | Total precipitation for period 2 (mm) | 47.8–92.1 | 68 | 68.70 | 9.66 |
| sg_06 | Total precipitation for period 3 (mm) | 200.9–346.2 | 297.4 | 290.52 | 33.38 |
| sg_07 | Total precipitation for period 4 (mm) | 153.1–258 | 228.4 | 221.83 | 25.75 |
| sg_08 | GDD above base temperature for Period 1 | 0–6 | 1 | 1 | 1.10 |
| sg_09 | GDD above base temperature for Period 2 | 194–268 | 236 | 234.27 | 16.06 |
| sg_10 | GDD above base temperature for Period 3 | 702–1317 | 1005 | 1043.80 | 116.34 |
| sg_11 | GDD above base temperature for Period 4 | 444–1113 | 771 | 809.54 | 127.64 |
| sg_12 | Annual Mean Temperature (°C) | −5.46–1.64 | −1.51 | −1.09 | 1.46 |
| sg_13 | Annual Minimum Temperature (°C) | −10.17–−3.96 | −6.68 | −6.53 | 1.39 |
| sg_14 | Annual Maximum Temperature (°C) | −0.75–7.23 | 3.99 | 4.34 | 1.58 |
| sg_15 | Mean Temperature for Period 3 (°C) | 11.12–13.16 | 12.05 | 12.16 | 0.38 |
| sg_16 | Temperature Range for Period 3 (°C) | 21.92–25.67 | 23.99 | 24.04 | 0.67 |
| Species Information | R2 Overall Importance | Specific R2S | Environmental Variable |
|---|---|---|---|
| Bryoria glabra, Lichen | 0.878 | 0.1620 | sg_04 |
| 0.1210 | Longitude | ||
| 0.0770 | sg_06 | ||
| 0.0697 | sg_07 | ||
| 0.0634 | TDS | ||
| Usnea species, Lichen | 0.661 | 0.1570 | bio_17 |
| 0.1380 | bio_19 | ||
| 0.0533 | sg_13 | ||
| 0.0302 | DNH3 | ||
| 0.0212 | sg_03 | ||
| Lecidella euphorea, Lichen | 0.640 | 0.1060 | sg_04 |
| 0.1030 | Longitude | ||
| 0.0480 | DNH3 | ||
| 0.0472 | sg_06 | ||
| 0.0403 | sg_07 | ||
| Cladina mitis, Lichen | 0.595 | 0.1350 | HSO3 |
| 0.0695 | Longitude | ||
| 0.0643 | DNO | ||
| 0.0625 | TDS | ||
| 0.0405 | DNO2 | ||
| Lecanora circumborealis, Lichen | 0.526 | 0.0967 | Longitude |
| 0.0779 | sg_04 | ||
| 0.0341 | WSO4 | ||
| 0.0331 | HSO3 | ||
| 0.0265 | sg_06 | ||
| Arctostaphylos uva-ursi, Vascular | 0.523 | 0.1480 | Longitude |
| 0.0796 | sg_01 | ||
| 0.0641 | HSO3 | ||
| 0.0533 | sg_12 | ||
| 0.0222 | DNO | ||
| Lecanora cenisia, Lichen | 0.488 | 0.0869 | HSO3 |
| 0.0665 | sg_13 | ||
| 0.0341 | sg_11 | ||
| 0.0322 | sg_03 | ||
| 0.0282 | WNO3 | ||
| Stereocaulon grande, Lichen | 0.477 | 0.0831 | sg_02 |
| 0.0607 | sg_13 | ||
| 0.0503 | sg_03 | ||
| 0.0324 | sg_14 | ||
| 0.0249 | sg_11 | ||
| Evernia mesomorpha, Lichen | 0.477 | 0.0991 | Longitude |
| 0.0368 | Latitude | ||
| 0.0340 | DSO2 | ||
| 0.0242 | DNO | ||
| 0.0229 | TDS | ||
| Vaccinium uliginosum, Vascular | 0.452 | 0.0657 | sg_01 |
| 0.0414 | sg_13 | ||
| 0.0384 | WNO3 | ||
| 0.0326 | sg_03 | ||
| 0.0321 | sg_10 | ||
| Polypodium virginianum, Vascular | 0.451 | 0.0964 | bio_19 |
| 0.0493 | bio_12 | ||
| 0.0458 | Longitude | ||
| 0.0398 | bio_17 | ||
| 0.0376 | sg_04 | ||
| Viola adunca, Vascular | 0.429 | 0.2180 | sg_09 |
| 0.0641 | sg_14 | ||
| 0.0433 | sg_12 | ||
| 0.0156 | HSO3 | ||
| 0.0122 | Latitude | ||
| Cladonia gracilis, Lichen | 0.421 | 0.0433 | DNO |
| 0.0408 | DNO2 | ||
| 0.0289 | WSO4 | ||
| 0.0252 | Bio_15 | ||
| 0.0247 | Longitude |
| Environmental Variable | R2-Weighted Importance | Environmental Variable | R2-Weighted Importance |
|---|---|---|---|
| Longitude | 0.01610 | sg_09 | 0.00687 |
| HSO3 | 0.01110 | bio_19 | 0.00682 |
| sg_04 | 0.00843 | sg_12 | 0.00579 |
| DNO | 0.00784 | DSO2 | 0.00553 |
| Latitude | 0.00781 | DNO2 | 0.00547 |
| Elevation | 0.00751 | sg_13 | 0.00533 |
| bio_17 | 0.00729 | DHNO | 0.00502 |
| TDS | 0.00697 | ||
| Mean R2-Weighted Importance | 0.00467 | ||
| Environmental Variable | Correlation Coefficient (TDN) | Gradient Forest Threshold (eq ha−1 yr−1) | Inferred TDN Threshold (eq ha−1 yr−1) |
|---|---|---|---|
| DNO | 0.586 | 1–2 | 100 |
| DNO2 | 0.735 | 25 | 150 |
| DNH3 | 0.904 | 60 | 350 |
| 80–90 | 460 | ||
| 110 | 550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandinther, N.; Aherne, J. Ecological Risks from Atmospheric Deposition of Nitrogen and Sulphur in Jack Pine forests of Northwestern Canada. Nitrogen 2023, 4, 102-124. https://doi.org/10.3390/nitrogen4010008
Vandinther N, Aherne J. Ecological Risks from Atmospheric Deposition of Nitrogen and Sulphur in Jack Pine forests of Northwestern Canada. Nitrogen. 2023; 4(1):102-124. https://doi.org/10.3390/nitrogen4010008
Chicago/Turabian StyleVandinther, Nicole, and Julian Aherne. 2023. "Ecological Risks from Atmospheric Deposition of Nitrogen and Sulphur in Jack Pine forests of Northwestern Canada" Nitrogen 4, no. 1: 102-124. https://doi.org/10.3390/nitrogen4010008
APA StyleVandinther, N., & Aherne, J. (2023). Ecological Risks from Atmospheric Deposition of Nitrogen and Sulphur in Jack Pine forests of Northwestern Canada. Nitrogen, 4(1), 102-124. https://doi.org/10.3390/nitrogen4010008






