Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status
Abstract
:1. Introduction
2. Material and Methods
2.1. Relationship between Leaf N Concentration and Absolute SPAD Values
2.2. Optimal Corn Leaf N Concentration
3. Results and Discussion
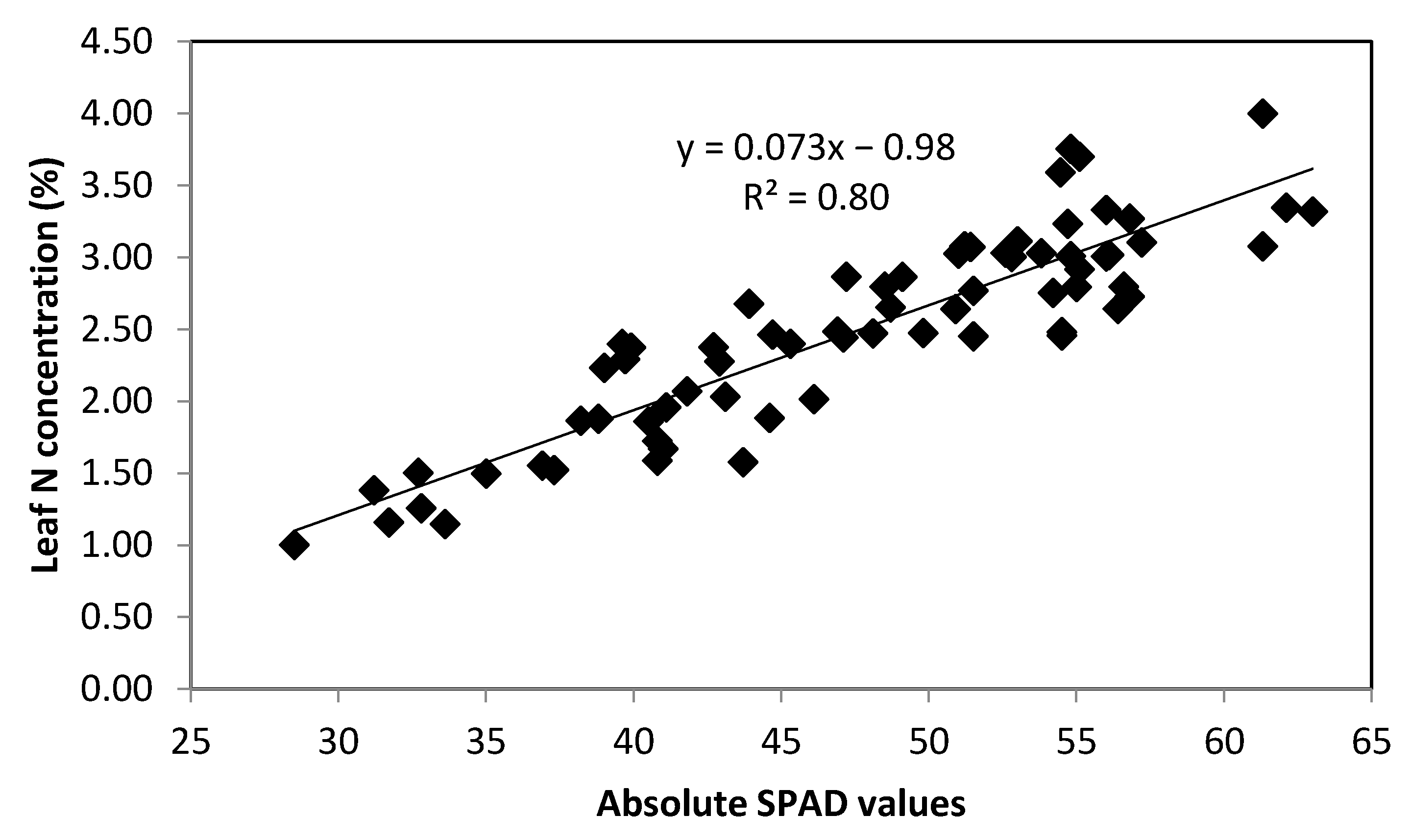
3.1. Relationship between Leaf N Concentration and Absolute SPAD Values
3.2. Optimal Corn Leaf N Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Hallberg, G.R. Nitrogen Management and Ground Water Protection; Follett, R.F., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1989; p. 74. [Google Scholar]
- Halvorson, A.D.; Schweissing, F.C.; Bartolo, M.E.; Reule, C.A. Corn response to nitrogen fertilization in a soil with high residual-Nitrogen. Agron. J. 2005, 97, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, C.A.; Wortmann, C.S. Corn Response to Nitrogen Rate, Row Spacing, and Plant Density in Eastern Nebraska. Agron. J. 2006, 98, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Raun, W.R.; Johnson, G.V. Improving Nitrogen Use Efficiency for Cereal Production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Hartz, T.K. Vegetable production best management practices to minimize nutrient loss. Horttechnology 2006, 16, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Schenk, M.K. Nutrient efficiency of vegetable crops. Acta Hortic. 2006, 700, 21–33. [Google Scholar] [CrossRef]
- Bishnu, P.K. SPAD value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 475. [Google Scholar]
- Edalat, M.; Naderi, R.; Egan, T.P. Corn nitrogen management using NDVI and SPAD sensor-based data under conventional vs. reduced tillage systems. J. Plant Nutr. 2019, 42, 2310–2322. [Google Scholar] [CrossRef]
- Friedman, J.M.; Raymond, E.H.; Mutters, R.G. Assessment of Leaf Color Chart Observations for Estimating Maize Chlorophyll Content by Analysis of Digital Photographs. Agron. J. 2016, 108, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Silva, G. Corn Leaf Greenness Affected by Nitrogen Rate, Row Spacing and Population Density; Michigan State University Extension: East Lansing, Michigan, USA, 2015. [Google Scholar]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-Based Leaf Nitrogen Estimation is Impacted by Environmental Factors and Crop Leaf Characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [Green Version]
- Parry, C.; Blonquist, J.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.A.; Francis, D.; Ferguson, S.; Wortmann, C.S. Using a Chlorophyll Meter to Improve N Management; NebGuide; Soil Resource Management; University of Nebraska: Lincoln, NE, USA, 2013. [Google Scholar]
- Rorie, R.L.; Purcell, L.C.; Karcher, D.E.; King, C.A. The Assessment of Leaf Nitrogen in Corn from Digital Images. Crop. Sci. 2011, 51, 2174–2180. [Google Scholar] [CrossRef] [Green Version]
- Rorie, R.L.; Purcell, L.C.; Karcher, D.E.; King, C.H. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agron. J. 2011, 103, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Beegle, D.; Zhu, Q.; Sripada, R. Improving in-season nitrogen recommendations for maize using an active sensor. Field Crop. Res. 2011, 120, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Shaver, T.M.; Khosla, R.; Westfall, D.G. Evaluation of two crop canopy sensors for nitrogen variability determination in irrigated maize. Precis. Agric. 2011, 12, 892–904. [Google Scholar] [CrossRef]
- Yu, H.; Wu, S.; Wang, J. Evaluation of SPAD and Dualex for In-Season Corn Nitrogen Status Estimation. Acta Agron. Sin. 2010, 5, 840–847. [Google Scholar]
- Chen, P.; Haboudane, D.; Wang, L.; Vigneault, P. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 chlorophyll meter for nitrogen management in corn. J. Plant Nutr. 2008, 21, 741–755. [Google Scholar] [CrossRef]
- Schepers, J.S.; Francis, D.D.; Vigil, M.; Below, F.E. Comparison of corn leaf nitrogen concentration and chlorophyll meter readings. Commun. Soil Sci. Plant Anal. 2008, 23, 17–20. [Google Scholar] [CrossRef]
- Miao, Y.; Mulla, D.J.; Randall, G.W.; Vetsch, J.A.; Vintila, R. Combining chlorophyll meter readings and high spatial resolution remote sensing images for in-season site-specific nitrogen management of corn. Precis. Agric. 2009, 10, 1573–1618. [Google Scholar] [CrossRef]
- Costa, C.; Dwyer, L.M.; Dutilleul, P.; Stewart, D.W.; Ma, B.L.; Smith, D.L. Inter-Relationships of applied Nitrogen, SPAD, And Yield of Leafy and non-leafy maize genotypes. J. Plant Nutr. 2006, 24, 1173–1194. [Google Scholar] [CrossRef]
- Martinez, D.; Guiamet, J. Distortion of the SPAD 502 chlorophyll meter readings by changes in irradiance and leaf water status. Agronomie 2004, 24, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Murdock, L.; Jones, S.; Bowley, C. Using a Chlorophyll Meter to Make Nitrogen Recommendations on Wheat. Cooperative Extension Service; University of Kentucky-College of Agriculture: Lexington, KY, USA, 1997. [Google Scholar]
- Piekielek, W. The Early-Season Chlorophyll Meter Test for Corn; Agronomy Facts 53; Penstate University: State College, PA, USA, 1995. [Google Scholar]
- Blackmer, T.M.; Schepers, J.S.; Varvel, G.E. Light Reflectance Compared with Other Nitrogen Stress Measurements in Corn Leaves. Agron. J. 1994, 86, 934–938. [Google Scholar] [CrossRef]
- Piekielek, W.; Fox, R.H. Use of a Chlorophyll Meter to Predict Sidedress Nitrogen Requirements for Maize. Agron. J. 1992, 84, 59–65. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Tollenaar, M.; Houwing, L. A nondestructive method to monitor leaf greenness in corn. Can. J. Plant Sci. 1991, 71, 505–509. [Google Scholar] [CrossRef]
- Abdendroth, L.J.; Elmore, R.W.; Marlay, S.R. Corn Growth and Development. PMR 1009; Iowa State University Extension: Ames, IA, USA, 2011. [Google Scholar]
- Zhang, J.; Blackmer, A.M. Monitoring nitrogen deficiencies in corn. Integrated Crop Manage. 1999. Available online: http://www.ipm.iastate.edu/ipm/icm/1999/5-5-1999/moncorn.html (accessed on 31 January 2008).
- Fox, R.H.; Piekielek, E.P.; Macneal, K.E. Comparison of late season diagnostic tests for predicting nitrogen status of corn. Agron. J. 2001, 93, 590–597. [Google Scholar] [CrossRef]
- Goodall, D.W.; Gregory, F.G. Chemical Composition of Plants as an Index of Their Nutritional Status; Imperial Bur. Hort. Plantation Crops. Tech. Commun. 17; IAB Central Branch: Wales, UK, 1947. [Google Scholar]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaton, N.A.; Mozaffari, M.; Espinoza, L.; Roberts, T.L.; Norman, R.J.; Kelley, J.R. Nitrogen rate recommendations for corn grown on clay and loamy soils. In Arkansas Soil Fertility Studies; Slaton, N.A., Ed.; Univiversity of Arkansas: Fayetteville, NC, USA, 2014; pp. 60–67. [Google Scholar]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Grasso, R.; Gallardo, M.; Thompson, R.B. Influence of time of day on measurement with chlorophyll meters and canopy reflectance sensors of different crop N status. Precis. Agric 2019, 20, 1087–1106. [Google Scholar] [CrossRef] [Green Version]
- Rhezali, A.; Rabii, M. Evaluation of a Digital Camera and a Smartphone Application, Using the Dark Green Color Index, in Assessing Maize Nitrogen Status. Commun. Soil Sci. Plant Anal. 2020, 51, 1–14. [Google Scholar] [CrossRef]
- Rhezali, A.; Purcell, L.C.; Roberts, T.L.; Greub, C.E. Predicting Nitrogen Requirements for Maize with the Dark Green Color Index under Experimental Conditions. Agron. J. 2018, 110, 1173–1179. [Google Scholar] [CrossRef]
| Authors | Nature of SPAD Values | Time of Sampling * | Outputs |
|---|---|---|---|
| [8] | Absolute | R1 and R2 |
|
| |||
| [9] | Not specified | Not specified |
|
| |||
| [10] | Absolute | R2 and R3 |
|
| [11] | Absolute | V7–V8 |
|
| |||
| [12] | Absolute | Not specified |
|
| |||
| [13] | Absolute | Multiple growth stages of wheat |
|
| |||
| [14] | Relative | After V6 |
|
| |||
| [15] | Relative | Tasseling |
|
| |||
| [16] | Relative | V3–V4 |
|
| [17] | Relative | V6–V7 |
|
| [18] | Relative | V12–V14 |
|
| [19] | Relative | 11–46 d after silking |
|
| [20] | Absolute | V6–V10 |
|
| |||
| [21] | Absolute | V7, R1,and R4 |
|
| |||
| [22] | Absolute | V8, R1–R3 |
|
| |||
| |||
| [23] | Absolute | V9, V11,R1, R3 |
|
| |||
| |||
| [24] | Absolute | Silking |
|
| |||
| [25] | Absolute | Not specified |
|
| [26] | Relative | Not specified |
|
| [27] | Relative | V6-V8 |
|
| |||
| |||
| [28] | Absolute | R1 |
|
| |||
| [29] | Absolute | V6 |
|
| |||
| |||
| [30] | Absolute | V10 |
|
|
| Year | Location | Soil Series | Planting Dates | Replications | N Rates (kg N ha−1) |
|---|---|---|---|---|---|
| 2014 | Fay 3 * | Captina silt loam § | 27 April | 4 | 0,72,144,216,288,360 |
| 2014 | Fay4 * | Pickwick silt loam µ | 27 April | 4 | 0,72,144,216,288,360 |
| 2014 | Kibler # | Roxanna sandy loam × | 25 May | 4 | 0,72,144,216,288,360 |
| 2015 | Fay 3 * | Captina silt loam § | 25 April | 4 | 0,72,144,216,288,360 |
| 2015 | Fay 4 * | Captina silt loam § | 26 April | 4 | 0,72,144,216,288,360 |
| 2015 | PineTree £ | Calloway silt loam ^ | 24 April | 4 | 0,72,144,216,288,360 |
| Treatments (kg N ha−1) | ||||||
|---|---|---|---|---|---|---|
| Application Times of N | T1 | T2 | T3 | T4 | T5 | T6 |
| Preplant | 0 | 36 | 36 | 36 | 36 | 36 |
| V6 | 0 | 36 | 54 | 90 | 126 | 162 |
| V8 | 0 | 0 | 54 | 90 | 126 | 162 |
| Year | Site | Equation * | Optimum N Rate (kg ha−1) | R2 | Yield Max (Mg ha−1) | Yield Min (Mg ha−1) |
|---|---|---|---|---|---|---|
| 2014 | Fay2 | y = −0.0001x2 + 0.01x + 10 | 76 | 0.84 | 11.8 | 9.8 |
| 2014 | Fay3 | y = −0.0001x2 + 0.02x + 4.7 | 105 | 0.53 | 10.5 | 4.5 |
| 2014 | Kibler | y = −0.0001x2 + 0.06x + 3.8 | 300 | 0.99 | 12.3 | 3.62 |
| 2015 | Fay2 | y = −0.0001x2 + 0.05x + 5.4 | 250 | 0.98 | 12.9 | 5.2 |
| 2015 | Fay3 | y = −0.0001x2 + 0.06x + 5.4 | 300 | 0.99 | 13.4 | 5.1 |
| 2015 | PineTree | y = −0.0001x2 + 0.07x + 2.9 | 350 | 0.84 | 12.6 | 2.7 |
| Variable | Source | Df | p-Values |
|---|---|---|---|
| Yield | Preplant N | 1 | <0.0001 |
| Treatments | 5 | 0.0075 | |
| Treatments × preplant N | 5 | 0.06 | |
| R2 | 0.74 |
| Corn Yield (Mg ha−1) | ||
|---|---|---|
| Treatments | 0 kg N ha−1 (Preplant) | 67 kg N ha−1 (Preplant) |
| 0 kg N ha−1 | 11.0 b | 12.0 a |
| 302 kg ha−1 | 10.9 b | 12.5 a |
| V6 Std Rec | 10.6 b | 12.0 a |
| V8 Std Rec | 10.7 b | 12.8 a |
| V10 Std Rec | 10.4 b | 12.5 a |
| V12 Std Rec | 10.3 b | 12.2 a |
| Leaf N Concentration (%) | ||||
|---|---|---|---|---|
| Growth Stages | 0 kg N ha−1 (Preplant) | 67 kg N ha−1 (Preplant) | ||
| Mean | C.I. | Mean | C.I. | |
| V6 | 3.72 | 3.58–3.85 | 4.09 | 3.66–4.51 |
| V8 | 3.29 | 2.90–3.67 | 3.28 | 2.90–3.65 |
| V10 | 2.95 | 2.56–3.33 | 2.89 | 2.65–3.12 |
| V12 | 2.63 | 2.21–3.04 | 2.97 | 2.59–3.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhezali, A.; Aissaoui, A.E. Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status. Nitrogen 2021, 2, 298-307. https://doi.org/10.3390/nitrogen2030020
Rhezali A, Aissaoui AE. Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status. Nitrogen. 2021; 2(3):298-307. https://doi.org/10.3390/nitrogen2030020
Chicago/Turabian StyleRhezali, Abdelaziz, and Abdellah El Aissaoui. 2021. "Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status" Nitrogen 2, no. 3: 298-307. https://doi.org/10.3390/nitrogen2030020
APA StyleRhezali, A., & Aissaoui, A. E. (2021). Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status. Nitrogen, 2(3), 298-307. https://doi.org/10.3390/nitrogen2030020





