Abstract
Nitrogen (N) cycling in mangroves is complex, with rapid turnover of low dissolved N concentrations, but slow turnover of particulate N. Most N is stored in soils. The largest sources of N are nearly equal amounts of mangrove and benthic microalgal primary production. Dissolved N fluxes between the forests and tidal waters show net uptake, indicating N conservation. N2-fixation is underestimated as rapid rates measured on tree stems, aboveground roots and cyanobacterial mats cannot currently be accounted for at the whole-forest scale due to their extreme patchiness and the inability to extrapolate beyond a localized area. Net immobilization of NH4+ is the largest ecosystem flux, indicating N retention. Denitrification is the largest loss of N, equating to 35% of total N input. Burial equates to about 29% of total inputs and is the second largest loss of N. Total inputs slightly exceed total outputs, currently suggesting net N balance in mangroves. Mangrove PON export equates to ≈95% of PON export from the world’s tropical rivers, but only 1.5% of the entire world’s river discharge. Mangrove N2O emissions, denitrification, and burial contribute 0.4%, 0.5–2.0% and 6%, respectively, to the global coastal ocean, which are disproportionate to their small worldwide area.
1. Introduction
Nitrogen (N) is the most important nutrient element in fostering growth, reproduction, productivity, and other energetic and physiological processes that enable ecosystems to thrive. Mangrove forests and their associated waterways are major coastal ecosystems that live along the world’s subtropical and tropical coastlines, requiring nitrogen and other nutrients like all other ecosystems [1]. Mangroves are an important ecological and economic resource, offering a wide variety of ecosystem goods and services, such as being important breeding sites and nursery grounds for birds, fish, crustaceans, amphibians, shellfish, reptiles, and mammals [2]. These tidal forests are a potentially renewable resource of wood and accumulate sediment, carbon, contaminants and nutrients, such as N. Mangroves also provide a vital livelihood for coastal inhabitants and offer some protection against coastal erosion and catastrophic events, such as tsunamis [1,2]. Thus, it is important to understand how these tropical ecosystems function biogeochemically.
In mangrove ecosystems where N is often limiting, native flora and fauna and their associated food webs have evolved a variety of mechanisms to conserve N. These retention mechanisms and strategies include, but are not restricted to: (1) highly efficient solute uptake between trees, microbes and soil N pools; (2) high N-use efficiency and high rates of leaf resorption; (3) low rates of N loss, such as dissolved N export and nitrous oxide (N2O) emissions in proportion to N inputs; (4) export of highly refractory N in the form of humic and fulvic acids (tannins); (5) rapid rates of nitrogen fixation at the soil surface and on various forest components (bark, downed wood, prop roots, pneumatophores, cyanobacterial mats); and (6) a large reservoir of dead roots belowground [1,2].
Living in a low-N environment, mangroves rely greatly on the efficiency of the microbial machinery existing in their soils and tidal waters to process and conserve N in its various forms. Numbers, diversity and productivity of soil and planktonic bacterial communities are high, resulting in rapid uptake, transformation, and release of particulate and dissolved inorganic and organic N [1,3]; dissolved N pools turn over rapidly, usually in a matter of minutes to days [4]. The experimental addition of nutrients to mangrove trees in the field and laboratory indicate rapid uptake and utilization, although complex patterns often result due to various interactive factors, such as differences in forest stands in species composition, soil type (carbonate sand, terrigenous silt/clay, quartz sand, etc.), intertidal position, soil fertility, salinity, forest age, and tree and forest development stage [5,6,7].
Despite our knowledge of N transformation and utilization processes and rates in mangrove ecosystems, a comprehensive picture at the ecosystem-level has yet to emerge. To this day, only one complete N budget exists for a mangrove ecosystem [4] and no attempt has been made to construct a N mass balance for the world’s mangrove ecosystems. This paper details such an attempt as a model tool to identify what is and what is not known about N cycling processes in mangroves. First, we will assess the standing stocks of N in the forests and associated waterways, prior to examining functional processes, including the significance of N retention and the contribution of mangroves to N flow in the global coastal ocean.
2. Nitrogen Concentrations and Standing Stocks
2.1. Dissolved N Concentrations in Tidal Waters and Porewater
Dissolved organic and inorganic N in mangrove tidal waters are dominated by dissolved organic nitrogen (DON), followed in descending order by ammonium (NH4+), nitrate (NO3−) and nitrite (NO2−). In nearly all cases, except for some organically polluted environments, concentrations of all four species are in the micromolar (μM) range. DON, NH4+, NO2− and NO3− concentrations in unpolluted waterways range from 0.1 to 60 μM, 0 to 120 μM, 0 to 5 μM and 0 to 37 μM, respectively [3,4]. The major drivers of change in concentrations are rainfall, land runoff, intrusions of offshore water, groundwater and porewater inputs, leaching from litter, temperature, anthropogenic inputs, and plankton metabolic activities, especially of phytoplankton and bacterioplankton [1].
In soil porewater, dissolved N concentrations also vary greatly, depending on soil particulate N concentrations and composition, soil texture, redox status, presence of sulfides and other reductants, microbial mineralization processes, salinity, bioturbation, rate of soil accumulation, benthic community composition, and, most significantly, uptake and release from mangrove roots [1]. The chemical composition of DON in tidal waters and in porewater is not well characterized, but some evidence indicates that most DON is composed of humic acids (range: 47–91%) and to a lesser extent, of aromatic compounds, peptides and amino acids [8].
Figure 1 illustrates the importance of tree uptake on NH4+ porewater concentrations, showing an inverse relationship between the vertical soil depth changes in porewater NH4+ concentrations and live fine root biomass in three Kandelia candel forests in southern China [9]. A Pearson product-moment correlation (r) of porewater ammonium versus root biomass of −0.476 is significant (p < 0.00784). Earlier data [10,11] have similarly indicated an effect of NH4+ uptake by mangrove roots on porewater concentrations and profiles. Additional evidence indicates that adjacent mudflat sediments usually have higher porewater concentrations of NH4+ than in mangrove soils, suggesting that the difference is due to tree uptake [11]. These data are circumstantial, but support experimental data showing a strong preference by mangroves for ammonium (and nitrate) for their growth and nutrition [12,13,14,15]. Nitrite and nitrate are often found in mangrove porewater [4,11], but concentrations show very irregular patterns with increasing soil depth.
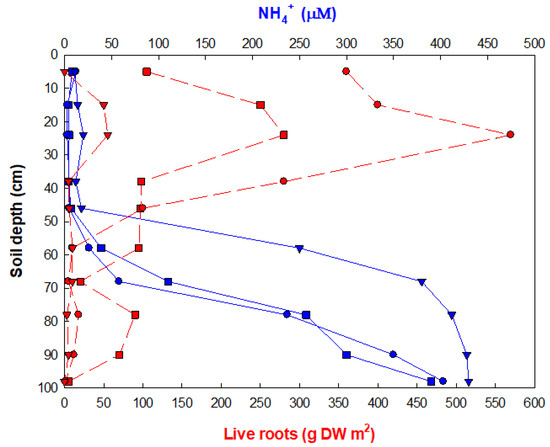
Figure 1.
Vertical profiles of porewater extractable ammonium (NH4+, μM) compared with live root biomass (g dry weight m2) in three Kandelia candel forests in the Jiulongjiang estuary, China [9].
2.2. Total N Concentrations and C/N Ratios in Forest Components and Soil
Concentrations of total nitrogen (TN) in leaves, stems, branches, roots, litter, and soil vary among forests due to many factors, including species composition, forest age, allochthonous N inputs, extent of organic pollution (if any), climate, temperature, salinity, physiological condition, nutritional status, soil composition, porewater nutrient concentrations, micronutrient availability, rate of soil organic matter accumulation, intertidal position, tidal regime, redox status, presence of sulfides and other reductants, microbial mineralization processes, bioturbation, and benthic community composition [1,4]. On average, TN concentrations in mangrove forests are very similar to those in tropical terrestrial forests for leaves and wood, and lower (Table 1) than terrestrial forest soil N (mean = 0.45% DW) and roots (mean = 0.86% DW), but higher (Table 1) than the C/N ratio (g/g) of below-ground biomass (mean = 33.2) (terrestrial forest references [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]).

Table 1.
Mean (±1 standard error, SE) and median total nitrogen (TN) concentrations (as percentage of dry weight, DW) of various above- and belowground mangrove components, including soils to a depth of 1 m. The mean C/N ratios (g/g) are as follows: soil C/N = 11.3; live wood C/N = 130.4; belowground root C/N = 80.9; litter C/N = 79.4. The C/N values for biomass were calculated based on relative percentage of biomass DW in the respective components (i.e., stems + branches for wood estimate) and dead and live root and rhizome DW for belowground estimates using the carbon data and references in [39]. N data from [4,9,10,11,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112] and earlier references within.
2.3. Global Mean N Stocks
Mean TN stock of global mangrove forests (Figure 2) totals 52.03 Mg N ha−1, with 96% of total TN stock contained in soils to a depth of 1 m (mean = 50.0 Mg N ha−1). In comparison, mean TN stocks in tropical terrestrial forests (rain forests, other moist forests, and peat swamps) total 22.33 Mg N ha−1 (Figure 2). Like mangroves, 91% of N stocks are vested in soil (mean = 20.3 Mg N ha−1), suggesting similar investment of N by both mangroves and terrestrial forests in various forest components and belowground storage.
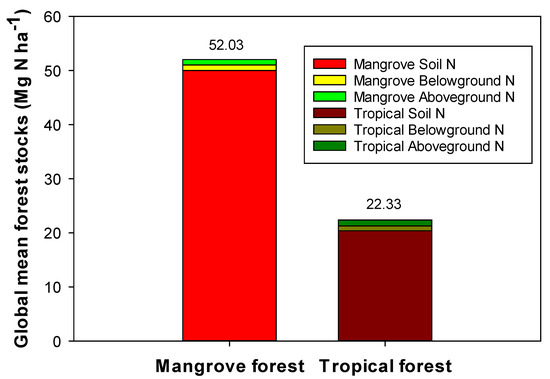
Figure 2.
Mean TN stocks in mangrove above- and belowground biomass and soils to a depth of 1 m. Biomass and soil dry weight data extrapolated to N using N content or C/N ratios (g/g) in Table 1. Tropical terrestrial forest data are presented for comparison and includes data from moist forests, peat swamp forests, and rain forests with N, C and C/N (g/g) data [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,113,114,115,116,117,118,119,120] and earlier references within.
3. The Nitrogen Cycle in Soils
The soil nitrogen cycle (Figure 3) is composed of a series of complex transformation processes, nearly all conducted by specialized bacterial and archaeal groups, such as ammonium-oxidizers, cyanobacteria, nitrate-reducers, and nitrite-oxidizers.
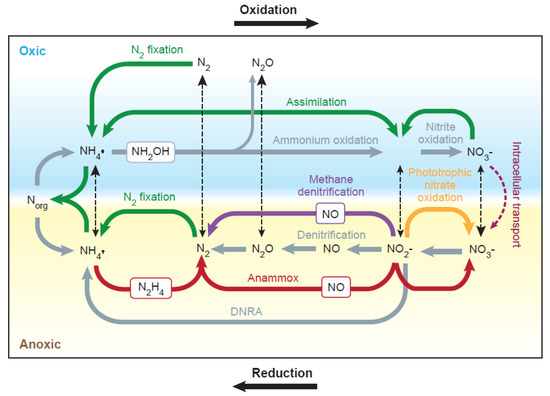
Figure 3.
The nitrogen cycle showing all oxidizing and reducing transformation pathways that occur in oxidized and anoxic environments, particularly in marine sediments and waterlogged saline soils. Ammonification (shown in gray on left side) is the microbial breakdown of organic nitrogen (NORG) into ammonium (NH4+); the reverse process is NH4+ immobilization (green arrows on left). Nitrification (gray arrows in top side) is the biological oxidation of NH4+ to NO2− followed by the oxidation of the NO2− to NO3−. Black dotted arrows depict transport processes between oxic and anoxic environments. Abbreviations: DNRA = dissimilatory nitrate reduction to ammonium; Anammox = anaerobic ammonium oxidation; N2 fixation = nitrogen fixation; NO = nitric oxide; N2O = nitrous oxide; N2 = nitrogen gas; NH2OH = hydroxylamine, an intermediate in biological nitrification; N2H4 = hydrazine, the intermediate in the anaerobic oxidation of ammonium (anammox) process. Reproduced with permission from [121].
Fungi are also involved in organic matter decomposition and in carbon and nitrogen cycling processes, especially on litter and in roots and rhizomes [122,123,124]. In mangrove soils, a vast menagerie of highly diverse bacterial, archaeal, fungal and protistan communities exist, comprised mostly of members of the phyla Proteobacteria and Bacteroidetes, Bathyarchaeota and Euryarchaeota, Ascomycota, and Sarcondina, Mastigophora, Ciliophora and Myxomycota. Interkingdom biotic factors shape both community structure and function of all four kingdoms. Aside from fully oxic and anoxic conditions, mangrove soils provide microaerophilic environments, such as in and near biogenic structures and in rhizospheres where microaerophiles carry out nitrogen reactions such as ammonium oxidation [125]. All of these microbial types and communities vary greatly in composition and function in relation to such factors as temperature, mangrove species composition, tides, pH, salinity, and soil fertility, redox status, and soil type [126,127].
Several microbial groups and transformation processes have only recently been discovered (DNRA, anammox, methane denitrification), but are often quantitatively important in N cycling. For example, a nitrite-dependent anaerobic methane-oxidizing bacterium was recently discovered in mangrove soils of the Zhangjiang estuary in China [128]. This “Candidatus Methylomirabilis oxyfera-like” bacterium is unique in linking the carbon and nitrogen cycles. It is conceivable that new microbe species and new N transformation pathways await discovery in mangrove environments.
At the ecosystem-scale, other N processes are important in mangroves, such as the exchange of dissolved and particulate nitrogen (DN, PN) between the forests and tidal waterways and with the adjacent coastal ocean, dry and wet deposition of N, groundwater inputs, sedimentation and burial, and assimilation and retention of N by flora and fauna.
3.1. Forest Soil N Transformations
3.1.1. Nitrogen Fixation
N2-fixation rates are highest on algal/bacterial crusts growing on tree stems (Table 2), although the few measurements preclude meaningful statistical analyses. One-way analysis of variance on ranks (H = 46.082; p < 0.001) followed by Dunn’s method (Q) indicate significant differences between rates measured on the soil surface and the other forest vegetation. Rates of N2-fixation on pneumatophores and prop roots are significantly greater than on the soil surface and vegetation components (Q values = 3.757–4.743; p < 0.001), except for belowground roots and rhizomes. Although few measurements have been made on vegetation and cyanobacterial mats, N2-fixing microbial communities associated with roots, stem surfaces, and cyanobacterial mats may provide significant input of N to mangrove forests.

Table 2.
Rates of nitrogen fixation (mg N m−2 d−1) at the mangrove soil surface, cyanobacteria mats, aboveground roots (pneumatophores and prop roots), belowground roots and rhizomes, litter and senescent leaves lying on the forest floor, and microbial crusts on the bark of tree stems [1,4,9,55,56,57,58,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161] and earlier references within. Rates presented on a g−1 DW/WW basis were converted to m−2 assuming 1 g matter = 1 cm−3. Ethylene rates were converted to N using the theoretical factor of 3 C2H2 molecules = 1 N molecule [162].
3.1.2. Within-Soil Transformations
Denitrification has been measured often in mangrove soils, mostly within the upper 5–20 cm (only complete (to N2 production) denitrification rates were considered, Table 3). Gross rates of soil ammonification are significantly greater than net ammonification rates (one-way ANOVA on ranks, H = 33.483; Dunn’s Q = 5.786; p < 0.001). There are no significant differences between gross and net rates of soil nitrification, although mean rates of gross nitrification are nearly three times greater than net rates (Table 3), indicating significant N immobilization. Although some studies found that anammox is a minor transformation process in mangrove soils compared to complete denitrification (references in Table 3), the available data (Table 3) show no significant differences between rates of both processes (one-way ANOVA on ranks, H = 0.788; p = 0.375). Rates of microbial N metabolism depend on many drivers, including temperature, soil fertility, microbial community structure, plant metabolism and root activities, bioturbation, intertidal position, and salinity. There is evidence that other N-transforming bacterial and archaeal groups (see Section 3) are present in mangrove soils, but no rate data are available for processes, such as methane denitrification, nitrite oxidation and phototrophic nitrate oxidation.

Table 3.
Rates of N transformation processes (mg N m−2 d−1) in mangrove soils. All rates are to soil depths of 5–20 cm. Rates presented on a g−1 DW/WW basis were converted to m−2 assuming 1 g soil = 1 cm−3. Abbreviations: DNRA= dissimilatory nitrate reduction to ammonium; anammox = anaerobic ammonium oxidation. Source: [9,15,55,56,57,58,129,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192].
3.1.3. NO, N2O and DN Fluxes from Surface Soils
Only two measurements at one site have been made of NO fluxes across the soil–air interface (Table 4); both indicate negligible release. Rates of N2O flux show a net mean flux to the atmosphere, although some measurements indicate net uptake by mangrove soils (Table 4).

Table 4.
Estimates of net NO and N2O gas and dissolved nitrogen fluxes across the mangrove soil surface. Units = mg N m−2 d−1. Negative values indicate fluxes into the soil and positive values indicate fluxes from the soil to the overlying tidal water/ atmosphere. Source: [9,164,165,167,169,176,184,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210].
Rates of DON, NO2− + NO3−, and NH4+ (Table 4) flux vary widely among forests, due mostly to differences between fluxes measured in light (clear) versus dark (opaque) chambers. Most measurements from light bottles show net uptake of solutes indicating utilization of dissolved nutrients in overlying tidal waters by microalgae, cyanobacteria, and other autotrophs on the mangrove soil surface. This fact is reflected in the mean rates of exchange being negative for all three dissolved N forms. Due to high variation, all three solutes appear to be utilized equally by benthic autotrophs.
4. Tidal Water N Processes
4.1. N2O Fluxes
Fluxes of N2O in mangrove tidal waters have been measured in more than 30 mangrove-fringed estuaries, tidal creeks, and waterways for a total of 62 measurements [211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226]. Net flux is to the atmosphere, averaging (±1 SE) 0.11 ± 0.03 mg N m−2 d−1 with a median of 0.02 mg N m−2 d−1 and ranging from net uptake (−0.06 mg N m−2 d−1) to net release (1.32 mg N m−2 d−1).
4.2. Tidal Exchange
Rates of exchange between mangrove forests and adjacent tidal waterways vary greatly within and among sites, as reflected in the wide range of estimates for NH4+, NO2− + NO3− and DON flux (Table 5). Despite the variability, net exchange is into the mangroves for all DN species based on mean rates; median rates indicate little net exchange (Table 5). The behavior of DN in transport between tidal waters and mangroves depends on a wide range of factors, including tidal prism, geomorphology, climate, seasonal weather patterns, the ratio of forest to waterway area, temperature, salinity, pH, dissolved oxygen levels, and plankton metabolism [1,3,4].

Table 5.
Rates of NH4+, DON and NO2− + NO3− exchange between mangrove forests and adjacent tidal waterways, including mangrove-fringed estuaries. Source: [1,4,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241] and references within. Import into the mangroves is shown as a negative value and export to adjacent tidal waters is a positive value. Units = mg N m−2 water d−1.
5. An Ecosystem-Level View of Mangrove Forests: A N Budget
Using the data in the preceding tables, a preliminary N budget for the world’s mangrove forests was constructed (Figure 4). The mean values used in the budget are not absolute, but the budget is an instructive research tool to pinpoint the major and minor pathways and transformations of N flow in mangrove ecosystems, and will help to identify where further research is needed.
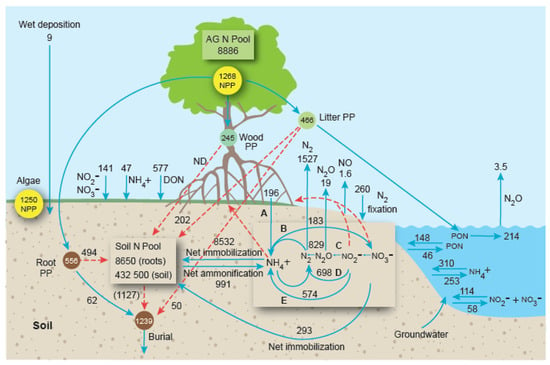
Figure 4.
Nitrogen cycling in the world’s mangrove ecosystems. Mean fluxes = Gg N a−1; mean standing stocks = Gg N. The model assumes a global mangrove area of 86,495 km2 [242]. Soil N transformations are lettered as: (A) root + rhizome N2-fixation; (B) net nitrification; (C) denitrification; (D) anammox; (E) dissimilatory nitrate reduction to ammonium. Dashed red arrows represent mean values estimated indirectly (by difference); solid blue arrows represent mean values based on empirical measurements (see text for explanation and references). The N pool (both roots and soil) in soils to a depth of 1 m is presented as a box on left in the forest floor. N transformation in soils to depths of 5–20 cm is presented as a box on the right in the forest floor. Unquantified inputs and outputs of dissolved nitrogen from land-derived groundwater and organic matter inputs from adjacent marine waters and catchments are not represented. Abbreviations: ND = no data; AG N Pool = aboveground forest N biomass pool; PP = primary production; NPP = net primary production; NO2− + NO3− = nitrite plus nitrate; NH4+ = ammonium; N2 = gaseous nitrogen; N2O = nitrous oxide; NO = nitric oxide; PON = particulate organic nitrogen.
The budget was constructed based on several assumptions: (1) the current (2014) global mangrove area is 86,495 km2 [242]; (2) although mangrove tidal creeks and waterways are only a small fraction of total mangrove area, the total global mangrove area was used to calculate all tidal exchanges because the data includes many measurements taken from mangrove-fringed estuaries that are likely in toto to be of equivalent area; (3) litter export (PON) was estimated by converting litter C export [243] to N assuming a litter C/N of 79.4 (Table 1); (4) wood, litter, root and benthic microalgal NPP were estimated by converting the C values in [243] using the C/N ratios (g/g) in the Table 1 legend and using a microalgal C/N ratio (g/g) of 12 [244]; (5) standing stocks of soil and belowground roots and aboveground forest were derived from Figure 2; (6) the input of the total belowground root + soil pool (441,150 Gg N) to N burial (1127 Gg a−1) was estimated by the difference between the mean N burial value (1239 Gg a−1) minus the inputs from root production (62 Gg a−1) and litter production (50 Gg a−1); (7) wet N deposition was estimated from the only two studies available [4,245]; and (8) the differences between gross and net ammonification and nitrification represent N immobilization.
Not included in the budget are: (1) direct inputs from groundwater and upstream; (2) marine and terrigenous particle flux and deposition at the soil surface; (3) pelagic and benthic production; (4) dry deposition; (5) consumption and assimilation by fauna and flora; (6) N2-fixation on tree stems, cyanobacterial mats, aboveground roots, senescent leaves and litter; the latter are not included because of the inability to extrapolate these rates to more than a small area given the lack of knowledge of their areal coverage in a “typical” mangrove forest, and (7) rates of soil N transformations such as ammonification and denitrification likely account for only a part of total N flux in soils as most studies measured these rates only to soil depths of 5–20 cm. C mineralization is active to a soil depth of 1 m [243], so it is likely that significant N mineralization occurs in deeper soils.
Further, mangroves differ in their location and climate, and N-cycle pathways are significantly affected by physicochemical and biological factors, such as temperature, grazing, presence of biogenic structures, tides, pH, soil nutrients, soil type and rate of N input. Such variations have not, of course, been included in the mass balance, but must be kept in mind when considering the N cycle in mangroves. Thus, individual mangrove forests and ecosystems vary significantly from the global averages used here. For example, N cycling in a tropical mangrove ecosystem that is mature and luxuriant in terms of biomass and productivity is almost certainly to be more rapid and complex that N transformations in a subtropical fringing forest that is younger, less productive and smaller in terms of biomass.
Despite these significant shortcomings, the budget does suggest rapid rates of N cycling and transformations in mangrove forests, especially in the soil. Burial equates to about 29% of total N inputs. Net tidal exchange of dissolved N is into the forest or near zero, indicating N conservation. Anammox is as important a transformation process as denitrification. Denitrification equates to 35% of total N input, within the range found in other coastal ecosystems [246]. Net immobilization (8825 Gg N a−1) is the single largest transformation process, underscoring its significance in conserving N in mangrove soils. In terms of N, benthic microalgae appear to be the most important primary producers, although the cyanobacterial mats are not represented. Net uptake of dissolved N by soils from overlying tidal waters is a significant N conservation mechanism, likely reflecting uptake by algae, cyanobacteria and other autotrophs on the soil surface. Alongi [11] measured high rates of dissolved N uptake by stems, logs, prop roots, and twigs, supporting the idea that microbial communities on these surfaces also function as an N conservation mechanism.
The budget indicates a burial efficiency (burial/total input × 100) of 29% and a mineralization efficiency (gross ammonification-net ammonification/total input × 100) of 141%, which will be more realistically lower when other inputs (e.g., N2-fixation from stems, etc.) are eventually included. In any case, it appears that soil N is very efficiently mineralized, as found in individual forests in Asia [9,57,58,136]. For instance, in Chinese mangroves (Kandelia candel), burial efficiencies ranged from 8 to 31% and mineralization efficiencies from 69 to 92% [9]. In Thai mangroves, N burial efficiencies ranged from 4 to 12% and N mineralization efficiencies from 68 to 88% [57] and in Malaysian forests [136] burial and mineralization efficiencies ranged from 10 to 29% and 67 to 81%, respectively.
A mass balance of all inputs and outputs (Table 6) indicates a net positive gain of 957.9 Gg N a−1 which equals 12% of all inputs and outputs, well within the sum of systematic errors of the many measurements made of N cycling. Considering that several important processes are not included, it is possible that N flow in mangrove ecosystems may be in approximate balance. However, considering the high rates of N2-fixation on tree stems, cyanobacterial mats, aboveground roots, senescent leaves and litter, it is likely that ecosystem N inputs would need to be revised upwards when further studies with a proper sampling design incorporating the great spatial variability of this process (coupled to an appropriate computer algorithm) would warrant their inclusion in the N budget. The budget would be unbalanced with a larger net positive gain, but consumption and assimilation of these unquantified N2-fixers by mangrove-associated fauna would perhaps redress the imbalance. Many organisms such as gastropods and other benthic invertebrates commonly dwell on tree stems, cyanobacterial mats, prop roots, pneumatophores, leaves and litter and readily consume organic particles, micro- and macroalgae, bacteria, and detritus on these surfaces [1,2]. Further, as pointed out by Reis et al. [247], N cycling in mangroves is unbalanced when forests are subjected to additional nutrients from anthropogenic sources, such as effluents from aquaculture ponds and human and animal sewage, so only relatively pristine mangroves would be expected to exhibit balanced N flow.

Table 6.
A nitrogen mass balance of the world’s mangrove ecosystems. Units = Gg N a−1. Values are from Figure 4.
At the global scale, mangroves contribute variable percentages of N to the coastal ocean. Mangrove PON export (2474 kg N km−2 a−1) equates to 95% of PON export (2612 kg N km−2 a1) from the world’s major tropical rivers. However, most rivers in low latitudes have not been measured for PON export [248] so the true contribution is probably considerably smaller. Mangrove PON export accounts for only 1.5% of the entire world’s river discharge [249]. The contributions of mangrove N2O emissions, denitrification, and burial to the global coastal ocean [250] are modest (0.4%, 0.5–2.0% and 6%, respectively), but are disproportionate relative to their small area (0.31%) [251].
6. Conclusions
N cycling in mangrove forests and associated tidal waterways is complex, with rapid turnover of low concentrations of dissolved N but slow turnover of particulate N; 96% of the latter is stored in soils with a total N stock of global mangrove forests of 52.03 Mg N ha−1 which is considerably greater than their tropical terrestrial counterparts (22.33 Mg N ha−1). The largest sources of N are nearly equal amounts of mangrove net primary production and production of benthic microalgae, the latter being a major source of nutrition for most mangrove-associated fauna. These benthic autotrophs are likely responsible for the net uptake of dissolved N species, mostly in the form of DON. Tides exchange dissolved and particulate N, with net uptake of dissolved N by mangroves and net release of particulate N as litter. N2-fixation is an underestimated source of N; very high rates of N2-fixation have been measured on tree stems, aboveground roots, and cyanobacterial mats but due to their very high patchiness and the lack of data to extrapolate beyond a small area, their contribution is unquantifiable at the whole-forest scale. Net immobilization, estimated as the difference between gross and net ammonification and nitrification, is the single largest (8825 Gg N a−1) flux, reflecting its significance in conserving N. Denitrification is the largest loss pathway, equating to 35% of total N input, which is within the range measured in other coastal ecosystems. Burial in soil equates to about 29% of total N inputs and is the second largest loss of N. Overall, total inputs (4319 Gg N a−1) slightly exceed total outputs (3361 Gg N a−1) at present, suggesting net balance of N flow in mangrove ecosystems. Globally, mangrove PON export (2474 kg N km−2 a−1) equates to 95% of PON export (2612 kg N km−2 a−1) by the world’s major tropical rivers; mangrove PON export accounts for only 1.5% of the entire world’s river discharge. The contributions of mangrove N2O emissions, denitrification, and burial to the global coastal ocean are modest (0.4%, 0.5–2.0% and 6%, respectively), but are disproportionate relative to their small area.
Despite these findings, many aspects of nitrogen flow in mangroves need to be properly quantified. These include: (1) the need to develop formulae to extrapolate N2-fixation rates on stems, aboveground roots and cyanobacterial mats to the whole-forest scale; (2) measurement of N transformation processes in soils deeper than 20 cm; (3) direct groundwater inputs and inputs from upstream and marine sources, including deposition at the soil surface; (4) the contribution of fauna in N cycling, especially benthic crabs, epiphytes and plankton; and (5) wet and dry deposition.
Funding
This research received no external funding.
Acknowledgments
I thank the three anonymous reviewers for their thoughtful comments in improving this manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Alongi, D.M. The Energetics of Mangrove Forests; Springer Science: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in mangrove ecosystems. Annu. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Cycling and global fluxes of nitrogen in mangroves. Glob. Environ. Res. 2013, 17, 173–182. [Google Scholar]
- Alongi, D.M.; Boto, K.G.; Robertson, A.I. Nitrogen and Phosphorus Cycles. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; pp. 251–292. [Google Scholar]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Boto, K.G. Nutrients and mangroves. In Pollution in Tropical Aquatic Systems; Connell, D.M., Hawker, D.W., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 129–145. [Google Scholar]
- Lovelock, C.E.; Feller, I.C.; Ball, M.C.; Engelbrecht, B.M.J.; Ewe, M.L. Differences in plant function in phosphorus- and nitrogen-limited mangrove ecosystems. New Phytol. 2006, 172, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Tsutsuki, K.; Inoue, Y.; Maie, N.; Melling, L.; Jaffé, R. Composition of dissolved organic nitrogen in rivers associated with wetlands. Sci. Total Environ. 2014, 493, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Boto, K.G.; Wellington, J.T. Soil characteristics and nutrient status in northern Australian mangrove forests. Estuaries 1984, 7, 61–69. [Google Scholar] [CrossRef]
- Alongi, D.M. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J. Mar. Res. 1996, 54, 123–148. [Google Scholar] [CrossRef]
- Naidoo, G. Effects of salinity and nitrogen on growth and water relations in the mangrove, Avicennia marina (Forsk.) Vierh. New Phytol. 1987, 107, 317–325. [Google Scholar] [CrossRef]
- Naidoo, G. Effects of nitrate, ammonium and salinity on growth of the mangrove Bruguiera gymnorrhiza (L.) Lam. Aquat. Bot. 1990, 8, 209–219. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Lee, S.C.; Chen, T.H.; Tian, G.; Chiu, C.Y. Water salinity effects on growth and nitrogen assimilation rate of mangrove (Kandelia candel) seedlings. Aquat. Bot. 2017, 137, 50–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, Y.; Jiang, Z.; Ma, X.; Inyang, A.I.; Cheng, H. Effects of salt on root aeration, nitrification, and nitrogen uptake in mangroves. Forests 2019, 10, 1131. [Google Scholar] [CrossRef]
- Lalnunzira, C.; Tripathi, S.K. Leaf and root production, decomposition and carbon and nitrogen fluxes during stand development in tropical moist forests, north-east India. Soil Res. 2018, 56, 306–317. [Google Scholar] [CrossRef]
- Leuschner, C.; Harteveld, M.; Hertel, D. Consequences of increasing forest use intensity for biomass, morphology, and growth of fine roots in a tropical moist forest on Sulawesi, Indonesia. Agric. Ecosyst. Environ. 2009, 129, 474–481. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Raich, J.W.; Russell, A.E. Fine-root mass, growth and nitrogen content for six tropical tree species. Plant Soil 2007, 290, 357–370. [Google Scholar] [CrossRef]
- Asante, W.; Jengre, N. Carbon Stocks and Soil Nutrient Dynamics in the Peat Swamp Forests of the Amanzule Wetlands and Ankobra River Basin. In USAID Integrated Coastal and Fisheries Governance Program for the Western Region of Ghana; Nature Conservation and Research Centre: Accra, Ghana, 2012; p. 45. [Google Scholar]
- Vitousek, P.M.; Sanford, R.L., Jr. Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Syst. 1986, 17, 137–167. [Google Scholar] [CrossRef]
- Martin, A.R.; Erickson, D.L.; Kress, W.J.; Thomas, S.C. Wood nitrogen concentrations in tropical trees: Phylogenetic patterns and ecological correlates. New Phytol. 2014, 204, 484–495. [Google Scholar] [CrossRef]
- Herbohn, J.L.; Congdon, R.A. Ecosystem dynamics at disturbed and undisturbed sites in North Queensland wet tropical rain forest. III. Nutrient returns to the forest floor through litterfall. J. Trop. Ecol. 1998, 14, 217–229. [Google Scholar] [CrossRef]
- Msunaga, T.; Kubota, D.; Hotta, M.; Wakatsuki, T. Nutritional characteristics of mineral elements in tree species of tropical rain forest, West Sumatra, Indonesia. Soil Sci. Plant Nutr. 1997, 43, 405–418. [Google Scholar] [CrossRef]
- Paoli, G.D.; Curran, L.M. Soil nutrients limit fine litter production and tree growth in mature lowland forest of southwestern Borneo. Ecosystems 2007, 10, 503–518. [Google Scholar] [CrossRef]
- Sugihara, S.; Shibata, M.; Mvondo Ze, A.D.; Araki, S.; Funakawa, S. Effect of vegetation on soil C, N, P and other minerals in oxisols at the forest-savanna transition zone on central Africa. Soil Sci. Plant Nutr. 2014, 60, 45–59. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Piccolo, M.C.; Townsend, A.R.; Vitousek, P.M.; Cuevas, E.; McDowell, W.; Robertson, G.P.; Santos, O.C.; Treseder, K. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry 1999, 46, 45–65. [Google Scholar] [CrossRef]
- Schurr, E.A.G.; Matson, P.A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 2001, 128, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J.; Anderson, J.; Chai, P.; Vallack, H.W. Ecological studies in four contrasting lowland rain forests in Gunung Mulu National Park, Sarawak. I. Forest environment, structure and floristics. J. Ecol. 1983, 71, 237–260. [Google Scholar] [CrossRef]
- Shibata, M.; Sugihara, S.; Mvondo-Ze, A.D.; Taraki, S.; Funakawa, S. Nitrogen flux patterns through oxisols and ultisols in tropical forests of Cameroon, central Africa. Soil Sci. Plant Nutr. 2017, 63, 306–317. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Taylor, P.G.; Porder, S.; Asner, G.P. Topographic controls on soil nitrogen availability in a lowland tropical forest. Ecology 2015, 96, 1561–1574. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Tree species control rates of free-living nitrogen fixation in a tropical rain forest. Ecology 2008, 89, 2924–2934. [Google Scholar] [CrossRef]
- Tuah, S.J.; Jamal, Y.M.; Limin, S.H. Nutritional characteristics in leaves of plants native to tropical peat swamps and heath forests of central Kalimantan, Indonesia. Tropics 2003, 12, 224–232. [Google Scholar] [CrossRef]
- Powers, J.S.; Schlesinger, W.H. Relationships among soil carbon distributions and biophysical factors at nested spatial scales in rain forests of northeastern Costa Rica. Geoderma 2002, 109, 165–190. [Google Scholar] [CrossRef]
- Werner, C.; Kiese, R.; Butterbach-Bahl, K. Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. J. Geophys. Res. 2007, 112, D03308. [Google Scholar] [CrossRef]
- Koehler, B.; Corre, M.D.; Veldkamp, E.; Wullaert, H.; Wright, S.J. Immediate and long-term nitrogen oxide emissions from tropical forest soils exposed to elevated nitrogen input. Glob. Chang. Biol. 2009, 15, 2049–2066. [Google Scholar] [CrossRef]
- Neto, E.S.; Carmo, J.B.; Keller, M.; Martins, S.C.; Alves, L.F.; Vieira, S.A.; Piccolo, M.C.; Camargo, P.; Couto, H.T.Z.; Joly, C.A.; et al. Soil-atmosphere exchange of nitrous oxide, methane and carbon dioxide in a gradient of elevation in the coastal Brazilian Atlantic forest. Biogeosciences 2011, 8, 733–742. [Google Scholar] [CrossRef]
- Palmiotto, P.A.; Davies, S.J.; Vogt, K.A.; Ashton, M.S.; Vogt, D.J.; Ashton, P.S. Soil-related habitat specialization in dipterocarp rain forest tree species in Borneo. J. Ecol. 2004, 92, 609–623. [Google Scholar] [CrossRef]
- Vance, E.D.; Nadkarni, N.M. Microbial biomass and activity in canopy organic matter and the forest floor of a tropical cloud forest. Soil Biol. Biochem. 1990, 22, 677–684. [Google Scholar] [CrossRef]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Sci 2020, 2, 67. [Google Scholar] [CrossRef]
- Naidoo, G. Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aquat. Bot. 2009, 90, 184–190. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Ruess, R.W.; Feller, I.C. Fine root respiration in the mangrove Rhizophora mangle over variation in forest stature and nutrient availability. Tree Physiol. 2006, 26, 1601–1606. [Google Scholar] [CrossRef]
- Lee, R.Y.; Porubsky, W.P.; Feller, I.C.; Mc Kee, K.L.; Joye, S.B. Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochemistry 2008, 87, 181–198. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Ittekkot, V.; Patchineelam, S.R. Biogeochemistry of mangrove soil organic matter: A comparison between Rhizophora and Avicennia soils in south-eastern Brazil. Estuar. Coast. Shelf Sci. 1995, 40, 713–720. [Google Scholar] [CrossRef]
- Chen, R.; Twilley, R.R. A simulation model of organic matter and nutrient accumulation in mangrove wetland soils. Biogeochemistry 1999, 44, 93–118. [Google Scholar] [CrossRef]
- Krauss, K.W.; Doyle, T.W.; Twilley, R.R.; Rivera-Monroy, V.H.; Sullivan, J.K. Evaluating the relative contributions of hydroperiod and soil fertility on growth of south Florida mangroves. Hydrobiologia 2006, 569, 311–324. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Riul, P.; Cifuentes-Jara, M.; Manrow-Villalobos, M.; Horta, P.A.; Simonassi, J.C.; Fonseca, A.L.; Pagliosa, P.R. Global controls on carbon storage in mangrove soils. Nat. Clim. Chan. 2018, 3, 534–538. [Google Scholar] [CrossRef]
- Bala Krishna Prasad, M.; Ramanathan, A.L. Sedimentary nutrient dynamics in a tropical estuarine mangrove ecosystem. Estuar. Coast. Shelf Sci. 2008, 80, 60–66. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Clough, B.F. Below-ground decomposition of organic matter in forests of the mangroves Rhizophora stylosa and Avicennia marina along the arid coast of Western Australia. Aquat. Bot. 2000, 68, 97–122. [Google Scholar] [CrossRef]
- Alongi, D.M.; Christoffersen, P.; Tirendi, F. The influence of microbial-nutrient relationships in tropical mangrove sediments. J. Exp. Mar. Biol. Ecol. 1993, 171, 201–223. [Google Scholar] [CrossRef]
- Alongi, D.M.; de Carvalho, N.A. The effect of small-scale logging on stand characteristics and soil biogeochemistry in mangrove forests of Timor Leste. For. Ecol. Manag. 2008, 255, 1359–1366. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Dixon, P.; Trott, L.A.; Brunskill, G.J. Mineralization of organic matter in intertidal sediments of a tropical semi-enclosed delta. Estuar. Coast. Shelf Sci. 1999, 48, 451–467. [Google Scholar] [CrossRef]
- Alongi, D.M.; Ramanathan, A.L.; Kannan, L.; Tirendi, F.; Trott, L.A.; Bala Krishna Prasad, M. Influence of human-induced disturbance on benthic microbial metabolism in the Pichavaram mangroves, Vellar-Coleroon estuarine complex, India. Mar. Biol. 2005, 147, 1033–1044. [Google Scholar] [CrossRef]
- Alongi, D.M. Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol. 1988, 15, 59–79. [Google Scholar] [CrossRef]
- Alongi, D.M.; de Carvalho, N.A.; Amaral, A.L.; da Costa, A.; Trott, L.A.; Tirendi, F. Uncoupled surface and below-ground soil respiration in mangroves: Implications for estimates of dissolved inorganic carbon export. Biogeochemistry 2012, 109, 151–162. [Google Scholar] [CrossRef]
- Alongi, D.M.; Trott, L.A.; Tirendi, F.; McKinnon, A.D.; Undu, M.C. Growth and development of mangrove forests overlying smothered coral reefs, Sulawesi and Sumatra, Indonesia. Mar. Ecol. Prog. Ser. 2008, 370, 97–109. [Google Scholar] [CrossRef][Green Version]
- Alongi, D.M.; Tirendi, F.; Trott, L.A.; Xuan, T.T. Benthic decomposition rates and pathways in plantations of the mangrove Rhizophora apiculata in the Mekong delta, Vietnam. Mar. Ecol. Prog. Ser. 2000, 194, 87–101. [Google Scholar] [CrossRef]
- Alongi, D.M.; Trott, L.A.; Wattayakorn, G.; Clough, B.F. Below-ground nitrogen cycling in relation to net canopy production in mangrove forests of southern Thailand. Mar. Biol. 2002, 140, 855–864. [Google Scholar]
- Kristensen, E.; Holmer, M.; Banta, G.T.; Jensen, M.H.; Hansen, K. Carbon, nitrogen and sulfur cycling in sediments of the Ao Nam Bor mangrove forest, Phuket, Thailand: A review. Phuket Mar. Biol. Cent. Res. Bull. 1995, 60, 37–64. [Google Scholar]
- Nguyen, H.T.; Yoneda, R.; Ninomiya, I.; Harada, K.; Van Dao, T.; Sy, T.M.; Phan, H.N. The effects of stand-age and inundation on carbon accumulation in mangrove plantation soil in Namdinh, northern Vietnam. Tropics 2004, 14, 24–34. [Google Scholar] [CrossRef][Green Version]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H.; Zhang, K.; Davis, S.E., III; Ross, M. Sediment and nutrient deposition associated with Hurricane Wilma in mangroves of the Florida Coastal Everglades. Estuar. Coast. 2010, 33, 45–58. [Google Scholar] [CrossRef]
- Garcias-Bonet, N.; Delgado-Huertas, A.; Carrillo-de-Albornoz, P.; Anton, A.; Almahasheer, H.; Marbá, N.; Hendricks, I.E.; Krause-Jensen, D.; Duarte, C.M. Carbon and nitrogen concentrations, stocks, and isotopic compositions in Red Sea seagrass and mangrove sediments. Front. Mar. Sci. 2019, 6, 267. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Twilley, R.R.; Medina, E.; Moser, E.B.; Botero, L.; Francisco, A.M.; Bullard, E. Spatial variability of soil nutrients in disturbed riverine mangrove forests at different stages of regeneration in the San Juan River estuary, Venezuela. Estuaries 2004, 27, 44–57. [Google Scholar] [CrossRef]
- Gandaseca, S.; Pazi, A.M.M.; Zulkipli, M.N.S.; Hamzah, A.H.; Zaki, P.H.; Abdu, A. Assessment of nitrogen and phosphorus in mangrove forest soil at Awat-Awat Lawas Sarawak. Am. J. Agricult. For. 2016, 4, 136–139. [Google Scholar] [CrossRef]
- Sarker, S.; Masud-Ul-Alam, M.; Hossain, M.S.; Chowdhury, S.R.; Sharifuzzaman, S.M. A review of bioturbation and sediment organic geochemistry in mangroves. Geol. J. 2020. [Google Scholar] [CrossRef]
- Sofawi, A.B.; Nazri, M.N.; Rozainah, M.Z. Nutrient availability in mangrove soil: Anthropogenic, seasonal and depth variation factors. Appl. Ecol. Environ. Res. 2017, 15, 1983–1998. [Google Scholar] [CrossRef]
- Shilla, D.J.; Shilla, D.A. Assessment of the geochemical characteristics of water and surface sediments of Rufiji mangrove forest, Tanzania. Tanzan. J. Sci. 2020, 46, 482–497. [Google Scholar]
- Gutiérrez, J.C.S.; Ponce-Palafox, J.T.; Pineda-Jaimes, N.B.; Arenas-Fuentes, V.; Arredondo-Figueroa, J.L.; Cifuentes-Lemus, J.L. Comparison of the mangrove soil with different levels of disturbance in tropical Agua Brava Lagoon, Mexican Pacific. Appl. Ecol. Environ. Res. 2016, 14, 45–57. [Google Scholar] [CrossRef]
- Ramanathan, A.L.; Datta, D.K.; Ghosh, P.; Kaushal, S.; Murtudde, R. Tracing Nitrogen and Carbon Biogeochemical Processes in the Intertidal Mangrove Ecosystem (Sundarbans) of India and Bangladesh: Implications for the Global Environmental Change; Final Report for APN Project: ARCP2012-07CMY-Ramanathan; Asia-Pacific Network for Global Change Research: Kobe, Japan, 2012. [Google Scholar]
- Scharler, U.M.; Ulanowicz, R.E.; Fogel, M.L.; Wooller, M.J.; Jacobson-Meyers, M.E.; Lovelock, C.E.; Feller, I.C.; Frischer, M.; Lee, R.; McKee, K.; et al. Variable nutrient stoichiometry (carbon:nitrogen:phosphorus) across trophic levels determines community and ecosystem properties in an oligotrophic mangrove system. Oecologia 2015, 179, 863–876. [Google Scholar] [CrossRef]
- Nirmal Kumar, I.J.; Sajish, P.R.; Nirmal Kumar, R.; Bash, G.; Shailendra, V. Nutrient dynamics in an Avicennia marina (Forsk.) Vierh., mangrove forest in Vamleshwar, Gujarat, India. Not. Sci. Biol. 2011, 3, 51–56. [Google Scholar] [CrossRef]
- Nordhaus, I.; Salewski, T.; Jennerjahn, T.C. Interspecific variations in mangrove leaf litter decomposition are related to labile nitrogenous compounds. Estuar. Coast. Shelf Sci. 2017, 192, 137–143. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Schwendenmann, L.; Lundquist, C.J. Carbon and nitrogen stocks and below-ground allometry in temperate mangroves. Front. Mar. Sci. 2016, 3, 150. [Google Scholar] [CrossRef]
- Alongi, D.M.; Clough, B.F.; Dixon, P.; Tirendi, F. Nutrient partitioning and storage in arid-zone forests of the mangroves Rhizophora stylosa and Avicennia marina. Trees 2003, 17, 51–60. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F. The composition of sedimentary organic matter in relation to the dynamic features of the mangrove-fringed coast of French Guiana. Estuar. Coast. Shelf Sci. 2003, 56, 119–130. [Google Scholar] [CrossRef]
- Enamul Hoq, M.; Islam, M.L.; Paul, H.K.; Ahmed, S.U.; Islam, M.N. Decomposition and seasonal changes in nutrient constituents in mangrove litter of Sundarbans mangrove, Bangladesh. Ind. J. Mar. Sci. 2002, 31, 130–135. [Google Scholar]
- Haryadi, J.; Basukriandi, A. The study on mangrove litter as a source of nutrients for Blanakan mangrove pond, Subang, West Java. Indo. Aquacult. J. 2013, 8, 55–64. [Google Scholar]
- Nordhaus, I.; Salewski, T.; Jennerjahn, T.C. Food preferences on mangrove crabs related to leaf nitrogen compounds in the Segara Anakan Lagoon, Java, Indonesia. J. Sea Res. 2011, 65, 414–426. [Google Scholar] [CrossRef]
- Twilley, R.R.; Lugo, A.E.; Patterson-Zucca, C. Litter production and turnover in basin mangrove forests in southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
- Wafar, S.; Untawale, A.G.; Wafar, M. Litter fall and energy flux in a mangrove ecosystem. Estuar. Coast. Shelf Sci. 1997, 44, 111–124. [Google Scholar] [CrossRef]
- Hemati, Z.; Hossian, M.; Rozainah, M.Z. Determination of carbon and nitrogen in litter fall of mangrove ecosystem in Peninsular Malaysia. Pak. J. Bot. 2017, 49, 1381–1386. [Google Scholar]
- Ye, Y.; Chen, Y.P.; Chen, G.C. Litter production and litter elemental composition in two rehabilitated Kandelia obovata mangrove forests in Jiulongjiang estuary, China. Mar. Environ. Res. 2013, 83, 63–72. [Google Scholar] [CrossRef]
- Srisunont, C.; Jaiyen, T.; Tenrung, M.; Likitchaikul, M.; Srisunont, T. Nutrient accumulation by litterfall in mangrove forest at Klong Khone, Thailand. Sci. Technol. Asia 2017, 9–18. [Google Scholar] [CrossRef]
- Adame, M.F.; Zacaria, R.M.; Fry, B.; Chong, V.C.; Then, Y.H.A.; Brown, C.J.; Lee, S.Y. Loss and recovery of carbon and nitrogen after mangrove clearing. Ocean. Coast. Manag. 2018, 161, 117–126. [Google Scholar] [CrossRef]
- Lugo, A.E.; Medina, E.; Cuevas, E.; Laboy Nieves, E.N.; Schäeffer Novelli, Y. Ecophysiology of a mangrove forest in Jobos Bay, Puerto Rico. Carrib. J. Sci. 2007, 43, 200–219. [Google Scholar] [CrossRef]
- Pinto, L. Litterfall and its element content in the Pagbilao Mangrove Forest Reserve, Philippines. Mahasagar 1992, 25, 97–104. [Google Scholar]
- Pedrosa Fragoso, C.; Bernini, E.; Ferreira Araújo, B.; Gomes de Almeida, M.; Eduardo de Rezende, C. Mercury in litterfall and sediment using elemental and isotopic composition of carbon and nitrogen in the mangrove of southeastern Brazil. Estuar. Coast. Shelf Sci. 2018, 202, 30–39. [Google Scholar] [CrossRef]
- Bunt, J.S. Studies of Mangrove Litter Fall in Tropical Australia. In Mangrove Ecosystems in Australia: Structure, Function and Management; Clough, B.F., Ed.; Australian National University Press: Canberra, Australia, 1982; pp. 221–237. [Google Scholar]
- Woodroffe, C.D.; Bardsley, K.N.; Ward, P.J.; Hanley, J.R. Production of mangrove litter in a macrotidal embayment, Darwin Harbour, N.T., Australia. Estuar. Coast. Shelf Sci. 1988, 26, 581–598. [Google Scholar] [CrossRef]
- Thongtham, N.; Kristensen, E. Carbon and nitrogen balance of leaf-eating sesarmid crabs (Neoepisesarma versicolor) offered different food sources. Estuar. Coast. Shelf Sci. 2005, 65, 213–222. [Google Scholar] [CrossRef]
- Thongtham, N.; Kristensen, E.; Puangprasan, S.-Y. Leaf removal by sesarmid crabs in Bangrong mangrove forests, Phuket, Thailand; with emphasis on the feeding ecology of Neoepisesarma versicolor. Estuar. Coast. Shelf Sci. 2008, 80, 583–590. [Google Scholar] [CrossRef]
- Feller, I.C.; McKee, K.L.; Whigham, D.F.; O’Neill, J.P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 2002, 62, 145–175. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Rajkumar, M.; Sesh Serebiah, J.; Thivakaran, G.A. Seasonal variations in physico-chemical characteristics of water, sediment and soil texture in arid zone mangroves of Kachchh-Gujarat. J. Environ. Biol. 2008, 29, 725–732. [Google Scholar]
- Nabiul Islam Khan, M.; Suwa, R.; Hagihara, A. Carbon and nitrogen pools in a mangrove stand of Kandelia obovata (S.L.) Yong: Vertical distribution in the soil-vegetation system. Wetlands Ecol. Manag. 2007, 15, 141–153. [Google Scholar] [CrossRef]
- Mantiquilla, J.A.; Salmasan, S.F.D.; Obelidhon, M.K.A.; Abad, R.G. Nutrient status of Nipa (Nypa fruticans Wurmb.) in selected areas of Mindanao, the Philippines. Banwa B 2019, 14, art012. [Google Scholar]
- Medina, E.; Francisco, M. Osmolality and δ13C of leaf tissues of mangrove species from environments of contrasting rainfall and salinity. Estuar. Coast. Shelf Sci. 1997, 45, 337–344. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Tirendi, F.; Dixon, P. Nutrient capital in different aged forests of the mangrove Rhizophora apiculata. Bot. Mar. 2004, 47, 116–124. [Google Scholar] [CrossRef]
- Tognella, M.M.P.; Soares, M.L.G.; Cuevas, E.; Medina, E. Heterogeneity of elemental composition and natural abundance of stable isotopes of C and N in soils and leaves of mangroves at their southernmost West Atlantic range. Braz. J. Biol. 2016, 76, 994–1003. [Google Scholar] [CrossRef]
- Jayasekera, R. Chemical composition of the mangrove, Rhizophora mangle L. J. Plant Physiol. 1991, 138, 119–121. [Google Scholar] [CrossRef]
- Feller, I.C.; Lovelock, C.E.; McKee, K.L. Nutrient addition differentially affects ecological processes of Avicennia germinans in nitrogen versus phosphorus limited mangrove ecosystems. Ecosystems 2007, 10, 347–359. [Google Scholar] [CrossRef]
- Telave, A.B. Ecophysiological studies on Sonneratia L. from the coast of Maharashtra, India. Ind. J. Geo-Mar. Sci. 2015, 44, 1239–1244. [Google Scholar]
- Muzuka, A.N.N.; Shunula, J.P. Stable isotope compositions of organic carbon and nitrogen of two mangrove stands along the Tanzanian coastal zone. Estuar. Coast. Shelf Sci. 2006, 66, 447–458. [Google Scholar] [CrossRef]
- Bernini, E.; Silva, M.A.B.D.; Carmo, T.M.S.D.; Cuzzuol, G.R.F. Composição química do sediment e de folhas das espécies do manguezal do estuário do Rio São Mateus, Espírito Santo, Brasil. Rev. Brasil Bot. 2006, 29, 689–699. [Google Scholar] [CrossRef]
- Medina, E.; Giarrizzo, T.; Menezes, M.; Carvalho Lira, M.; Carvalho, E.A.; Peres, A.; Silva, B.; Vilhena, R.; Reise, A.A.; Braga, F.C. Mangal communities of the “Salgado Paraense”: Ecological heterogeneity along the Bragança peninsula assessed through soil and leaf analysis. Amazonia 2001, 16, 397–416. [Google Scholar]
- Lovelock, C.E.; Feller, I.C.; McKee, K.L.; Engelbrecht, B.M.J.; Ball, M.C. The effect of nutrient enrichment on growth, photosynthesis and hydraulic conductance of dwarf mangroves in Panama. Funct. Ecol. 2004, 18, 25–33. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Liu, X.-W.; Zhang, H.; Fan, H.-Q.; Lin, G.-H. Nutrient conservation strategies of a mangrove species Rhizophora stylosa under nutrient limitation. Plant Soil 2010, 326, 469–479. [Google Scholar] [CrossRef]
- Gritcan, I.; Duxbury, M.; Leuzinger, S.; Alfaro, A.C. Leaf stable isotope and nutrient status of temperate mangroves as ecological indicators to assess anthropogenic activity and recovery from eutrophication. Front. Plant Sci. 2016, 7, 1922. [Google Scholar] [CrossRef]
- Simpson, L.T.; Lovelock, C.E.; Cherry, J.A.; Feller, I.C. Short-lived effects of nutrient enrichment on Avicennia germinans decomposition in a saltmarsh-mangrove ecotone. Estuar. Coast. Shelf Sci. 2020, 235, 106598. [Google Scholar] [CrossRef]
- Ariyanto, D.; Gunawan, H.; Puspitasari, D.; Susanti Ningsih, S.; Jayanegara, A.; Hamim, H. The differences of the element content in Rhizophora mucronata leaves from Asahan Regency, North Sumatra, Indonesia. Pol. J. Natur. Sci. 2019, 34, 481–491. [Google Scholar]
- Li, M.S. Nutrient dynamics of a Futian mangrove forest in Shenzhen, South China. Estuar. Coast. Shelf Sci. 1997, 45, 463–472. [Google Scholar] [CrossRef]
- Bernini, E.; da Silva, M.A.B.; do Carmo, T.M.S.; Cuzzuol, G.R.F. Spatial and temporal variation of the nutrients in the sediment and leaves of two Brazilian mangrove species and their role in the retention of environmental heavy metals. Braz. J. Plant Physiol. 2010, 22, 177–187. [Google Scholar] [CrossRef]
- Ahmed, A.; Ohlson, M.; Hoque, S.; Moula, M.G. Chemical composition of leaves of a mangrove tree (Sonneratia apetala Buch.Ham.) and their correlation with some soil variables. Bangladesh J. Bot. 2010, 39, 61–69. [Google Scholar] [CrossRef]
- Mfilinge, P.L.; Atta, N.; Tsuchiya, M. Nutrient dynamics and leaf litter decomposition in a subtropical mangrove forest at Oura Bay, Okinawa, Japan. Trees 2002, 16, 172–180. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.P.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Couto, E.G.; Mund, E.E.; Amorim, R.S.S.; Siqueira, A.; Tielbörger, K.; Seifan, M. Soil carbon stocks in stream-valley-ecosystems in the Brazilian Cerrado agroscape. Agric. Ecosyst. Environ. 2012, 151, 70–79. [Google Scholar] [CrossRef]
- Descloux, S.; Chanudet, V.; Poilvé, H.; Grégoire, A. Co-assessment of biomass and soil organic carbon stocks in a future reservoir area located in Southeast Asia. Environ. Monit. Assess. 2011, 173, 723–741. [Google Scholar] [CrossRef]
- Laumonier, Y.; Edin, A.; Kanninen, M.; Munandar, A.W. Landscape-scale variation in the structure and biomass of the hill dipterocarp forest of Sumatra: Implications for carbon stock assessments. For. Ecol. Manag. 2010, 259, 505–513. [Google Scholar] [CrossRef]
- Yuen, J.Q.; Ziegler, A.D.; Webb, E.L.; Ryan, C.M. Uncertainty in below-ground carbon biomass for major land covers in Southeast Asia. For. Ecol. Manag. 2013, 310, 915–926. [Google Scholar] [CrossRef]
- Powers, J.S.; Treseder, K.K.; Lerdau, M.T. Fine roots, arbuscular mycorrhizal hyphae, and soil nutrients in four neotropical rain forests: Patterns across large geographic distances. New Phytol. 2005, 165, 913–921. [Google Scholar] [CrossRef]
- Cusack, D.F.; Markesteijn, L.; Condit, R.; Lewis, O.T.; Turner, B.L. Soil carbon stocks across tropical forests of Panama regulated by base cation effects on fine roots. Biogeochemistry 2018, 137, 253–266. [Google Scholar] [CrossRef]
- Thamdrup, B. New pathways and processes in the global nitrogen cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428. [Google Scholar] [CrossRef]
- Mukherji, S.; Haldar, S.; Ghosh, A. Investigation of the Structural and Functional Microbial Diversity in Indian Mangroves. In Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer Nature: Gland, Switzerland, 2019; pp. 93–130. [Google Scholar]
- Cheung, M.K.; Wong, C.K.; Chu, K.H.; Kwan, H.S. Community structure, dynamics and interactions of bacteria, archaea and fungi in subtropical coastal wetland sediments. Sci. Rep. 2018, 8, 14397. [Google Scholar] [CrossRef] [PubMed]
- Loganathachetti, D.S.; Poosakkannu, A.; Muthuraman, S. Fungal community assemblage of different soil compartments in mangrove ecosystem. Sci. Rep. 2017, 7, 8560. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.S.; Barboza, A.D.; Keijzer, R.M.; Laanbroek, H.J. Tide as steering factor in structuring archaeal and bacterial ammonia-oxidizing communities in mangrove forest soils dominated by Avicennia germinans and Rhizophora mangle. Microb. Ecol. 2018, 75, 997–1008. [Google Scholar] [CrossRef]
- Li, R.; Tong, T.; Wu, S.; Chai, M.; Xie, S. Multiple factors govern the biogeographic distribution of archaeal community in mangrove across China. Estuar. Coast. Shelf Sci. 2019, 231, 106414. [Google Scholar] [CrossRef]
- Bai, S.; Li, J.; He, Z.; Van Nostrand, J.D.; Tian, Y.; Lin, G.; Zhou, J.; Zheng, T. GeoChip-based analysis of the functional gene diversity and metabolic potential of soil microbial communities in mangroves. Appl. Microbiol. Biotechnol. 2013, 97, 7035–7048. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Y.; Lin, L.; Lin, X.; Hetharua, B.; Zhao, W.; Zhou, M.; Zhan, Q.; Xu, H.; Zheng, T.; et al. Molecular and stable isotope evidence for the occurrence of nitrite-dependent anaerobic methane-oxidizing bacteria in the mangrove sediment of Zhangjiang estuary, China. Appl. Microbiol. Biotechnol. 2018, 102, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Jensen, M.H.; Banta, G.T.; Hansen, K.; Holmer, M.; King, G.M. Transformation and transport of inorganic nitrogen in sediments of a southeast Asian mangrove forest. Aquat. Microb. Ecol. 1998, 15, 165–175. [Google Scholar] [CrossRef]
- Viner, A.B. The Status and Transport of Nutrients through the Purari River (Papua New Guinea). In Purari River Hydroelectric Scheme Environmental Studies; Publication of the Environment and Conservation: Waigani, Papua New Guinea, 1979; Volume 9, pp. 1–28. [Google Scholar]
- Kyaruzi, J.J.; Kyewalyanga, M.S.; Muruke, M.H.S. Cyanobacteria composition and impact of seasonality on their in situ nitrogen fixation rate in a mangrove ecosystem adjacent to Zanzibar town. West. Indian Ocean J. Mar. Sci. 2003, 2, 35–44. [Google Scholar] [CrossRef]
- van der Valk, A.G.; Attiwill, P.M. Acetylene reduction in an Avicennia marina community in southern Australia. Aust. J. Bot. 1984, 32, 157–164. [Google Scholar]
- Lee, R.Y.; Joye, S.B. Seasonal patterns of nitrogen fixation and denitrification in oceanic mangrove habitats. Mar. Ecol. Prog. Ser. 2006, 307, 127–141. [Google Scholar] [CrossRef]
- Joye, S.B.; Lee, R.Y. Benthic microbial mats: Important sources of fixed nitrogen and carbon to the Twin Cays, Belize ecosystem. Atoll Res. Bull. 2004, 528, 1–26. [Google Scholar] [CrossRef]
- Whigham, D.F.; Verhoeven, J.T.A.; Samarkin, V.; Megonigal, P.J. Responses of Avicennia germinans (Black Mangrove) and the soil microbial community to nitrogen addition in a hypersaline wetland. Estuar. Coast. 2009, 32, 926–936. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Chong, V.C.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Brunskill, G.J. Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Mar. Geol. 2004, 208, 383–402. [Google Scholar] [CrossRef]
- Twilley, R.R.; Rivera-Monroy, V.H.; Rovai, A.S.; Castañeda-Moya, E.; Davis, S. Mangrove Biogeochemistry at Local and Global Scales Using Ecogeomorphic Approaches. In Coastal Wetlands: An Integrated Ecosystem Approach; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 717–785. [Google Scholar]
- Morell, J.M.; Corredor, J.E. Sediment trapping in a mangrove lagoon. Estuar. Coast. Shelf Sci. 1993, 37, 203–212. [Google Scholar] [CrossRef]
- Das, S.; Ganguly, D.; Mukherjee, A.; Chakraborty, S.; De, T.K. Exploration of N2 fixation and denitrification processes in the Sundarbans mangrove ecosystem, India. Ind. J. Geo-Mar. Sci. 2020, 49, 740–747. [Google Scholar]
- Pelegri, S.P.; Rivera-Monroy, V.H.; Twilley, R.R. A comparison of nitrogen fixation (acetylene reduction) among three species of mangrove litter, sediments, and pneumatophores in south Florida, USA. Hydrobiologia 1997, 356, 73–79. [Google Scholar] [CrossRef]
- Shiau, Y.-J.; Lin, M.-F.; Tan, C.-C.; Tian, G.; Chiu, C.-Y. Assessing N2 fixation in estuarine mangrove soils. Estuar. Coast. Shelf Sci. 2017, 189, 84–89. [Google Scholar] [CrossRef]
- Romero, I.C.; Jacobson, M.; Fuhrman, J.A.; Fogel, M.; Capone, D.G. Long-term nitrogen and phosphorus fertilization effects on N2 fixation rates and nifH gene community patterns in mangrove sediments. Mar. Ecol. 2012, 33, 117–127. [Google Scholar] [CrossRef]
- Vovides, A.G.; Lόpez-Portillo, J.; Bashan, Y. N2-fixation along a gradient of long-term disturbance in tropical mangroves bordering the Gulf of Mexico. Biol. Fertil. Soils 2011, 47, 567–576. [Google Scholar] [CrossRef]
- Zuberer, D.A.; Silver, W.S. Biological nitrogen fixation (acetylene reduction) associated with Florida mangroves. Appl. Environ. Microbiol. 1978, 35, 567–575. [Google Scholar] [CrossRef]
- Sheridan, R.P. Epicaulous, nitrogen-fixing microepiphytes in a tropical mangal community, Guadeloupe, French West Indies. Biotropica 1991, 23, 530–541. [Google Scholar] [CrossRef]
- Mann, F.D.; Steinke, T.D. Biological nitrogen fixation (acetylene reduction) associated with blue-green algal (cyanobacterial) communities in the Beachwood Mangrove Nature Reserve. I. The effect of environmental factors on acetylene reduction activity. S. Afr. Tydskr. Plantk. 1989, 55, 438–446. [Google Scholar] [CrossRef]
- Mann, F.D.; Steinke, T.D. Biological nitrogen fixation (acetylene reduction) associated with blue-green algal (cyanobacterial) communities in the Beachwood Mangrove Nature Reserve. II. Seasonal variation in acetylene reduction activity. S. Afr. J. Bot. 1993, 59, 1–8. [Google Scholar]
- Pelagri, S.P.; Twilley, R.R. Heterotrophic nitrogen fixation (acetylene reduction) during leaf-litter decomposition of two mangrove species from south Florida, USA. Mar. Biol. 1998, 131, 53–61. [Google Scholar]
- Sengupta, A.; Chaudhuri, S. Ecology of heterotrophic dinitrogen fixation in the rhizosphere of mangrove plant community at the Ganges River estuary in India. Oecologia 1991, 87, 560–564. [Google Scholar] [CrossRef]
- Vovides, A.G.; Bashan, Y.; Lόpez-Portillo, J.; Guevara, R. Nitrogen fixation in preserved, reforested, naturally regenerated and impaired mangroves as an indicator of functional restoration in mangroves in an arid region of Mexico. Restor. Ecol. 2010, 19, 236–244. [Google Scholar] [CrossRef]
- Sheridan, R.P. Nitrogen fixation by epicaulous cyanobacteria in the Pointe de la Saline mangrove community, Guadeloupe, French West Indies. Biotropica 1992, 24, 571–574. [Google Scholar] [CrossRef]
- Lugomela, C.; Bergman, B. Biological N2-fixation on mangrove pneumatophores: Preliminary observations and perspectives. Ambio 2002, 31, 612–623. [Google Scholar] [CrossRef]
- Potts, M.; Whitton, B.A. Nitrogen fixation by blue-green algal communities in the intertidal zone of the lagoon of Aldabra Atoll. Oecologia 1977, 27, 275–283. [Google Scholar] [CrossRef]
- Potts, M. Nitrogen fixation (acetylene reduction) associated with communities of heterocystous and non-heterocystous blue-green algae in mangrove forests of Sinai. Oecologia 1979, 39, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Boto, K.G.; Robertson, A.I. The relationship between nitrogen fixation and tidal exports of nitrogen in a tropical mangrove system. Estuar. Coast. Shelf Sci. 1990, 31, 531–540. [Google Scholar] [CrossRef]
- Woitchik, A.F.; Ohowa, B.; Kazungu, J.M.; Rao, R.G.; Goeyens, L.; Dehairs, F. Nitrogen enrichment during decomposition of mangrove leaf litter in an east African coastal lagoon (Kenya): Relative importance of biological nitrogen fixation. Biogeochemistry 1997, 39, 15–35. [Google Scholar] [CrossRef]
- Uchino, F.; Hambali, G.G.; Yatazawa, M. Nitrogen-fixing bacteria from warty lenticellate bark of a mangrove tree, Bruguiera gymnorrhiza (L.) Lamk. Appl. Environ. Microbiol. 1984, 47, 44–48. [Google Scholar] [CrossRef]
- Zuberer, D.A.; Silver, W.S. N2-fixation (acetylene reduction) and the microbial colonization of mangrove roots. New Phytol. 1979, 82, 467–471. [Google Scholar] [CrossRef]
- Toledo, G.; Bashan, Y.; Soeldner, A. Cyanobacteria and black mangroves in northwestern Mexico: Colonization, and diurnal and seasonal nitrogen fixation on aerial roots. Can. J. Microbiol. 1995, 41, 999–1011. [Google Scholar] [CrossRef]
- Gotto, J.W.; Taylor, B.F. N2 fixation associated with decaying leaves of the red mangrove (Rhizophora mangle). Appl. Environ. Microbiol. 1976, 31, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Qashqari, M.S.; Garcias-Bonet, N.; Fusi, M.; Booth, J.M.; Daffonchio, D.; Duarte, C.M. High temperature and crab density reduce atmospheric nitrogen fixation in Red Sea mangrove sediments. Estuar. Coast. Shelf Sci. 2020, 232, 106487. [Google Scholar] [CrossRef]
- Capone, D.G. Determination of Nitrogenase Activity in Aquatic Samples Using the Acetylene Reduction Procedure. In Aquatic Microbial Ecology; Kemp, P.F., Sherr, B.F., Sherr, E.B., Cole, J.J., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 621–632. [Google Scholar]
- Wang, H.-T.; Su, J.-Q.; Zheng, T.-L.; Yang, X.-R. Impacts of vegetation, tidal process, and depth on the activities, abundances, and community compositions of denitrifiers in mangrove sediment. Appl. Microbiol. Biotechnol. 2014, 98, 9375–9387. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.O.; Loka Bharathi, P.A. Denitrification: An important pathway for nitrous oxide production in tropical mangrove sediments (Goa, India). J. Environ. Qual. 2010, 39, 1507–1516. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Loka Bharathi, P.A. Nitrate levels modulate denitrification activity in tropical mangrove sediments (Goa, India). Environ. Monit. Assess. 2011, 173, 117–125. [Google Scholar] [CrossRef]
- Kristensen, E.; Mangion, P.; Tang, M.; Flindt, M.R.; Holmer, M.; Ulomi, S. Microbial carbon oxidation rates and pathways in sediments of two Tanzanian mangrove forests. Biogeochemistry 2011, 103, 143–158. [Google Scholar] [CrossRef]
- Gao, G.-F.; Li, P.-F.; Zhong, J.-X.; Shen, Z.-J.; Chen, J.; Li, Y.-T.; Isabew, A.; Zhu, X.-Y.; Ding, Q.-S.; Zhang, S.; et al. Spartina alterniflora invasion alters soil bacterial communities and enhances soil N2O emissions by stimulating soil denitrification in mangrove wetland. Sci. Total Environ. 2019, 653, 231–240. [Google Scholar] [CrossRef]
- Pérez-Villalona, H.; Cornwell, J.C.; Ortiz-Zayas, J.R.; Cuevas, E. Sediment denitrification and nutrient fluxes in the San José Lagoon, a tropical lagoon in the highly urbanized San Juan Bay estuary, Puerto Rico. Estuar. Coast. 2015, 38, 2259–2278. [Google Scholar] [CrossRef]
- Senthilkumar, B. Biogeochemical and Biophysical Aspects of Pichavarum Mangrove Ecosystem, South India. Ph.D. Thesis, Anna University, Chennai, India, 2007. [Google Scholar]
- Rivera-Monroy, V.H.; Twilley, R.R.; Boustany, R.G.; Day, J.W.; Vera-Herrera, F.; del Carmen Ramirez, M. Direct denitrification in mangrove sediments in Terminos Lagoon, Mexico. Mar. Ecol. Prog. Ser. 1995, 126, 97–109. [Google Scholar] [CrossRef]
- Guan, Q.S.; Cao, W.Z.; Wang, M.; Wu, G.J.; Wang, F.F.; Jiang, C.; Tao, Y.R.; Gao, Y. Nitrogen loss through anaerobic ammonium oxidation coupled with iron reduction in a mangrove wetland. Eur. J. Soil Sci. 2018, 69, 732–741. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, P.; Lin, X.; Lin, L.; Hetharua, B.; Zhang, Y.; Tian, Y. Nitrogen loss by anaerobic ammonium oxidation in a mangrove wetland of the Zhangjiang estuary, China. Sci. Total Environ. 2020, 698, 134291. [Google Scholar] [CrossRef]
- Reis, C.R.G.; Nardoto, G.B.; Rochelle, A.L.C.; Vieira, S.A.; Oliveira, R.S. Nitrogen dynamics in subtropical fringe and basin mangrove forests inferred from stable isotopes. Oecologia 2017, 183, 841–848. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.Ø.; Holmboe, N.; Holmer, M.; Thongtham, N. Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquat. Microb. Ecol. 2000, 22, 199–213. [Google Scholar] [CrossRef]
- Nedwell, D.B.; Blackburn, T.H.; Wiebe, W.J. Dynamic nature of the turnover of organic carbon, nitrogen and sulphur in the sediments of a Jamaican mangrove forest. Mar. Ecol. Prog. Ser. 1994, 110, 223–231. [Google Scholar] [CrossRef]
- Cao, W.; Guan, Q.; Li, Y.; Wang, M.; Liu, B. The contribution of denitrification and anaerobic ammonium oxidation to N2 production in mangrove sediments of southeast China. J. Soils Sediments 2017, 17, 1767–1776. [Google Scholar] [CrossRef]
- Krishnan, K.P.; Loka Bharathi, P.A. Organic carbon and iron modulate nitrification rates in mangrove swamps of Goa, south west coast of India. Estuar. Coast. Shelf Sci. 2009, 84, 419–426. [Google Scholar] [CrossRef]
- Dunn, R.J.K.; Welsh, D.T.; Jordan, M.A.; Waltham, N.J.; Lemckert, C.J.; Teasdale, P.R. Benthic metabolism and nitrogen dynamics in a sub-tropical coastal lagoon: Microphytobenthos stimulate nitrification and nitrate reduction through photosynthetic oxygen evolution. Estuar. Coast. Shelf Sci. 2012, 113, 272–282. [Google Scholar] [CrossRef]
- Xiao, K.; Wu, J.; Li, H.; Hong, Y.; Wilson, A.M.; Jiao, J.J.; Shananan, M. Nitrogen fate in a subtropical mangrove swamp: Potential association with seawater-groundwater exchange. Sci. Total Environ. 2018, 635, 586–597. [Google Scholar] [CrossRef]
- Amano, T.; Yoshinaga, I.; Yamagishi, T.; Van Thuoc, C.; The Thu, P.; Ueda, S.; Kato, K.; Sako, Y.; Suwa, Y. Contribution of anammox bacteria to benthic nitrogen cycling in a mangrove forest and shrimp ponds, Haiphong, Vietnam. Microb. Environ. 2011, 26, 1011040240. [Google Scholar] [CrossRef]
- Purvaja, R.; Ramesh, R.; Ray, A.K.; Rixen, T. Nitrogen cycling: A review of the processes, transformations and fluxes in coastal ecosystems. Curr. Sci. 2008, 94, 1419–1438. [Google Scholar]
- Luvizotto, D.M.; Araujo, J.E.; de Cássia, P.; Silva, M.; Dias, A.C.F.; Kraft, B.; Tegetmeye, H.; Strous, M.; Andreote, F.D. The rates and players of denitrification, dissimilatory nitrate reduction to ammonia (DNRA) and anaerobic ammonia oxidation (anammox) in mangrove soils. An. Acad. Bras. Cienc. 2019, 91, e20180373. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Sobey, M.N.; Smith, C.J.; Rusmana, I.; Phillips, W.; Stott, A.; Osborn, A.M.; Nedwell, D.B. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol. Oceangr. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Michotey, V.D.; Guasco, S.; Bonin, P.C.; Loka Bharathi, P.A. Denitrification prevails over anammox in tropical mangrove sediments. Mar. Environ. Res. 2012, 74, 9–19. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, L.; Zhang, Z.; Chen, F.; Gao, D.; Yin, G.; Han, P.; Dong, H.; Liang, X.; Yang, Y.; et al. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in sediments of the Indus estuary. Estuar. Coast. Shelf Sci. 2020, 243, 106925. [Google Scholar] [CrossRef]
- Meyer, R.L.; Risgaard-Petersen, N.; Allen, D.E. Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Appl. Environ. Microbiol. 2005, 71, 6142–6149. [Google Scholar] [CrossRef]
- Cao, W.; Yang, J.; Li, Y.; Liu, B.; Wang, F.; Chang, C. Dissimilatory nitrate reduction to ammonium conserves nitrogen in anthropogenically affected subtropical mangrove sediments in southeast China. Mar. Pollut. Bull. 2016, 110, 155–161. [Google Scholar] [CrossRef]
- Molnar, N.; Welsh, D.T.; Marchand, C.; Deborde, J.; Meziane, T. Impacts of shrimp farm effluent on water quality, benthic metabolism and N-dynamics in a mangrove forest (New Caledonia). Estuar. Coast. Shelf Sci. 2013, 117, 12–21. [Google Scholar] [CrossRef]
- Dunn, R.J.K.; Welsh, D.T.; Jordan, M.A.; Teasdale, P.R.; Lemckert, C.J. Influence of natural amphipod (Victoriopisa australiensis) (Chilton, 1923) population densities on benthic metabolism, nutrient fluxes, denitrification and DNRA in sub-tropical estuarine sediment. Hydrobiologia 2009, 628, 95–109. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Bonin, P.C.; Michotey, V.D.; Garcia, N.; Loka Bharathi, P.A. Nitrogen-limited mangrove ecosystems conserve N through dissimilatory nitrate reduction to ammonium. Sci. Rep. 2012, 2, 419. [Google Scholar] [CrossRef]
- Iizumi, H. Soil nutrient dynamics. In Workshop on Mangrove Ecosystem Dynamics; Cragg, S., Polunin, N., Eds.; UNESCO: New Delhi, India, 1986; pp. 171–180. [Google Scholar]
- Chen, R.; Twilley, R.R. Patterns of mangrove forest structure and soil nutrient dynamics along the Shark River estuary, Florida. Estuaries 1999, 22, 955–970. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.-H.; Xiao, Q.; Yang, Z.-H.; Huang, S.-K.; Wang, J.; Wu, Y.-G.; Dong, X.-J.; Pei, Z.-P.; Zheng, H.-L. Diurnal variation in nitric oxide emission flux from a mangrove wetland in Zhangjiang River estuary, China. Estuar. Coast. Shelf Sci. 2010, 90, 212–220. [Google Scholar] [CrossRef]
- Castillo, J.A.A.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G., III. Soil greenhouse gas fluxes in tropical mangrove forests and in land uses on deforested mangrove lands. Catena 2017, 159, 60–69. [Google Scholar] [CrossRef]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef]
- Padhy, S.R.; Bhattacharya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuations in three mode of greenhouse gas emission in relation to soil labile carbon pools in degraded mangrove, Sundarban, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, B.; Yu, D.; Ye, Y.; Tam, N.F.Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Chauhan, R.; Datta, A.; Ramanathan, A.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Konnerup, D.; Betancourt-Portela, J.M.; Villamil, C.; Parra, J.P. Nitrous oxide and methane emissions from the restored mangrove ecosystem of the Ciénaga de Santa Marta, Colombia. Estuar. Coast. Shelf Sci. 2014, 140, 43–51. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Artur, A.G.; Taniguchi, C.A.K.; da Silva, M.R.S.; do Nascimento, J.C.; Nόbrega, G.N.; Otero, X.L.; Ferreira, T.O. Hidden contribution of shrimp farming effluents to greenhouse gas emissions from mangrove soils. Estuar. Coast. Shelf Sci. 2019, 221, 8–14. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Wang, H.; Liao, G.; D’Souza, M.; Yu, X.; Yang, J.; Yang, X.; Zheng, T. Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Ulumuddin, Y.I.; Pramudji, S.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Ramanathan, A.L.; Adhya, T.K. Assessment of methane and nitrous oxide flux from mangroves along the east coast of India. Geofluids 2008, 8, 321–332. [Google Scholar] [CrossRef]
- Bauza, J.F.; Morell, J.M.; Corredor, J.E. Biogeochemistry of nitrous oxide production in the red mangrove (Rhizophora mangle) forest sediments. Estuar. Coast. Shelf Sci. 2002, 55, 697–704. [Google Scholar] [CrossRef]
- Allen, D.E.; Dalal, R.C.; Rennenberg, H.; Meyer, R.L.; Reeves, S.; Schmidt, S. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Muñoz-Hincapié, M.; Morell, J.M.; Corredor, J.E. Increase of nitrous oxide flux to the atmosphere upon nitrogen addition to red mangroves sediments. Mar. Pollut. Bull. 2002, 44, 992–996. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A.; Brown, B. High greenhouse gas emissions mitigation benefits from mangrove rehabilitation in Sulawesi, Indonesia. Ecosyst. Serv. 2019, 40, 101035. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Ou, D.; Tam, N.F.T.Y.; Chen, S.; Zhang, Q.; Chen, B.; Ye, Y. Increased nitrous oxide emissions from intertidal soil receiving wastewater from dredging shrimp pond sediments. Environ. Res. Lett. 2020, 15, 094015. [Google Scholar] [CrossRef]
- Krithika, K.; Purjava, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224. [Google Scholar]
- Müller, D.; Bange, H.W.; Warneke, T.; Rixen, T.; Müller, M.; Mujahid, A.; Notholt, J. Nitrous oxide and methane in two tropical estuaries in a peat-dominated region of northwestern Borneo. Biogeosciences 2016, 13, 2415–2428. [Google Scholar] [CrossRef]
- Bange, H.W.; Sim, C.H.; Bastian, D.; Kallert, J.; Kock, A.; Mujahid, A.; Müller, M. Nitrous oxide (N2O) and methane (CH4) in rivers and estuaries of northwestern Borneo. Biogeosciences 2019, 16, 4321–4335. [Google Scholar] [CrossRef]
- Barnes, J.; Ramesh, R.; Purvaja, R.; Rajkumar, A.N.; Sentil Kumar, B.; Krithika, K.; Ravichandran, K.; Uher, G.; Upstill-Goddard, R. Tidal dynamics and rainfall control N2O and CH4 emissions from a pristine mangrove creek. Geophys. Res. Lett. 2006, 33, L15405. [Google Scholar] [CrossRef]
- Maher, D.T.; Sippo, J.Z.; Tait, D.R.; Holloway, C.; Santos, I.R. Pristine mangrove creek waters are a sink of nitrous oxide. Sci. Rep. 2016, 6, 25701. [Google Scholar] [CrossRef] [PubMed]
- Divia, J.I.; Neetha, V.; Hariharan, G.; Kakolee, B.; Purvaja, V.; Ramesh, R. N2O flux from South Andaman mangroves and surrounding creek waters. Int. J. Ocean. Oceanogr. 2013, 7, 73–82. [Google Scholar]
- Murray, R.; Erler, D.; Rosentreter, J.; Maher, D.; Eyre, B. A seasonal source and sink of nitrous oxide in mangroves: Insights from concentration, isotope, and isotopomer measurements. Geochim. Cosmochim. Acta 2018, 238, 169–192. [Google Scholar] [CrossRef]
- Reading, M.J.; Santos, I.R.; Maher, D.T.; Jeffrey, L.C.; Tait, D.R. Shifting nitrous oxide source/sink behaviour in a subtropical estuary revealed by automated time series observations. Estuar. Coast. Shelf Sci. 2017, 194, 66–76. [Google Scholar] [CrossRef]
- Sturm, K.; werner, U.; Grinham, A.; Yuan, Z. tidal variability in methane and nitrous oxide emissions along a subtropical estuarine gradient. Estuar. Coast. Shelf Sci. 2017, 192, 159–169. [Google Scholar] [CrossRef]
- Rao, G.D.; Sarma, V.V.S.S. Contribution of N2O emissions to the atmosphere from Indian monsoonal estuaries. Tellus B 2013, 65, 19660. [Google Scholar] [CrossRef]
- Murray, R.; Erler, D.; Rosentreter, J.; Wells, N.; Eyre, B. Seasonal and spatial controls on N2O concentrations and emissions in low-nitrogen estuaries: Evidence from three tropical systems. Mar. Chem. 2020, 221, 103779. [Google Scholar] [CrossRef]
- Chen, N.; Wu, J.; Zhou, X.; Chen, Z.; Lu, T. Riverine N2O production, emissions and export from a region dominated by agriculture in Southeast Asia (Jiulong River). Agric. Ecosyst. Environ. 2015, 208, 37–47. [Google Scholar] [CrossRef]
- Lin, H.; Dai, M.; Kao, S.J.; Wang, L.; Roberts, E.; Yang, J.Y.T.; Huang, T.; He, B. Spatiotemporal variability of nitrous oxide in a large eutrophic estuarine system: The Pearl River estuary, China. Mar. Chem. 2016, 182, 14–24. [Google Scholar] [CrossRef]
- Liu, X.L.; Bai, L.; Wang, Z.L.; Li, J.; Yue, F.J.; Li, S.L. Nitrous oxide emissions from river network with variable nitrogen loading in Tianjin, China. J. Geochem. Expl. 2015, 157, 153–161. [Google Scholar] [CrossRef]
- Wells, N.S.; Maher, D.T.; Erlet, D.V.; Hipsey, M.; Rosentreter, J.A.; Eyre, B.D. Estuaries as sources and sinks of N2O across a land-use gradient in subtropical Australia. Glob. Biogeochem. Cycle. 2018, 32, 877–894. [Google Scholar] [CrossRef]
- Manjrekar, S.; Uskaikar, H.; Morajkar, S. Seasonal production of nitrous oxide in a tropical estuary off western India. Reg. Stud. Mar. Sci. 2020, 39, 101418. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Johnstone, R.W. Porewater profiles and nutrient sediment-water exchange in a tropical mangrove waterway, Mapopwe Creek, Chwaka Bay, Zanzibar. Afr. J. Ecol. 2002, 40, 172–178. [Google Scholar] [CrossRef]
- Jiang, S.; Müller, M.; Jin, J.; Wy, Y.; Zhu, K.; Zhang, G.; Mujahid, A.; Rixen, T.; Fakharuddin Muhamad, M.; Sien Aun Sia, E.; et al. Dissolved inorganic nitrogen in a tropical estuary in Malaysia: Transport and transformation. Biogeosciences 2019, 16, 2821–2836. [Google Scholar] [CrossRef]
- Terada, K.; Koibuchi, Y.; Isobe, M. Rainfall effect on sediment and nutrient fluxes in a small mangrove river, Okinawa, Japan. J. Mar. Res. 2017, 75, 1–17. [Google Scholar] [CrossRef]
- Davis, S.E., III; Lirman, D.; Wozniak, J.R. Nitrogen and Phosphorus Exchange among Tropical Coastal Ecosystems. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Amsterdam, The Netherlands, 2009; pp. 9–43. [Google Scholar]
- Davis, S.E., III; Childers, D.L.; Day, J.W., Jr.; Rudnick, D.T.; Sklar, F.H. Wetland-water column exchanges of carbon, nitrogen, and phosphorus in a Southern Everglades dwarf mangrove. Estuaries 2001, 24, 610–622. [Google Scholar] [CrossRef]
- Boto, K.G.; Bunt, J.S. Tidal export of particulate organic matter from a northern Australian mangrove system. Estuar. Coast. Shelf Sci. 1981, 13, 247–255. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Day, J.W.; Twilley, R.R.; Vera-Herrera, F.; Coronado-Molina, C. Flux of nitrogen and sediment in a fringe mangrove forest in Terminos Lagoon, Mexico. Estuar. Coast. Shelf Sci. 1995, 40, 139–160. [Google Scholar] [CrossRef]
- Davis, S.E., III; Childers, D.L.; Day, J.W., Jr.; Rudnick, D.T.; Sklar, F.H. Nutrient dynamics in vegetated and unvegetated areas of a Southern Everglades mangrove creek. Estuar. Coast. Shelf Sci. 2001, 52, 753–768. [Google Scholar] [CrossRef]
- Barboza, C.D.N.; Paes, E.T.; de Andrade Jandre, K.; Marques, A.N., Jr. Concentrations and fluxes of nutrients and suspended organic matter in a tropical estuarine system: The Tinharé-Boipeba Islands Archipelago (Baixo Sul Baiano, Brazil). J. Coast. Res. 2014, 30, 1197–1209. [Google Scholar] [CrossRef]
- Tay, H.W.; Bryan, K.R.; Pilditch, C.A.; Park, S.; Hamilton, D.P. Variations in nutrient concentrations at different time scales in two shallow tidally dominated estuaries. Mar. Freshw. Res. 2012, 63, 95–109. [Google Scholar] [CrossRef]
- Valiela, I.; Elmstrom, E.; Lloret, J.; Stone, T.; Camilli, L. Tropical land-sea couplings: Role of watershed deforestation, mangrove estuary processing, and marine inputs on N fluxes in coastal Pacific Panama. Sci. Total Environ. 2018, 630, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Monroy, V.H.; Twilley, R.R.; Davis, S.E., III; Childers, D.L.; Simard, M.; Chambers, R.; Jaffe, R.; Boyer, J.N.; Rudnick, D.T.; Zhang, K.; et al. The role of the Everglades mangrove ecotone region (EMER) in regulating nutrient cycling and wetland productivity in South Florida. Crit. Rev. Environ. Sci. Technol. 2011, 41 (Suppl. S1), 633–669. [Google Scholar] [CrossRef]
- Ray, R.; Majumder, N.; Das, S.; Chowdhury, C.; Kumar Jana, T. Biogeochemical cycle of nitrogen in a tropical mangrove ecosystem, east coast of India. Mar. Chem. 2014, 167, 33–43. [Google Scholar] [CrossRef]
- Adame, M.F.; Virdis, B.; Lovelock, C.E. Effect of geomorphological setting and rainfall on nutrient exchange in mangroves during tidal inundation. Mar. Freshw. Res. 2010, 61, 1197–1206. [Google Scholar] [CrossRef]
- Adame, M.F.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century. Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling in the world’s mangrove ecosystems revisited: Significance of non-steady state diagenesis and subsurface linkages between the forest floor and the coastal ocean. Forests 2020, 11, 977. [Google Scholar] [CrossRef]
- Hillebrand, H.; Sommer, U. The nutrient stoichiometry of benthic microalgal growth: Redfield proportions are optimal. Limnol. Oceangr. 1999, 44, 440–446. [Google Scholar] [CrossRef]
- Cerόn, R.M.; Cerόn, J.G.; Muriel, M.; Anguebes, F.; Ramirez, M.; Zavala, J.; Carballo, C.; Escoffie, R.C. Spatial and Temporal Distribution of Throughfall Deposition of Nitrogen and Sulfur in the Mangrove Forests Associated with Terminos Lagoon. In Current Air Quality Issues; Nejadkoorki, F., Ed.; Intech Open Ltd: London, UK, 2015; pp. 147–164. [Google Scholar]
- Seitzinger, S.; Harrison, J.A.; Böhlke, J.K.; Bouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Van Drecht, G. Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef]
- Reis, C.R.; Nardoto, G.B.; Oliveira, R.S. Global overview on nitrogen dynamics in mangroves and consequences of increasing nitrogen availability for these systems. Plant Soil 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Alvarez-Cobelas, M.; Angeler, D.G.; Sánchez-Carrillo, S. Export of nitrogen from catchments: A worldwide analysis. Environ. Pollut. 2008, 156, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Beusen, A.H.W.; Bouwman, A.F.; Van Beck, L.P.H.; Mogollόn, J.M.; Middelburg, J.J. Global riverine N and P transport to ocean increased during the 20th century despite increased retention along the aquatic continuum. Biogeosciences 2016, 13, 2441–2451. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon balance in salt marsh and mangrove ecosystems: A global synthesis. J. Mar. Sci. Eng. 2020, 8, 767. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).