Influence of Mixed Acids on Coal Fractal Characteristics and Permeability
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Experimental Steps
2.2.1. Low-Temperature N2 Adsorption on Coal Samples
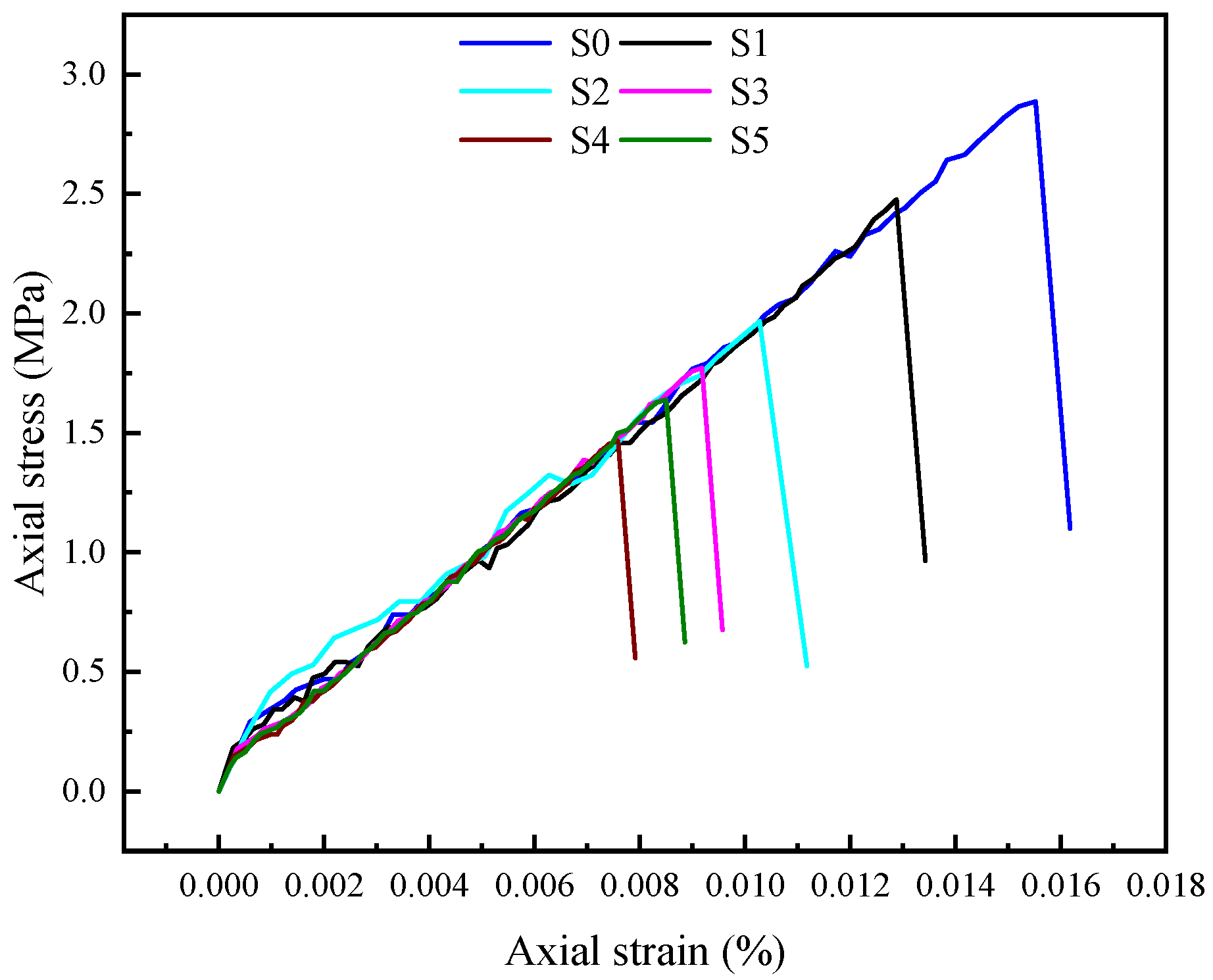
2.2.2. Static Brazilian Split Tensile Test on Coal Samples
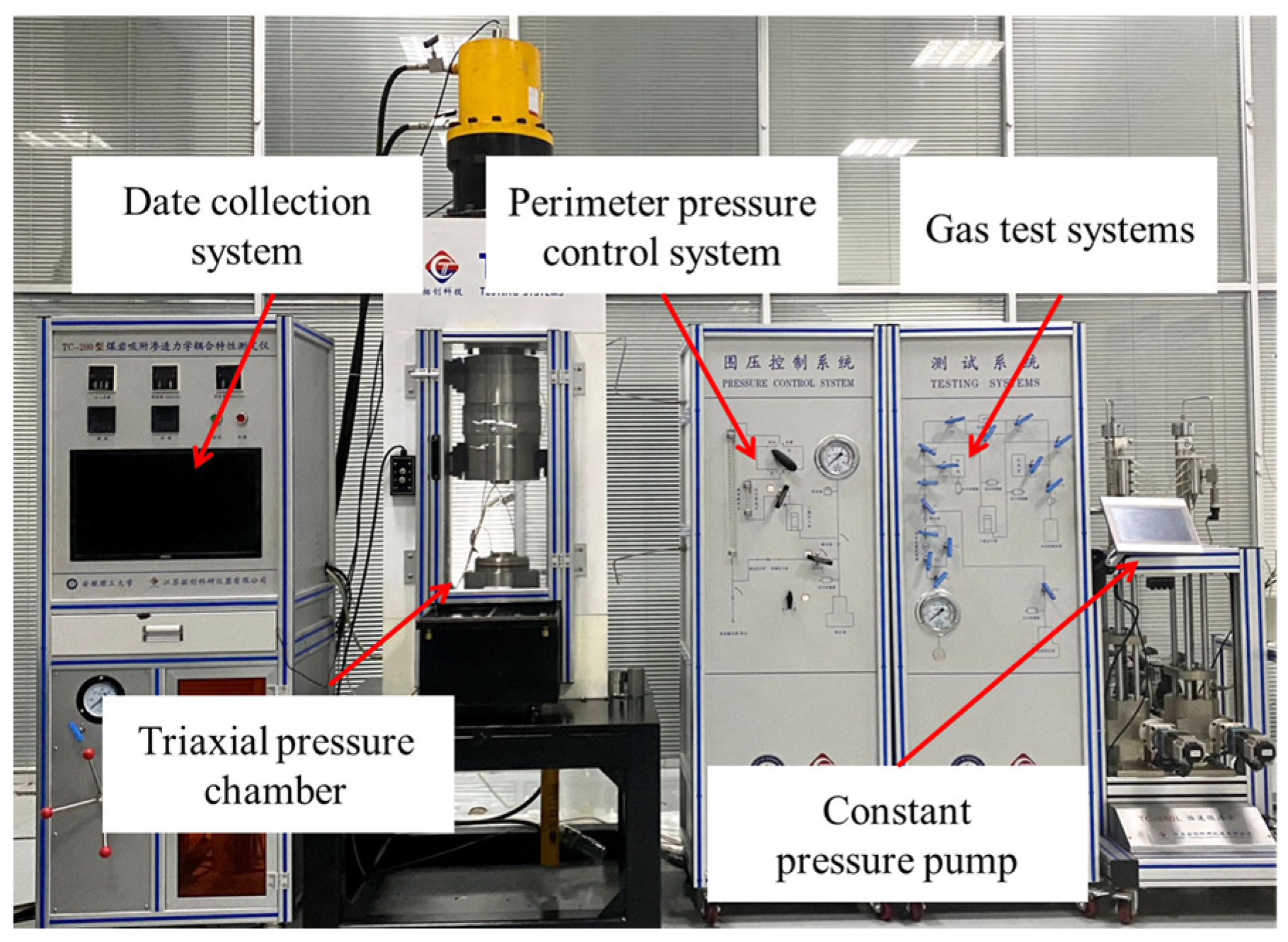
2.2.3. Permeability Test on Coal Samples
- (1)
- Wrap the coal sample with heat-shrink tubing and install the sample in the triaxial pressure chamber after checking the air tightness.
- (2)
- Control the perimeter pressure system so that the perimeter pressure reaches a predetermined value.
- (3)
- When the perimeter pressure is kept constant, adjust the gas control system to apply air pressure to the coal sample for permeability measurement. The formula for the permeability of the coal sample according to Darcy’s law is [32] as follows in Equation (1):
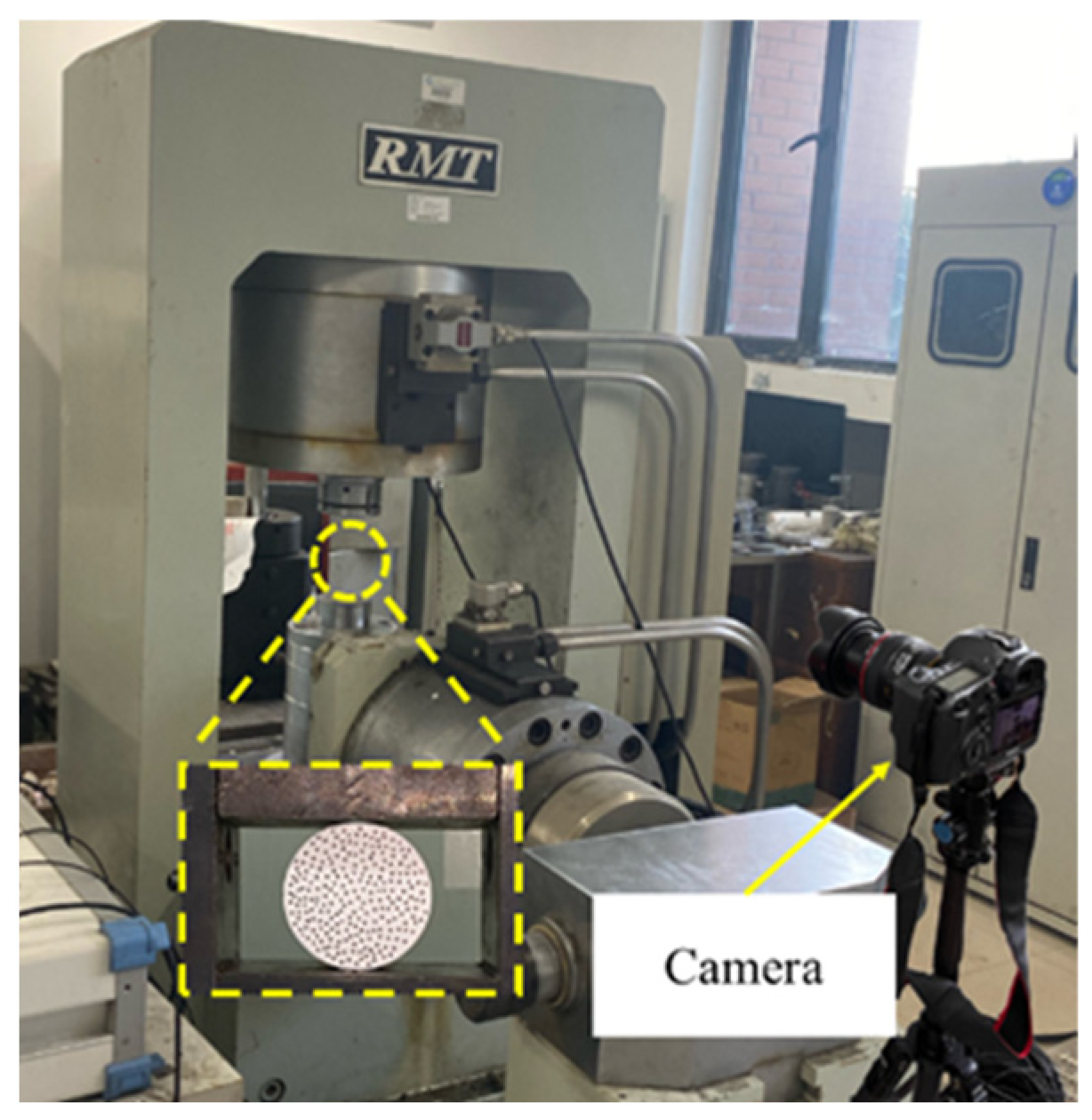
2.2.4. DIC Test on Coal Samples
2.2.5. SEM Test
3. Results
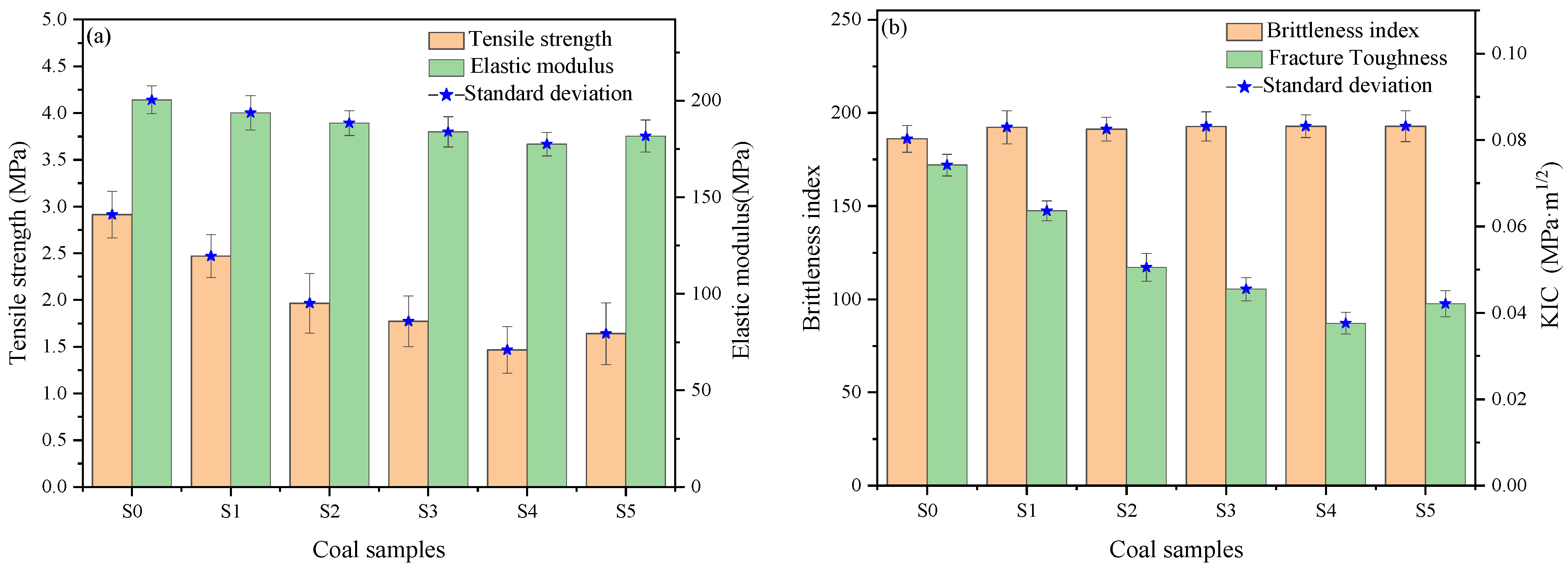
3.1. Mechanical Performance Analysis
3.2. Pore Structure Analysis
3.2.1. Pore Type
3.2.2. Pore Size
3.2.3. Pore Size Distribution
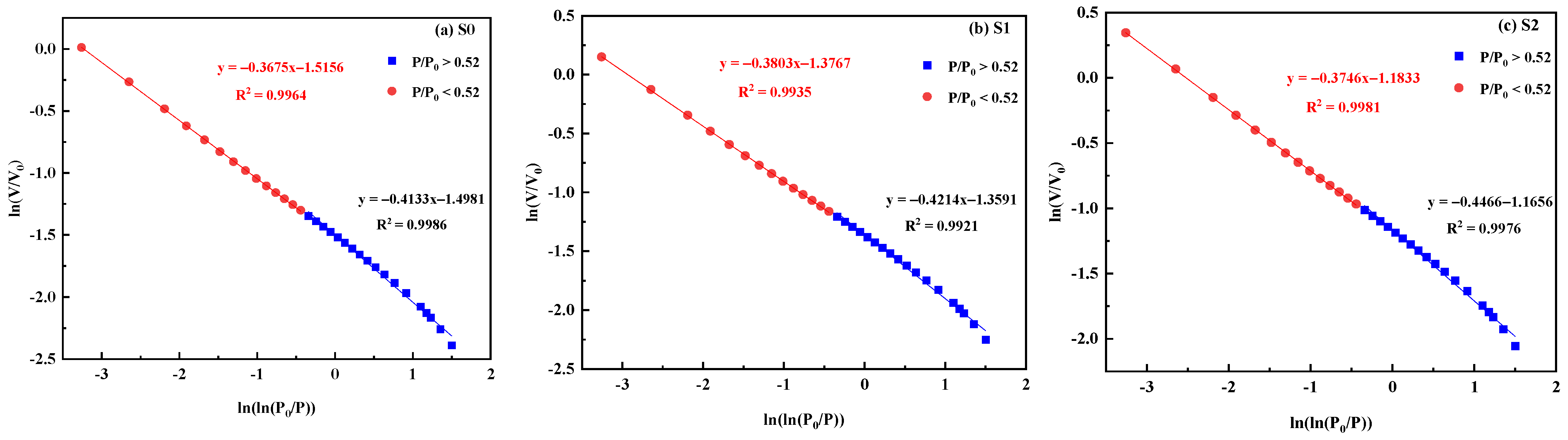
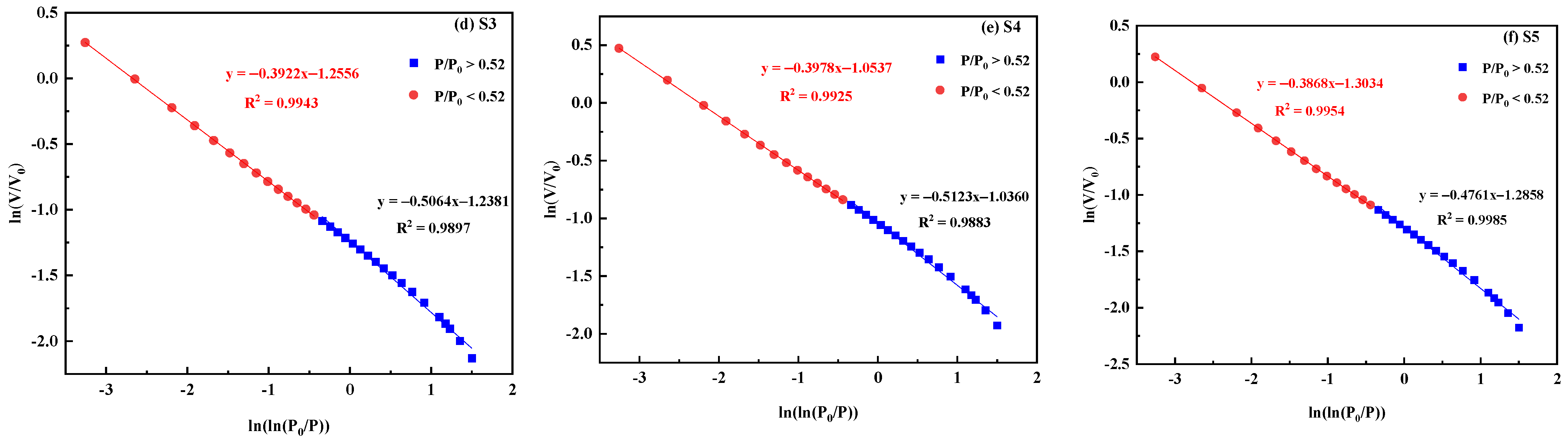
3.2.4. Pore Fractal Characterization
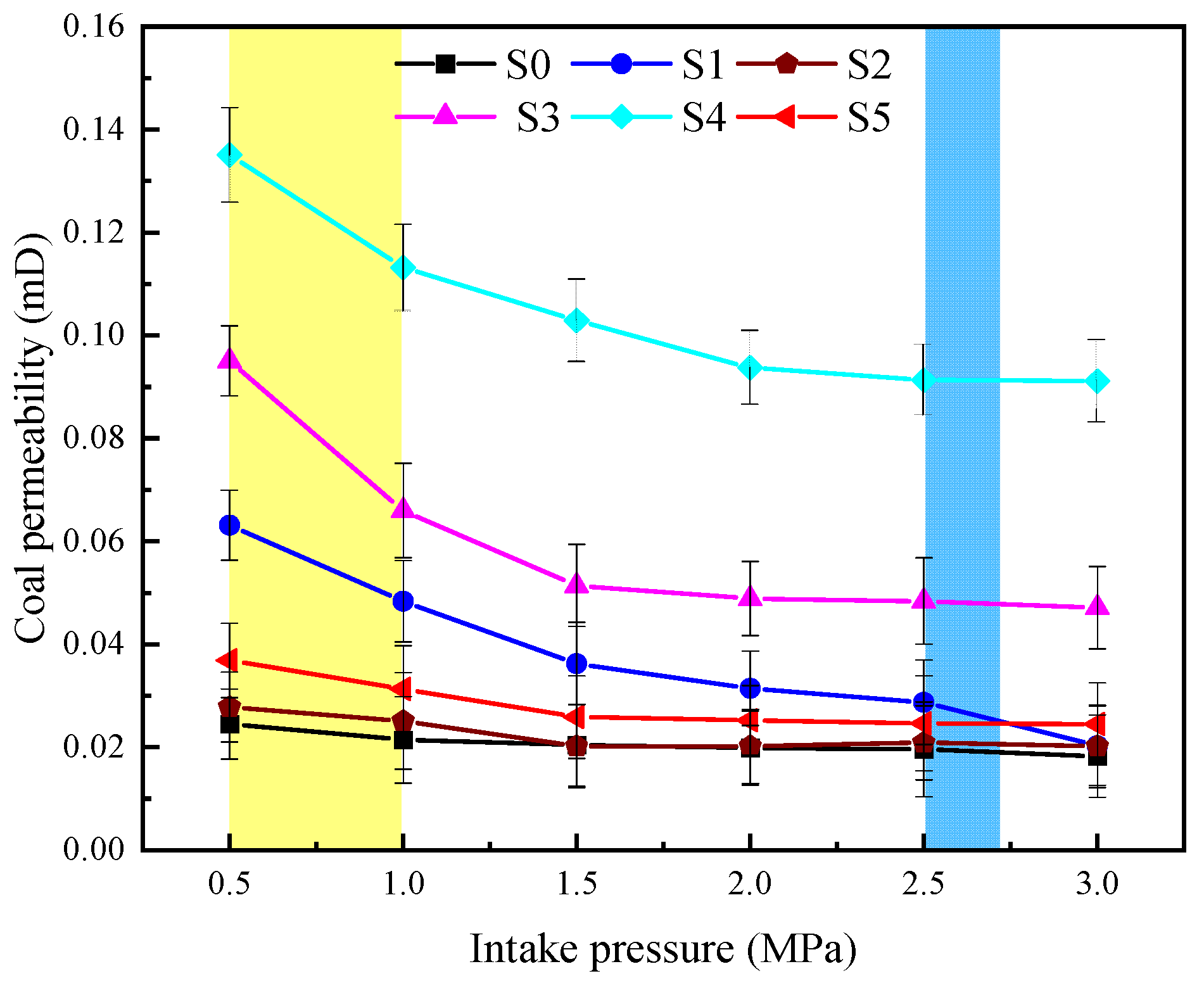
3.3. Gas Permeability Analysis
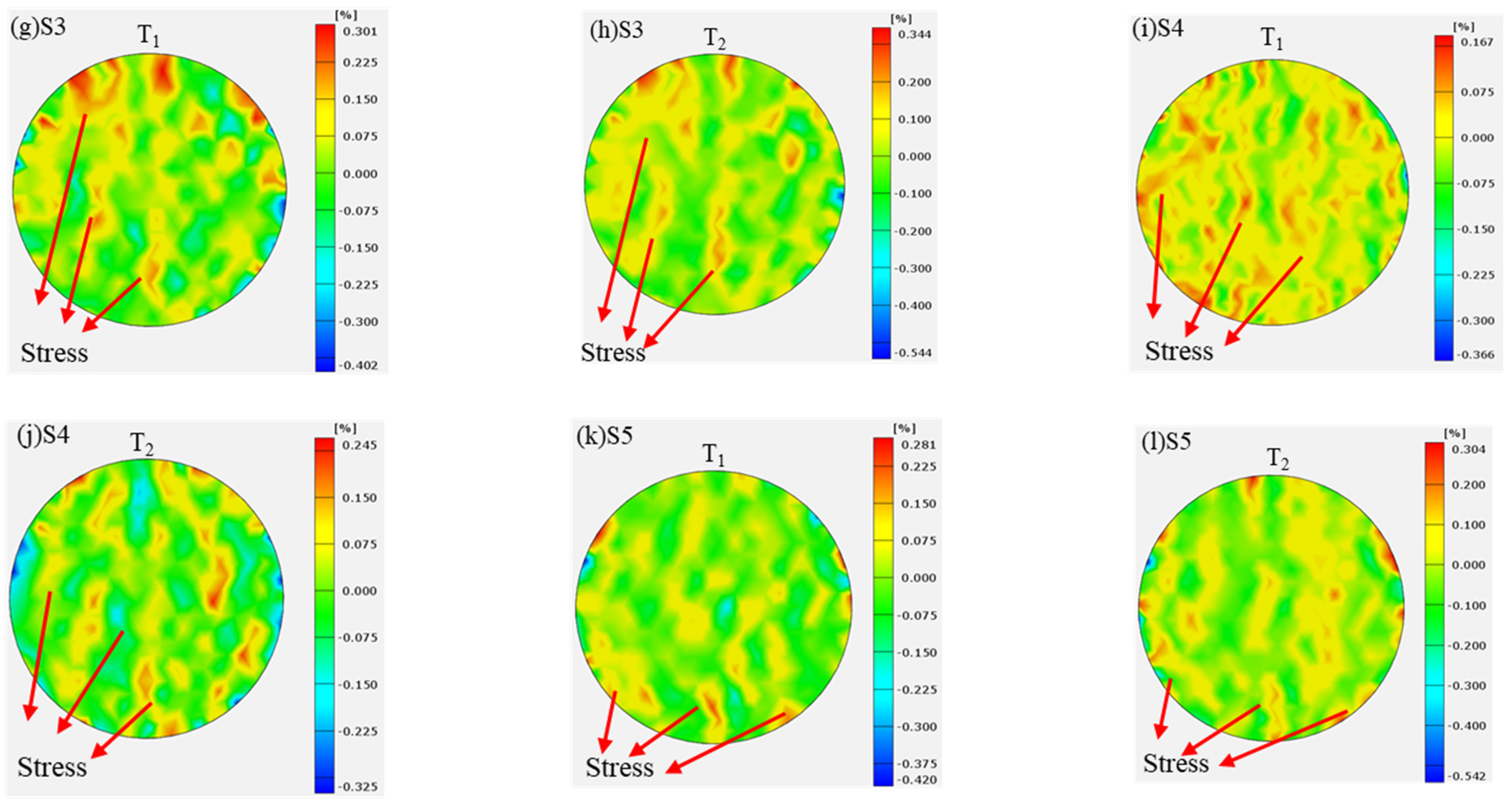
3.4. DIC Analysis
3.5. SEM Test Results and Analysis
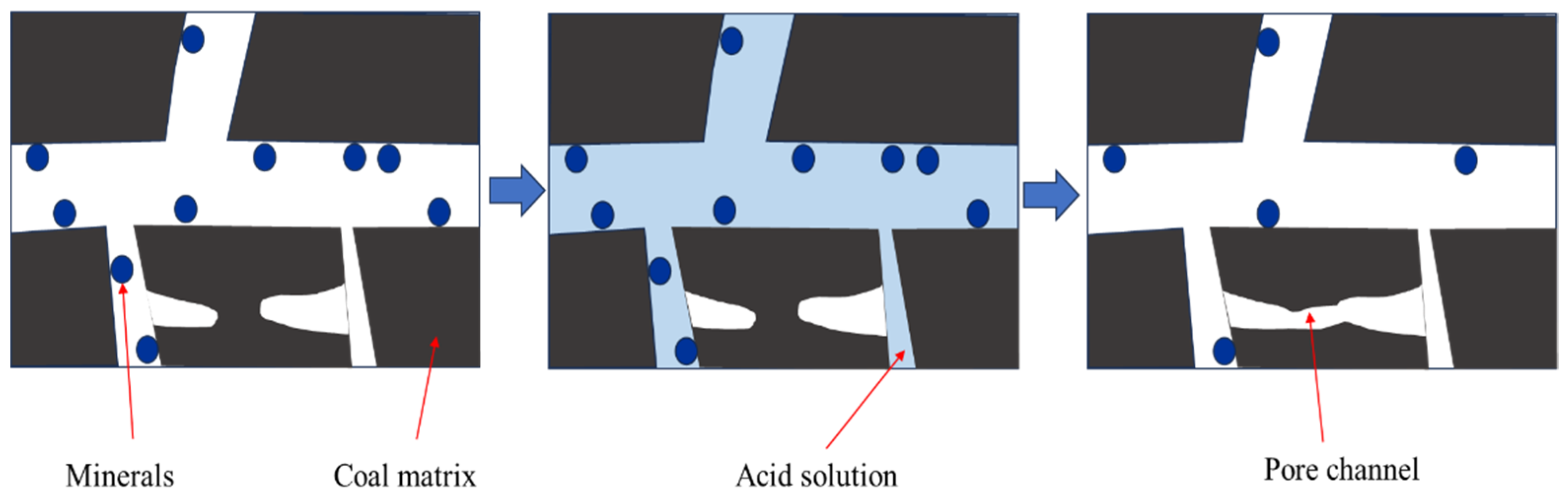
3.6. Mechanism of Penetration Enhancement of Coal by Mixed Acid Solutions
4. Conclusions
- (1)
- After treatment with different ratios of acid solutions, the fractal dimension D1 and fractal dimension D2 of the coal samples decreased, indicating that the pore connectivity was improved, the tortuosity of the gas diffusion paths was reduced, and the gas transportation efficiency was enhanced.
- (2)
- The Brazilian splitting experiments indicated that the main damage mode of coal samples was brittle damage. After modification by the mixed acid solution, all coal sample groups exhibited reductions in both splitting tensile strength and elastic modulus, demonstrating that the mixed acid treatment significantly degraded their mechanical properties.
- (3)
- The treatment with mixed acid solution caused the internal microscopic pore structure and macroscopic mechanical properties of the coal to alter, and the permeability of the coal samples treated with mixed acid solution was obviously greater than that of the raw coal.
- (4)
- Under the action of mixed acid solution, the evolution of cracks in the coal samples showed two different processes. The strain change in the original coal was more concentrated, and the concentrated area was extended along the axial direction, while the internal strain of the treated coal samples showed a discrete distribution when subjected to force.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Shen, J.; Wang, G.; Li, K.; Fang, X. Experimental study on the effect of high-temperature nitrogen immersion on the nanoscale pore structure of different lithotypes of coal. Energy 2023, 284, 128596. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A. 5-Coalbed Methane: Reserves, Production, and Future Outlook. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–109. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhang, H.D.; Zhou, Z.; Ge, Z.L.; Chen, C.J.; Hou, Y.D.; Ye, M.L. Current status and effective suggestions for efficient exploitation of coalbed methane in China: A review. Energy Fuels 2021, 35, 9102–9123. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.S.; Tang, S.H.; Elsworth, D. Re-evaluating adsorbed and free methane content in coal and its ad-and desorption processes analysis. Chem. Eng. J. 2022, 428, 131946. [Google Scholar] [CrossRef]
- Lu, Y.; Ge, Z.; Yang, F.; Xia, B.; Tang, J. Progress on the hydraulic measures for grid slotting and fracking to enhance coal seam permeability. Int. J. Min. Sci. Technol. 2017, 27, 867–871. [Google Scholar] [CrossRef]
- Mi, W.; Wen, H.; Fan, S.; Wei, G.; Liu, M.; Wang, S.; He, H.; Wu, X.; Cheng, X.; Liu, B.; et al. Pilot test of high-pressure water jet slotting with liquid CO2 injection to displace CH4 and improve coal seam permeability. Fuel 2023, 351, 128822. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Wang, K. Mechanism of solvent extraction-induced changes to nanoscale pores of coal before and after acidification. Fuel 2022, 310, 122467. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, X.; Li, B.; Zhou, A. Quantitative study on the role of desorption gas on coal-gas outbursts: Energy contribution and dynamic characteristics. Process Saf. Environ. 2023, 171, 437–446. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Yin, X.; Liu, H.; Liu, B. Mechanical characterization of intermittent weak interlayer based on DIC and acoustic emission technique. Theor. Appl. Fract. Mec. 2023, 127, 104097. [Google Scholar] [CrossRef]
- Ni, G.; Li, S.; Rahman, S.; Xun, M.; Wang, H.; Xu, Y.; Xie, H. Effect of nitric acid on the pore structure and fractal characteristics of coal based on the low-temperature nitrogen adsorption method. Powder Technol. 2020, 367, 506–516. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Fu, X.; Yu, K.; Zeng, F.B. An experimental study on the effects of acidization on coal permeability: Implications for the enhancement of coalbed methane production. Energy 2023, 280, 128145. [Google Scholar] [CrossRef]
- Luo, M.; Cui, S.; Zhou, X.; Li, S.; Fan, C. Experimental study on permeability enhancement of coal seam with high mineral content by acid fracturing. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 7770–7783. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Tian, J.; Li, T.; Wang, W.; Pan, J.; Niu, Q.; Feng, S.; Hao, H.; Zhang, Y. Permeability enhancement of low rank coal through acidization using H2S solution: An experimental study in the Kuqa-Bay Coalfield, Xinjiang, China. J. Petrol. Sci. Eng. 2020, 185, 106476. [Google Scholar] [CrossRef]
- Balucan, R.D.; Turner, L.G.; Steel, K.M. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas Sci. Eng. 2016, 33, 973–987. [Google Scholar] [CrossRef]
- Kousar, K.; Walczak, M.S.; Ljungdahl, T.; Wetzel, A.; Oskarsson, H.; Restuccia, P.; Ahmad, E.A.; Harrison, N.M.; Lindsay, R. Corrosion inhibition of carbon steel in hydrochloric acid: Elucidatingthe performance of an imidazoline-based surfactant. Corros. Sci. 2021, 180, 109195. [Google Scholar] [CrossRef]
- Ramírez-Pérez, J.F.; Zamudio-Rivera, L.S.; Servín-Nájera, A.G.; Soto-Castruita, E.; Cerón-Camacho, R.; Cisneros-Dévora, R.; Oviedo-Roa, R.; Martínez-Magadán, J.M. Quantum modeling design of imidazoline-based corrosion inhibitors for oil industry applications. Mater. Today Commun. 2021, 27, 102466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, N.; Feng, C.; Zhang, Q.; Yu, J.; Jiao, Y.; Xu, Y.; Chen, J. Citric acid modified β-cyclodextrin for the synthesis of water-stable and recoverable CD-MOF with enhanced adsorption sites: Efficient removal of Congo red and copper ions from wastewater. J. Environ. Chem. Eng. 2023, 11, 111413. [Google Scholar] [CrossRef]
- Yang, B.; Xue, W.; Zhao, X.; Wang, W.; Zhu, H.; Luo, L.; Huang, H.; Zhong, C. Synergistic dicarboxylate sites of natural citric acid modified MOF-808 for the deep removal of Pb2+ in water. J. Mol. Liq. 2022, 366, 120235. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Z.; Li, R.; Liu, X.; Wang, H.; Gong, S. Effects of acid-based fracturing fluids with variable hydrochloric acid contents on the microstructure of bituminous coal: An experimental study. Energy 2022, 244, 122621. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Bai, G.; Bi, D.; Liu, W. An experimental investigation of the effect of acid stimulation on gas extraction from coal. AIP Adv. 2020, 10, 115309. [Google Scholar] [CrossRef]
- Guo, Z.; Cao, Y.; Dong, S.; Zhang, Z. Experimental studies on the enhancement of permeability of anthracite by acidizing: A case study in the Daning block, Southern Qinshui Basin. ACS Omega 2021, 6, 31112–31121. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Yuan, H.; Abi, E.; Han, Y.; Li, H.; Pu, Y. Deformation and acoustic emission characteristics of hard rock under different unloading rates. Alex. Eng. J. 2023, 77, 581–591. [Google Scholar] [CrossRef]
- Pang, P.; Han, H.; Hu, L.; Guo, C.; Gao, Y.; Xie, Y. The calculations of pore structure parameters from gas adsorption experiments of shales: Which models are better? J. Nat. Gas Sci. Eng. 2021, 94, 104060. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Lu, Y.; Liu, T.; Lu, J.; Shi, S.; Ye, Q.; Jia, Z.; Wang, Z. Influence of microwave-assisted oxidant stimulation on pore structure and fractal characteristics of bituminous coal based on low-temperature nitrogen adsorption. Fuel 2022, 327, 125173. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Cai, J.; Chen, S.; Li, X. Study of Key Factors for Efficient Coalbed Methane Extraction: Pore Structure, Desorption Rate and Seepage Characteristics. Langmuir 2025, 41, 6020–6036. [Google Scholar] [CrossRef]
- Lin, H.; Bai, Y.; Bu, J.; Li, S.; Yan, M.; Zhao, P.; Qin, L. Comprehensive fractal model and pore structural features of medium-and low-rank coal from the zhunnan coalfield of Xinjiang, China. Energies 2019, 13, 7. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Yang, Y.; Miao, G.; Han, Y. Effects of oxidation on physical and chemical structure of a low rank sub-bituminous coal during the spontaneous combustion latency. Energy 2023, 272, 127122. [Google Scholar] [CrossRef]
- Hu, P.; Ren, J.; Hu, X.; Yang, H. Comparison of two starch-based flocculants with polyacrylamide for the simultaneous removal of phosphorus and turbidity from simulated and actual wastewater samples in combination with FeCl. Int. J. Biol. Macromol. 2021, 167, 223–232. [Google Scholar] [CrossRef]
- Pi, Z.; Dong, Z.; Li, R.; Wang, Y.; Li, G.; Zhang, Y.; Peng, B.; Meng, L.; Fu, S.; Yin, G. Low-field NMR experimental study on the effect of confining pressure on the porous structure and connectivity of high-rank coal. ACS Omega 2022, 7, 14283–14290. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, S.; Xue, S.; Jiang, B.; Chen, Z. Effects of chemical solvents on coal pore structural and fractal characteristics: An experimental investigation. Fuel 2022, 327, 125246. [Google Scholar] [CrossRef]
- Zhou, X.; He, Q.; Hou, H. Investigation on Adsorption Pore and Fractal Analyses of Low-Rank Coals in the Northern Qaidam Basin. ACS Omega 2024, 9, 9823–9834. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Cheng, Y.; Nemcik, J. Laboratory quantification of coal permeability reduction effect during carbon dioxide injection process. Process Saf. Environ. 2021, 148, 638–649. [Google Scholar] [CrossRef]
- Zheng, C.; Liao, F.; Xue, S.; Jiang, B.; Gong, X.; Han, B.; Chen, Z. Experimental investigation on pores and permeability variation characteristics of coal treated by chemical solvents. Geoenergy Sci. Eng. 2023, 223, 211514. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Cai, X.; Rui, Y.; Tan, L. Water saturation effects on mechanical performances and failure characteristics of rock-concrete disc with different interface dip angles. Constr. Build. Mater. 2022, 324, 126684. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, R.; Zhang, J.; Li, Y.; Li, Z.; Xing, X.; Zhang, Q.; Feng, G. Brazilian splitting testing of the restorative properties of eco-friendly epoxy resin on cracked granite samples with various widths. Constr. Build. Mater. 2023, 401, 132907. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Liu, H.; Jin, Z.; Yue, T.; Zhang, H. Investigation on the influences of CO2 adsorption on the mechanical properties of anthracite by Brazilian splitting test. Energy 2022, 259, 125053. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xia, K.; Li, X.B.; Li, H.B.; Ma, G.W.; Zhao, J.; Zhou, Z.L.; Dai, F. Suggested methods for determining the dynamic strength parameters and mode-I fracture toughness of rock materials. Int. J. Rock Mech. Min. Sci. 2007, 49, 105–112. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Jin, L.; Wei, Y.; Ou, S.; Wang, T.; Xu, J.; Liu, X.; Tao, G. Surface pore characteristics of original coal dust produced in underground mining sites and their impact on the moisture content. Process Saf. Environ. 2022, 167, 284–298. [Google Scholar] [CrossRef]
- Zhou, D.; Lv, Z.; Cao, Y.; Liu, G.; Zhang, X.; Shi, B.; Zhang, J.; Liu, S. Fractal Evolution Characteristics of Pore Structure in Coal-Acidified Stimulation. Fractal. Fract. 2025, 9, 62. [Google Scholar] [CrossRef]
- Dou, H.; Xie, J.; Xie, J.; Sun, G.; Li, Z.; Wang, Z.; Miao, Y. Study on the mechanism of the influence of HNO3 and HF acid treatment on the CO2 adsorption and desorption characteristics of coal. Fuel 2022, 309, 122187. [Google Scholar] [CrossRef]
- He, X.Q.; Mo, W.L.; Ma, Y.Y.; Wei, X.Y. Effect of mineral removal on the structure and pyrolysis characteristics of subbituminous coal. J. Energy Inst. 2024, 117, 101773. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Li, Z.; Li, J.; Zhang, X.; Liu, L.; Yan, D.; Zhou, Y. Influence of soluble organic matter on mechanical properties of coal and occurrence of coal and gas outburst. Powder Technol. 2018, 332, 8–17. [Google Scholar] [CrossRef]
- Fukun, X.; Lei, S.; Xufei, Z.; Kai, X. Experimental Study on Percolation Rate Characteristics of Gas-Filled Coal Bodies Based on True Triaxial Condition. Shock Vib. 2021, 2021, 6165198. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, Z.; Wang, K.; Qu, B.; Yuan, L.; Xu, C. Coal permeability evolution characteristics: Analysis under different loading conditions. Greenh. Gas 2020, 10, 347–363. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Ding, Z.; Wang, B.; Li, P. A dynamic evolution model of coal permeability during enhanced coalbed methane recovery by N2 injection: Experimental observations and numerical simulation. RSC Adv. 2021, 11, 17249–17258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, D.; Xu, H.; Tao, S.; Li, S.; Yang, G.; Yu, J. Pore and fracture characteristics of different rank coals in the eastern margin of the Ordos Basin, China. J. Nat. Gas Sci. Eng. 2015, 26, 1264–1277. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, Y.; Pan, Z. Classification methods of pore structures in coal: A review and new insight. J. Nat. Gas Sci. Eng. 2023, 110, 204876. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, X.; Jiang, X.; Jiang, X. Solvent Extraction of Superfine Pulverized Coal. Part 2. Free-Radical Characteristics. Energy Fuels 2021, 19, 35. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zhang, X.; Li, P.; Li, J. Effect of microstructure and chemical composition of coal on methane adsorption. J. Nat. Gas Sci. Eng. 2020, 82, 103507. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, B.; Yang, W.; Li, H.; Lin, M. Fracture and pore development law of coal under organic solvent erosion. Fuel 2022, 307, 121815. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.X.; Zhao, Y.; Liao, X.; Li, J.; Zhao, Z.; Liu, Q. Multi-scale pore structure transformation of shale under mixed acid acidification method. Arab. J. Chem. 2023, 16, 104937. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Shao, Y. Effect of demineralization on Yimin lignite by experiments and molecular simulation techniques. J. Mol. Struct. 2022, 1269, 133837. [Google Scholar] [CrossRef]


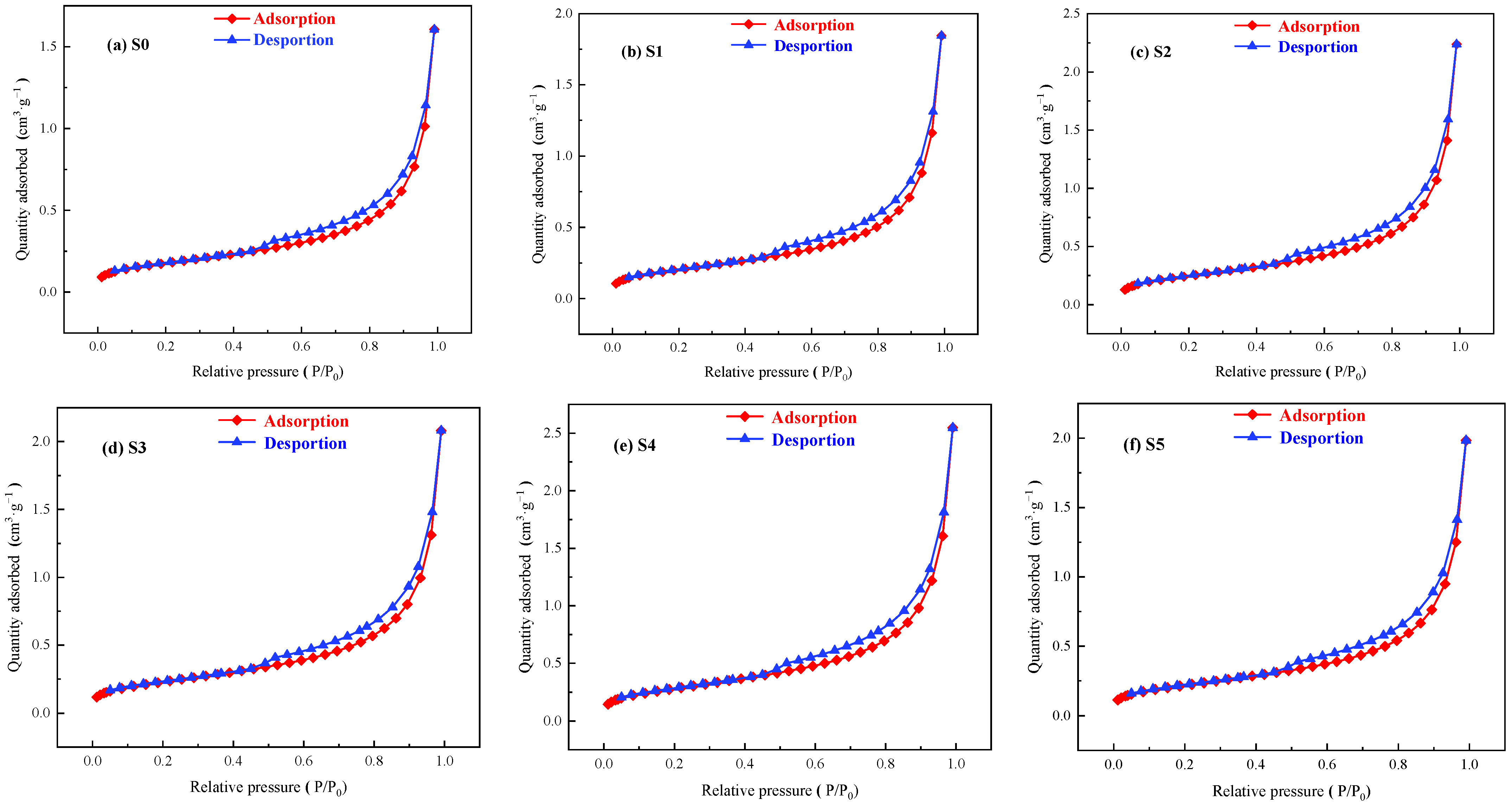
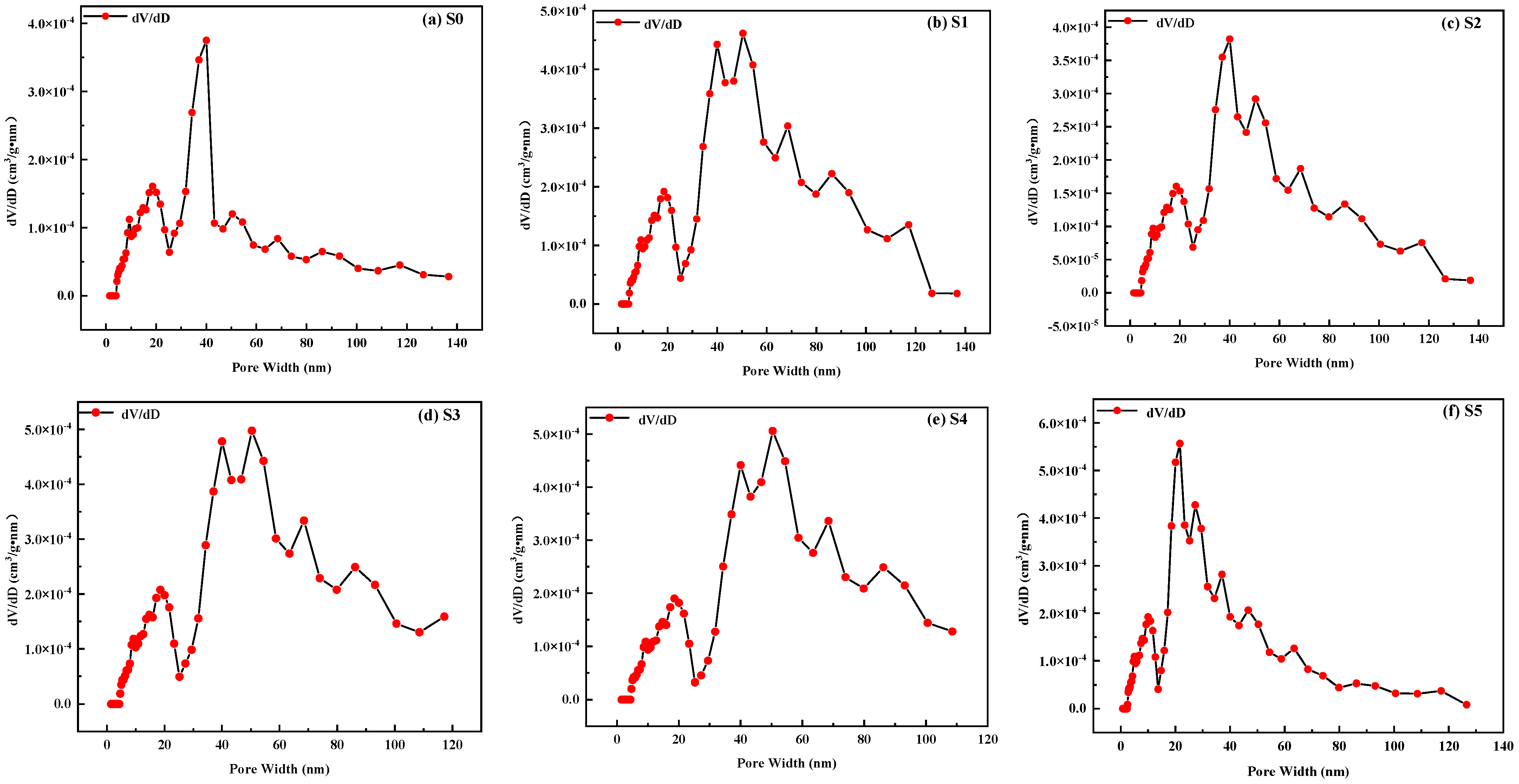



| Number Proportions | S0 | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
| HCl | 0 | 0 | 1 | 2 | 1 | 1 |
| Imidazoline surfactants | 0 | 0 | 1 | 1 | 1 | 2 |
| Citric acid | 0 | 0 | 1 | 1 | 2 | 1 |
| Samples | Volume (10−3 cm3/g) | TPV (10−3 cm3/g) | Average Pore Size (nm) | ||
|---|---|---|---|---|---|
| Micropore | Transition Pore | Mesopore | |||
| S0 | 0.91 | 6.53 | 0.88 | 8.32 | 14.3 |
| S1 | 0.88 | 6.77 | 1.12 | 8.77 | 14.6 |
| S2 | 0.85 | 6.89 | 1.24 | 8.98 | 15.1 |
| S3 | 0.82 | 7.02 | 1.28 | 9.12 | 15.3 |
| S4 | 0.79 | 7.11 | 1.32 | 9.22 | 16.2 |
| S5 | 0.84 | 7.07 | 1.19 | 9.10 | 14.9 |
| Coal Sample | Fractal Dimension | |||
|---|---|---|---|---|
| D1 | R2 | D2 | R2 | |
| S0 | 2.5867 | 0.9986 | 2.6325 | 0.9964 |
| S1 | 2.5786 | 0.9921 | 2.6197 | 0.9935 |
| S2 | 2.5534 | 0.9976 | 2.6254 | 0.9981 |
| S3 | 2.4936 | 0.9897 | 2.6078 | 0.9943 |
| S4 | 2.4877 | 0.9883 | 2.6022 | 0.9925 |
| S5 | 2.5239 | 0.9985 | 2.6132 | 0.9954 |
| Samples | T1 (s) | T2 (s) |
|---|---|---|
| S0 | 3.26 | 12.15 |
| S1 | 3.26 | 9.87 |
| S2 | 3.26 | 8.24 |
| S3 | 3.26 | 7.66 |
| S4 | 3.26 | 7.14 |
| S5 | 3.26 | 7.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Cai, F.; Zhang, Q. Influence of Mixed Acids on Coal Fractal Characteristics and Permeability. Fractal Fract. 2025, 9, 386. https://doi.org/10.3390/fractalfract9060386
Fan J, Cai F, Zhang Q. Influence of Mixed Acids on Coal Fractal Characteristics and Permeability. Fractal and Fractional. 2025; 9(6):386. https://doi.org/10.3390/fractalfract9060386
Chicago/Turabian StyleFan, Jiafeng, Feng Cai, and Qian Zhang. 2025. "Influence of Mixed Acids on Coal Fractal Characteristics and Permeability" Fractal and Fractional 9, no. 6: 386. https://doi.org/10.3390/fractalfract9060386
APA StyleFan, J., Cai, F., & Zhang, Q. (2025). Influence of Mixed Acids on Coal Fractal Characteristics and Permeability. Fractal and Fractional, 9(6), 386. https://doi.org/10.3390/fractalfract9060386





