Abstract
To address the detrimental effects of high mud content on cemented sand and gravel (CSG) materials, this study focuses on CSG materials with different contents of alkali-activated rice husk ash (RHA). The microscopic enhancement mechanism of mechanical properties of CSG materials with alkali-activated RHA were studied through the experiments. Based on the changes in microstructure, pore sizes, and mineral composition, the microstructural features of CSG materials are quantitatively characterized using pore characteristic parameters and fractal dimensions, revealing the mechanism by which alkali-activated RHA improves the mechanical properties of CSG materials. The results indicate that alkali-activated RHA effectively enhances the mechanical properties of CSG materials. As the RHA content increases, the compressive strength and elastic modulus initially increase and then decrease, while the failure strain first decreases and then increases. The failure mode transitions from splitting failure to shear failure, with the optimal mechanical performance at 5% RHA content. Microscopic experimental analysis found that adding an appropriate amount of RHA can promote the generation of cementitious substances, improve the internal pore structure, and increase the fractal dimension. However, excessive RHA can adsorb moisture, inhibit part of the hydration reactions and reduce the fractal dimension. Under the action of alkali activators, the activity of RHA is enhanced, generating more cementitious materials and significantly improving the pore-filling effect within the material, especially affecting the capillary pores.
1. Introduction
Cemented sand and gravel (CSG) materials are a new type of dam construction materials designed according to the concept of suitable materials and structures. Its main raw materials include riverbed materials [1], excavation materials, artificial sand and gravel, water, and a small amount of cementitious materials, which are usually mixed using conventional technology and equipment. CSG materials have the advantages of less cement consumption, high construction efficiency, and strong material properties, and they have been widely used in water conservancy engineering [2,3].
CSG materials are usually composed of water, coarse and fine aggregates, cement, and fly ash mixed in a certain proportion, and its mechanical properties are closely related to water consumption, aggregate gradation, sand ratio, and cementitious material content [4,5]. Reasonable mix proportions and the handling of raw materials are key factors affecting materials’ mechanical properties. However, the cemented sand and gravel excavated directly from the riverbed often have a high mud content, and the mud attached to the raw materials can negatively impact the material properties, which usually does not meet the engineering application standards [6]. Gong et al. [7] found that the mechanical properties of cemented sand and gravel were negatively correlated with mud content, and that the compressive strength and flexural strength of CSG materials are significantly reduced with the increase in mud content. Excessive mud content suppresses the hydration reaction, and reduces the generation of cementitious substances, which in turn affects the bonding strength of CSG materials. The above-mentioned research results indicate that excessive mud content weakens the overall bonding effect of CSG materials, reducing density and strength. At present, it is stipulated in the material criteria for technical guideline for cemented granular material dams, that the mud content in CSG materials should not exceed 5% [8], otherwise, it will adversely affect the strength and stability of the materials. In order to comply with the technical guidelines for cemented granular material dams, during the construction process, local sand and gravel aggregates need to be screened and washed to reduce the mud content, but the screening and washing process is not only time-consuming and labor-intensive, but also increases construction costs, failing to fully leverage the advantages of CSG materials. Although existing studies have identified the detrimental effects of high mud content on the performance of CSG materials, systematic research on preserving the strength of CSG materials while effectively solidifying the mud remains insufficient.
Some researchers have found that RHA, as a geopolymer material with high activity and large specific surface area [9,10,11], is rich in large amounts of amorphous silica. The addition of RHA can effectively solidify mud-containing materials, thereby improving the microstructure of the materials and enhancing their mechanical properties [12,13,14], which has been widely used in practical engineering. Li et al. [15] studied the solidified lime soil of RHA and found that when the content of RHA was 4%, a large amount of hydrated silicate hydrates were generated on the soil surface, resulting in the densest microstructure and optimal mechanical properties. Li et al. [16] studied the RHA-cement-solidified sludge and found that the compressive strength initially increases and then decreases with the increase in RHA content. The addition of RHA generates a large amount of C-S-H gel that connects the aggregate particles and enhances the bonding effect of the material. Chen et al. [17] studied the performance of cured soft clay of RHA and found that the porous particle structure of RHA can effectively fill the pores and improve the compactness of the material. Meanwhile, the incorporation of RHA causes pozzolanic and hydration reactions, generating cementitious materials that fill and improve the pore structure, leading to improvements in the compressive and shear strength as well as durability of the RHA-solidified soft clay. However, RHA is classified as a low-calcium geopolymer rich in silica, which results in RHA-solidified materials exhibiting low early strength and high late strength characteristics [18]. In order to enhance the solidification effect of RHA, domestically and internationally, researchers have found that using alkali activators mixed with RHA can increase its activity and promote the generation of cementitious materials, thereby significantly improving the solidification performance of RHA [19,20]. Zhao et al. [21] studied alkali-activated RHA and blast furnace slag cementitious materials and found that alkali activators can enhance the pozzolanic activity of the cementitious materials and generate C-A-S-H cementitious substances that increases compressive strength. The optimal mechanical performance is achieved at a 10% RHA content. Li et al. [22] studied alkali-activated RHA fiber-reinforced soil and found that the cementitious materials generated upon the addition of RHA enhanced the plasticity of the soil. Under the action of alkali activator, although no new phases formed within the samples, more cementitious substances were produced, further improving the microstructure. The above research results show that RHA exhibits superior performance as a solidifying material, and alkali activators can effectively enhance the solidification effect of RHA [23].
The CSG materials have low content of cementitious materials, uneven distribution of aggregate gradation, resulting in more complex internal pore structures [24]. Using scanning electron microscope (SEM), energy dispersive spectroscopy (EDS), nuclear magnetic resonance (NMR), and other techniques, it is difficult to quantitatively reveal the internal microstructure and mechanical mechanism of materials. In recent years, researchers both domestically and internationally have found that the fractal dimension, as an important representation parameter of fractal theory, can quantitatively describe changes in pore structure characteristics by quantifying the complexity of an object into fractal dimensions [25,26]. Zhao et al. [27] studied gravel materials of different grades and found that there is a linear relationship between the fractal dimension and the strength and damage index. Zhang et al. [28] studied roller compacted concrete with different large, crushed stone content and established a mathematical model for determining the mechanical properties and damage degree based on fractal dimensions, revealing the influence of large, crushed stone content on mechanical properties and frost resistance of roller compacted concrete (RCC) from a microscopic perspective. The above research results indicate that studying the effects of changes in material microstructure on mechanical properties through variations in fractal dimension is feasible.
To address the issue of reduced strength caused by high mud content in CSG materials, this study conducted unconfined compressive tests to deeply investigate the macroscopic mechanical properties of CSG materials with different ratios of alkali activators and RHA. Microscopic techniques such as SEM, EDS, NMR, and X-ray Diffraction (XRD) were employed to analyze the changes in the microstructure of the materials and combined with fractal theory to quantitatively characterize the internal microstructure [29,30], revealing the mechanism by which alkali-activated RHA enhances the mechanical properties of CSG materials from a microscopic perspective, and providing a theoretical basis for addressing the negative impact of high mud content on the mechanical properties of CSG materials.
2. Materials and Methods
2.1. Materials
2.1.1. Aggregate
The aggregates used in the experiment were taken from the riverbed gravel soil at a construction site in Linzhou City, Henan Province China, with a sampling depth of approximately 2 m. The soil sample type is silty gravel, containing about 58% gravel and 16% fine-grained soil, with a few particles exceeding 60 mm in diameter. The aggregate particle gradation curve is shown in Figure 1, and the proportion of different particle sizes is shown in Table 1. Test results indicate that the mud content of the material reaches 23.56%, which far exceeds the requirement in the technical guideline for cemented granular material dams stating that “the mud content in cemented sand and gravel should not exceed 5%”.
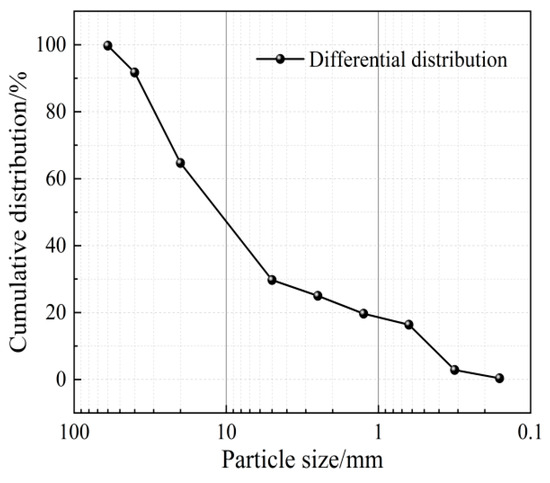
Figure 1.
Aggregate particle gradation curve.

Table 1.
Proportions of different particle sizes in the test aggregate.
2.1.2. Cement
Cement is the most commonly used hydraulic cementing material in domestic and international engineering. It has the advantages of fast setting time and high strength, and its physical properties have a significant impact on the strength of CSG materials. In this experiment P.O 42.5 ordinary Portland cement produced by Tianrui Cement Co., Ltd., located in Zhengzhou, Henan Province, China was used, and its specific morphology is shown in Figure 2. Performance indicators and compositional components of cement are shown in Table 2. All comply with the requirements of the national standard General Portland Cement (GB 175-2007) [31].

Figure 2.
Morphology of cementitious materials. (a) Cement. (b) Fly ash. (c) Rice husk ash.

Table 2.
Performance indicators and compositional components of cement.
2.1.3. Fly Ash and Rice Husk Ash
The fly ash used in the experiment is the dry discharge second level fly ash of the thermal power plant; its appearance is gray-black powder. The RHA used in the experiment was purchased from an agricultural materials wholesale center in Heilongjiang Province, China, and its appearance is black powder. Their specific morphology is shown in Figure 2, and the main chemical composition and content are shown in Table 3.

Table 3.
Main chemical components of fly ash and rice husk ash.
2.1.4. Alkali Activator
The alkali activator is prepared by mixing NaOH flaky granules and sodium silicate powdery granules. The purity of NaOH granules is more than 96%, while the mass fractions of SiO2 and Na2O in sodium silicate are both 21%. After being uniformly mixed with the corresponding ratio of water, it is kept sealed for 24 h before use.
2.2. Test Mix Ratio
To investigate the solidification effect of alkali-activated rice husk ash (RHA) on cemented sand and gravel (CSG) materials under high mud content conditions, the experiment was designed with three different combinations: (1) CSG materials, (2) CSG materials and RHA, (3) CSG materials with RHA and alkali activator. The CSG material had a mud content of 23.56%, and the group without RHA or alkali activator served as the control (CSG-R0). Four proportions of RHA were designed 0%, 5%, 10%, and 15% (mass ratio). The optimal mass ratio of alkali activator to rice husk ash was determined as 0.16:1 through extensive preliminary experiments. The specific experimental design mix ratios are shown in Table 4. All samples are cubic with dimensions of 150 mm × 150 mm × 150 mm. After the samples are prepared and demolded, they are placed into the standard curing room, with a curing period of 28 days.

Table 4.
CSG test mix ratio.
2.3. Experimental Methods
2.3.1. Uniaxial Compression Test
The uniaxial compression test was conducted according to the “Standard for geotechnical testing method” (GB/T 5013-2019) [32]. Three specimens were selected for each test group, and the average of the test results was taken. The test was performed using a pressure machine produced by Shanghai Sansi Zongheng Machinery Manufacturing Co., Ltd., located in Shanghai, China, with the loading method of the equipment controlled by displacement at a loading rate of 0.5 mm/min and the maximum test load of 5000 kN.
2.3.2. Scanning Electron Microscopy and Energy Dispersive Spectroscopy Analysis
The experimental equipment used includes a Sigma 300 scanning electron microscope produced by Zeiss in Oberkochen, Baden-Württemberg, Germany, an Xplore 30 energy dispersive spectrometer produced by Oxford Instruments plc, Abingdon, UK, and a CMC-1500 ion sputtering instrument produced by Shanghai Hezao Instrument Co., Ltd., Shanghai, China. Qualitative and semi-quantitative analyses of the samples were conducted. Based on the morphological characteristics observed in the scanning electron microscope, typical points were selected in the well-developed central and edge areas for EDS scanning to analyze the elemental composition of the samples.
2.3.3. Nuclear Magnetic Resonance Test
The experimental instrument uses the MesoMR12-060H-I (MR) nuclear magnetic resonance equipment produced by Neway Analytical Instrument Co., Ltd., located in Suzhou Jiangsu Province, China. Before the experiment, the samples were subjected to vacuum saturation to remove impurities from the material pores, enhance signal strength, and improve the accuracy of the experimental data.
2.3.4. X-Ray Diffraction Test
The XRD experimental instrument uses the Rigaku SmartLab SE diffractometer produced by Rigaku Corporation, located in Tokyo, Japan, with a scanning range (2θ) set from 5° to 90° and a scanning speed of 2°/min for qualitative analysis of the materials. The phase composition was further determined by MDI Jade 6.5 analysis software.
3. Results and Discussion
3.1. Influence of Stress–Strain Curve
The stress–strain curves of alkali-activated RHA (CSG) samples are shown in Figure 3. As shown in Figure 3, the stress–strain curves of CSG samples with different mix ratios exhibit similar variation trends. The stress–strain curves can be generally divided into four stages: compaction stage, elastic stage, plastic stage, and failure stage. In the compaction stage, stress increases slowly as the internal pores of the samples are gradually compacted. In the elastic stage, the stress–strain curve is almost like a straight line, and the stress growth rate is relatively fast. During the plastic stage, stress increases slowly, and slight cracks begin to appear on the surface of the samples. When the stress reaches its peak value, the samples enter the failure stage, where noticeable cracks develop inside the samples, and stress rapidly decreases, exhibiting strain-softening characteristics. Taking the stress–strain curve with 5% RHA content as an example, without alkali activators, the slope of the elastic stage in the stress–strain curve increases compared to the control group without RHA addition, and the peak stress in the stress–strain curve rises. Under the action of alkali activators, the slope of the elastic stage of the stress–strain curve further increases, the stress growth rate accelerates, the peak stress significantly increases, and the overall brittleness of the material is increased. The specific experimental data are shown in Figure 4.
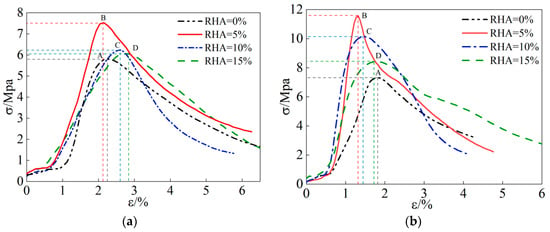
Figure 3.
Stress–strain curve of CSG materials. (a) Not alkali-activated. (b) Alkali-activated.
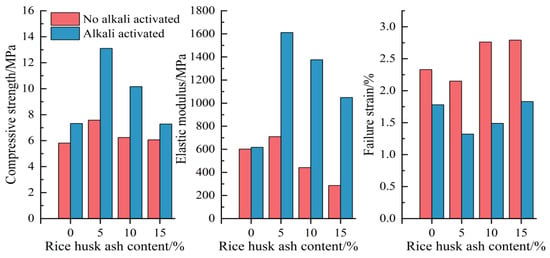
Figure 4.
Mechanical performance parameters of CSG materials.
As shown in Figure 4, with the increase in RHA content, the unconfined compressive strength of the CSG specimens exhibits a trend of first increasing and then decreasing, indicating the existence of an optimal threshold for the RHA content—the strength of the specimens reaches its peak at 5% RHA content. With the addition of alkali activators, the unconfined compressive strength of the CSG specimens significantly increases, reaching 7.32, 11.56, 10.16, and 7.28 MPa, respectively, compared to 5.81, 7.58, 6.24, and 6.06 MPa for the specimens without alkaline activation—the compressive strength increased by 25.9%, 72.8%, 62.8%, and 20.1%. This indicates that the addition of RHA can enhance the compressive strength of the CSG specimens, and alkali activators can further improve the mechanical properties of the material [33].
3.2. Variation Law of Elastic Modulus and Failure Strain
The elastic modulus is an important indicator for measuring the resistance of materials to elastic deformation, while the failure strain is a crucial parameter for assessing the material toughness, brittleness, and ductility under uniaxial compression. As shown in Figure 4, without the action of alkali activators, the elastic modulus first increases and then decreases with increasing content of RHA, whereas the failure strain first decreases and then increases. At 5% RHA content, the elastic modulus reaches its maximum value, while the failure strain reaches its minimum value, and the CSG specimens exhibit optimal mechanical properties. Under the action of alkali activators, the elastic modulus increases from 709.03 MPa to 1611.13 MPa, with an increase of approximately 127.2%, while the failure strain decreases from 2.15% to 1.32%, with a decrease of approximately 38.6%. These results indicate that the appropriate addition of RHA and alkali activators can significantly improve the mechanical properties of CSG materials, while excessive RHA may negatively impact overall performance [34].
3.3. Failure Mode
The failure modes of alkali-activated CSG samples are shown in Figure 5. As seen in Figure 5, during the stress loading process, CSG samples without RHA exhibit splitting failure, with vertical penetrating cracks generated in the samples, the direction of which is nearly parallel to the direction of stress loading. The surfaces of CSG samples incorporating RHA and alkali activators gradually transformed into X-shaped cracks, and the failure mode changed from splitting failure to shear failure. This phenomenon is consistent with the research findings of Li et al. [35]. The reason is that there is less cementitious material slurry in CSG samples, and there is a large difference in aggregate gradation, leading to weak resistance to deformation under stress. Adding RHA can fill the voids, improve the pore structure, and enhance the bonding effect between the aggregates. The addition of alkali activators promotes the generation of cementitious substances, allowing the samples to more effectively disperse stress under external loads and reduce stress concentration effects.
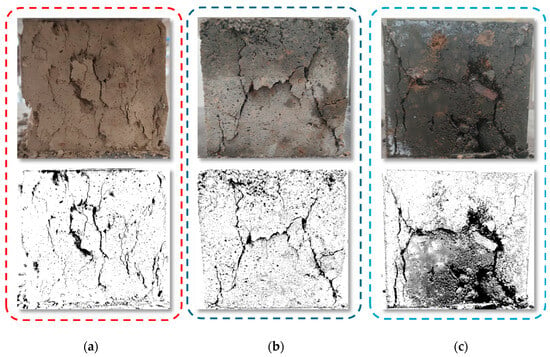
Figure 5.
Uniaxial failure mode of CSG materials. (a) Control group. (b) 5% RHA. (c) Alkali-activated 5% RHA.
4. Microstructural Analysis of Alkali-Activated RHA CSG Materials
4.1. Scanning Electron Microscope Analysis
To further understand the micro-mechanisms of alkali-activated RHA on CSG materials, qualitative and quantitative analyses of the samples were conducted using microscopic techniques such as SEM-EDS, NMR, and XRD. Due to the significant difference in aggregate gradation within CSG materials, the internal pores are relatively large. The classification of pore diameters in this study follows common concrete classification standards [36], namely, micropores (pore diameter ≤ 100 nm), capillary pores (pore diameter 100–1000 nm), and macropores (pore diameter > 1000 nm).
Due to the extensive number of SEM images, this paper only selects one control sample (CSG-R0) and the two samples with the best performance (CSG-R5 and CSG-AR5) for comparative analysis. Figure 6 shows the micro-morphology of CSG materials. As seen in Figure 6, the surface of CSG-R0 samples contains a large number of loose soil particles, with the framework particles being predominantly single grains. Some particles are connected by a small amount of reticulated cementing substances (C-S-H), while obvious voids exist between most aggregate particles, and the initial internal micro-cracks are noticeable. With the addition of RHA, on the one hand, the powdered RHA fills the pores as fine particles, enhancing the compactness of the material. On the other hand, RHA is rich in large amounts of active SiO2, which generates cementitious substances that connect the pores, resulting in solidified soil particles, exhibiting a certain clustering effect, reducing the number and width of initial micro-cracks within the material, and improving the microstructure of the material [37]. With the addition of alkali activators, an alkaline environment is created to enhance the pozzolanic activity of RHA and fly ash, promoting the generation of acicular and reticular cementitious substances. These amorphous, gel-like materials cover the surfaces of the original particles and form a dense network structure, further enhancing the material’s compactness and reducing the number of internal micro-cracks. This indicates that the incorporation of alkali activators significantly improves the microstructure of CSG materials.
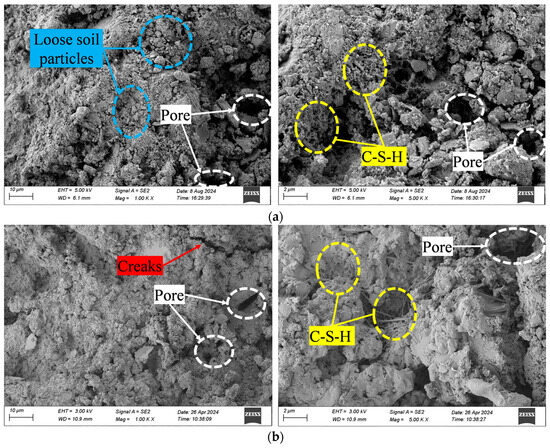
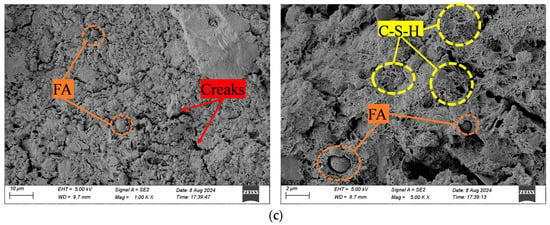
Figure 6.
SEM images of CSG materials. (a) CSG-R0 sample. (b) CSG-R5 sample. (c) CSG-AR5 sample.
4.2. Energy Dispersive Spectroscopy Analysis
To further investigate the chemical composition changes in alkali-activated RHA in CSG materials after reaction, the EDS images of CSG specimens are shown in Figure 7. The elemental content percentages under different mix ratios obtained through further processing are presented in Table 5. As seen in Table 5, with the increase in RHA content, the ratio of Ca/Si first decreases and then increases, with the lowest Ca/Si ratio occurring at an RHA content of 5%. The reason is that the active SiO2 in RHA reacts with Ca2+ ions to form C-S-H cementitious material which reduces the Ca/Si ratio. When excessive RHA is added, it inhibits the hydration reaction, resulting in an increase in the Ca/Si ratio [38,39]. With the addition of alkali activators, which provide a large number of OH− and Si2+ ions, the ratio remains the lowest at 5% RHA content. This indicates that under the action of alkali activators, the pozzolanic activity of RHA is significantly enhanced, leading to the production of more gel-like substances, thereby increasing the strength of the CSG samples.
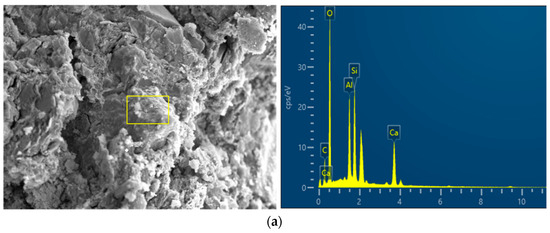
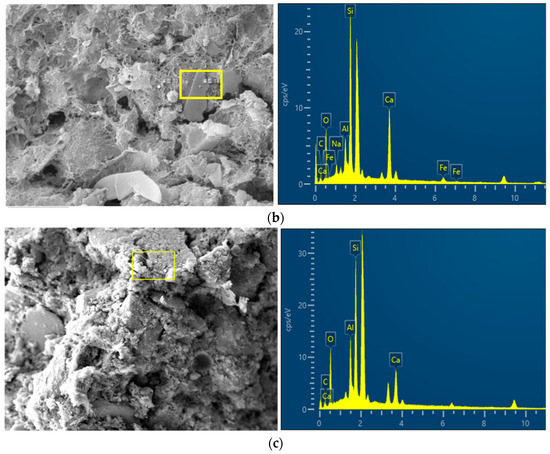
Figure 7.
EDS images of CSG materials. (a) CSG-R0 sample. (b) CSG-R5 sample. (c) CSG-AR5 sample.

Table 5.
Percentage of different elements’ content.
4.3. Changes in Pore Characteristic Parameters of Alkali-Activated RHA CSG Specimens
By analyzing the changes in pore characteristic parameters of CSG materials at different proportions, the impact of microstructural changes on the mechanical properties of CSG materials can be further quantified. The Image-Pro Plus 6.0 image software was utilized to process SEM images, and the measured pore characteristic parameters included surface porosity, average pore diameter, and fractal dimension. Surface porosity reflects the pore distribution of different samples observed under scanning electron microscopy—a larger surface porosity indicates a looser structure of the specimen. The average pore diameter reflects the size distribution of internal pores within the material. The fractal dimension of SEM images can represent the unevenness and roughness of the contours of measured objects in a two-dimensional plane, where a higher fractal dimension value signifies a more complex material structure. The fractal dimension was calculated by extracting the equivalent perimeter and equivalent area using the IPP software [40]. The specific calculation formula is as follows:
where L is the equivalent perimeter (μm) of the measured pore; D is the pore fractal dimension; A is the pore equivalent area (μm2); and C is a constant.
Figure 8 shows the pore characteristic parameters of CSG materials under different mix ratios. As can be seen from Figure 8, with the increase in RHA content, the surface porosity and average pore diameter first decrease and then increase, while the fractal dimension first increases and then decreases. On the one hand, RHA acts as fine particles that fill the pores; on the other hand, an appropriate amount of RHA undergoes pozzolanic reactions to generate cementitious substances, enhancing the overall compactness and complexity of the structure and forcing larger pores to transition into smaller ones. However, excessive addition of RHA leads to the incomplete hydration of some RHA particles, adversely affecting the generation of cementitious substances and negatively impacting the internal structure of the material, causing an increase in surface porosity and average pore diameter, while the fractal dimension decreases. With the addition of alkali activators, surface porosity and average pore diameter show a decreasing trend, while the fractal dimension shows an overall increasing trend. At 5% RHA content, the values of surface porosity and average pore diameter are the smallest, and the fractal dimension reaches its maximum value. The specific images and processed data of the fractal dimension are shown in Figure 9 and Figure 10, and Table 6, respectively.
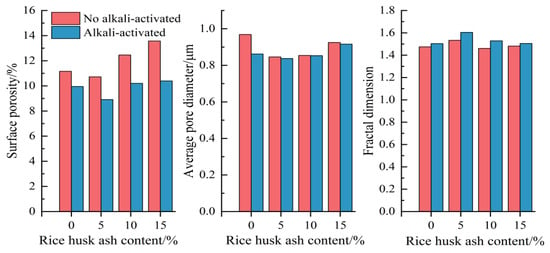
Figure 8.
Pore characteristic parameters of CSG materials.
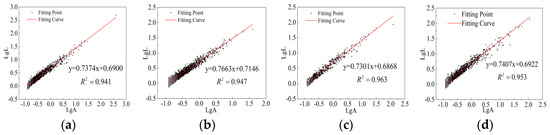
Figure 9.
Fractal dimensions of CSG samples without alkali activation. (a) 0%RHA. (b) 5%RHA. (c) 10%RHA. (d) 15%RHA.
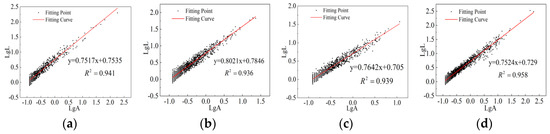
Figure 10.
Fractal dimensions of CSG samples under alkali activation. (a) 0%RHA. (b) 5%RHA. (c) 10%RHA. (d) 15%RHA.

Table 6.
Fractal dimension calculated based on scanning electron microscopy test.
4.4. Nuclear Magnetic Resonance Analysis
Nuclear magnetic resonance technology can analyze the pore distribution patterns of materials without causing damage. The T2 spectrum obtained from NMR experiments can effectively reflect the internal pore characteristics of CSG specimens. Figure 11a shows the T2 spectrum curves of CSG materials with different contents of RHA. As can be seen from Figure 11a, the T2 spectrum curve of CSG materials presents a three-peak distribution, with the first peak having a larger area. With the addition of RHA, the area of the first peak shows an increasing trend, while the area of the third peak significantly decreases. This indicates that the internal pores of the CSG samples can be roughly classified into three categories—micropores, capillary pores, and macropores—where micropores and capillary pores occupy higher proportions, while macropores occupy smaller proportions. Figure 11b presents the T2 spectrum curves of CSG materials with different contents of RHA under alkali activation. As can be seen from Figure 11b, with the addition of alkali activators, the overall shape of the T2 spectrum curve of the CSG samples remains unchanged and still exhibits a three-peak distribution. However, the first peak shifts to the right. The reason is that under the action of alkali activators, RHA reacts to generate additional cementitious substances, which may alter the original pore distribution within the material [41].
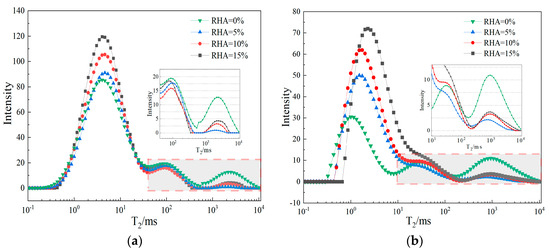
Figure 11.
T2 spectrum distribution curve of CSG materials. (a) Not alkali-activated. (b) Alkali-activated.
Figure 12a shows the pore proportions of CSG materials under different RHA contents. As can be seen from Figure 12a, with the increase in RHA content, the proportions of micropores and capillary pores show an overall upward trend, while the proportion of macropores and porosity first decreases and then increases. The reason is that a small amount of RHA is added to improve the pore structure, but when an excessive amount of RHA is added, it adheres to the surface of the aggregates without reacting, absorbing a large amount of water and inhibiting the progress of early hydration reactions. This, in turn, increases the local water–cement ratio of the particles within the material, resulting in an increase in the proportion of macropores and porosity [42]. Figure 12b presents the pore proportions of CSG materials with different contents of RHA under alkali activation. As shown in Figure 12b, with the addition of alkali activators, the proportions of micropores and macropores fluctuate slightly, while the proportions of capillary pores and porosity significantly decrease. This indicates that the addition of alkali activators promotes the generation of cementitious substances, with a more pronounced effect on improving capillary pores.
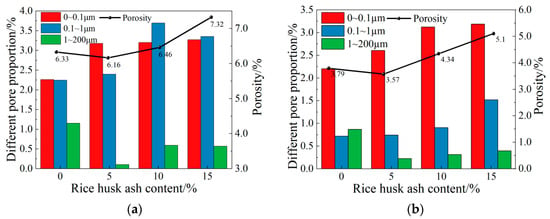
Figure 12.
Proportions of different pore distributions in CSG materials. (a) Not alkali-activated. (b) Alkali-activated.
By quantifying the complexity of SEM images into fractal dimension, the size of the fractal dimension can reflect the complexity of the internal structure of CSG materials. However, it only represents a two-dimensional cross-section and has associated uncertainty. By calculating the fractal dimension of the pore volume using nuclear magnetic resonance technology, the changes in the internal pore structure of CSG materials can be analyzed from a three-dimensional perspective, providing deeper quantitative insights into pore structure characteristics [43]. The specific calculation formula is as follows:
where Sv is the ratio of the cumulative pore volume to the total pore volume when the transverse relaxation time is less than T2; T2 is the relaxation time; Dv is the fractal dimension of pore volume; and T2 max is the maximum relaxation time.
Divided into three regions based on micropores, capillary pores, and macropores, the relationship between lgT2 and lgSv, plotted according to Equation (2), is shown in Figure 13 and Figure 14, with detailed data on the fractal dimension of pore volume presented in Table 7. According to the Euclidean fractal geometry description, the theoretical range of the fractal dimension of pore volume in nuclear magnetic resonance is between two and three. As shown in Table 7, the fractal dimensions of micropores are mostly outside the theoretical range and lack physical significance [44]; thus, fractal analysis is not performed on them. As can be seen from Figure 13, with the addition of RHA, the overall fractal dimension first increases and then decreases. When the content of RHA is 5%, the fractal dimension is the largest, indicating that the complexity of the pore structure initially increases and then decreases. The fractal dimensions of capillary pores and macropores range from 2.884 to 2.907 and 2.960 to 2.994, respectively. The fractal dimension of macropores is greater than that of capillary pores, indicating that the pore structure inside macropores is more complex. As can be seen from Figure 14, under the action of alkali activators, the fractal dimension of capillary pores shows an overall increasing trend, whereas the fractal dimension of macropores fluctuates slightly. This indicates that the addition of alkali activators promotes the formation of cementitious substances that fill the pores, significantly affecting capillary pores and increasing the complexity of their pore structure.
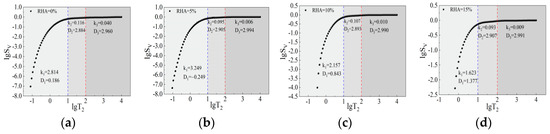
Figure 13.
Fractal dimension of pore volume of CSG specimens without alkali activation. (a) 0%RHA. (b) 5%RHA. (c) 10%RHA. (d) 15%RHA.
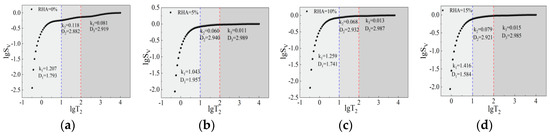
Figure 14.
Fractal dimension of pore volume of CSG specimens under alkali activation. (a) 0%RHA. (b) 5%RHA. (c) 10%RHA. (d) 15%RHA.

Table 7.
Fractal dimension of pore volume calculated using nuclear magnetic resonance.
4.5. The Relationship Between Fractal Dimension and Compressive Strength
To study the impact of pore structure changes on the mechanical properties of CSG materials, mathematical models were established correlating different types of fractal dimensions with compressive strength based on SEM and NMR experiments, and the changes in compressive strength were further predicted by fractal dimension. Since the micropore fractal dimensions obtained from nuclear magnetic resonance experiments lack physical significance, and the changes in macropore fractal dimensions are not significant, this paper selects the fractal dimension of capillary pores as the reference basis for the NMR fractal dimension. Figure 15 shows the fitting curves for different types of fractal dimensions versus compressive strength. As can be seen from Figure 15, the fractal dimension and compressive strength exhibit a binomial relationship, with the overall fitting accuracy of the curves being high, above 0.838. Compared to the fractal dimensions from the SEM experiment, the fitting effect of the capillary pore fractal dimensions from the NMR experiment is better. The reason is that the NMR test provides a comprehensive and systematic analysis of pore structure characteristics from a three-dimensional perspective, allowing for better capture of local detail changes [45].
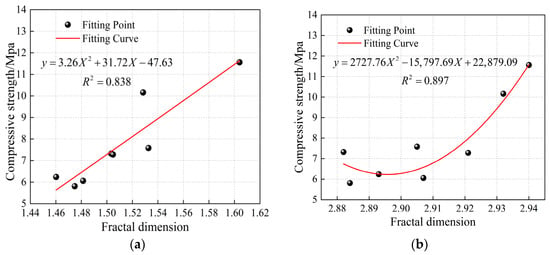
Figure 15.
Fitting curves of compressive strength of CSG specimens based on different types of fractal dimension. (a) SEM fractal dimension. (b) NMR fractal dimension.
4.6. X-Ray Diffraction Analysis
XRD diffraction analysis was performed on the CSG-R0, CSG-R5, and CSG-AR5 specimens. The main mineral components of the three groups of specimens are shown in Figure 16. As seen in Figure 16, the mineral compositions detected in the three groups are essentially identical, primarily consisting of quartz, montmorillonite, calcite, and other minerals indicating that the addition of RHA and alkali activators did not result in the formation of new phases within the specimens [46]. Compared to the control group, the incorporation of RHA leads to an increase in the SiO2 content, which is attributed to the presence of a large quantity of amorphous SiO2 in RHA. The unreacted SiO2 exists in its original form, resulting in an increase in the SiO2 diffraction peaks. After the addition of the alkali activator, a large amount of calcium silicate hydrate gel begins to form on the surface of crystalline SiO2, increasing the peak intensity of the hydration products while reducing the SiO2 diffraction peaks. This indicates that the alkali activator can enhance the activity of the RHA and promote the generation of cementitious substances, leading to changes in the mineral component content. Combined with SEM testing, it was found that a large number of network-structured gels were produced inside the specimens, and the surface of the fly ash underwent significant changes with the addition of the activator. This was consistent with XRD test results, confirming the generation of calcium silicate hydrate products and the solidification of the RHA with alkali activators.
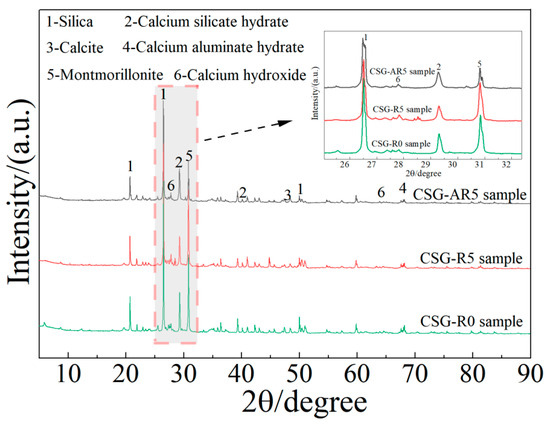
Figure 16.
XRD pattern of CSG materials.
4.7. Solidification Mechanism Study of Alkali-Activated RHA CSG Materials
By combining mechanical performance tests with microstructure analysis, it has been found that the solidifying effect of RHA on CSG materials is mainly due to its porous structure, high specific surface area, and the abundance of amorphous silica, which is manifested in physical filling and chemical reactions.
- (1)
- Physical filling
Figure 17 shows the solidification mechanism of alkali-activated RHA CSG materials. As shown in Figure 17, the CSG samples contain a small amount of cementitious materials and high porosity. After incorporating RHA, the powdered RHA can fill the capillary pores and macropores between coarse aggregates and soil particles, thereby reducing porosity and enhancing the compactness of the CSG materials. Additionally, the interface friction between RHA, soil particles, and cement is also increased.
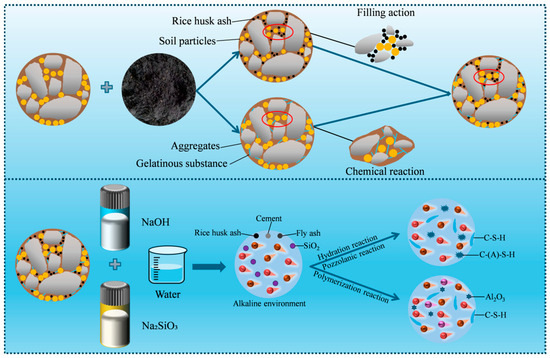
Figure 17.
Solidification mechanism of alkali-activated RHA CSG materials.
- (2)
- Chemical reactions
From the perspective of chemical reactions, the solidification mechanism of alkali-activated RHA CSG materials is mainly reflected in the following three aspects: hydration reaction, pozzolanic reaction, and polymerization reaction. When RHA, cement, soil, and water are mixed, the dicalcium silicate and tricalcium silicate contained in the cement undergo hydration reaction to form C-S-H gel. At the same time, the reactive amorphous SiO2 in RHA reacts with the generated Ca(OH)2 in a pozzolanic reaction, further producing C-S-H and C-A-S-H gel-like substances [47]. These gel-like substances exist in needle-like and network forms, providing solidification effects on the surrounding soil particles and significantly enhances their mechanical properties. And moderate amounts of RHA can accelerate the hydration process of cement-based cementitious materials, increase the early hydration rate, and further improve the early strength of specimens. Moreover, the alkali activator provides an alkaline environment and a large number of OH− and SiO32− ions, which undergo polymerization reactions with the active SiO2 in rice husk ash (RHA) and Al2O3 in the soil, forming aluminosilicate gels and establishing stable crystalline structures [48]. The specific chemical reaction equations are as follows:
Hydration reaction, Pozzolanic reaction
Polymerization reaction
5. Conclusions
Through examining the mechanical properties and microstructure of CSG materials with high mud content, combined with pore characteristic parameters and pore volume fractal dimensions, this study reveals the enhancement mechanism of action by which alkali-activated RHA enhances the mechanical properties of CSG materials from a microscopic perspective. The main conclusions are as follows:
RHA significantly enhances the macroscopic mechanical properties of CSG materials. As the content of RHA increases, the compressive strength and elastic modulus first increase and then decrease, while the failure strain first decreases and then increases. The failure mode transitions from splitting failure without RHA to shear failure. The addition of alkali activators enhances the pozzolanic activity of RHA and fly ash, further improving the overall strength of the CSG specimens. The mechanical properties are optimal at a 5% RHA content.
RHA improves the internal microstructure of the materials. The addition of an appropriate amount of RHA not only fills some of the small pores, but also generates needle-like and reticular cementitious substances under alkali activation, which help to solidify the loose fine-grained soil. However, excessive incorporation of RHA can inhibit part of the hydration reactions, resulting in a relative weakening of the bonding effect between particles. When the RHA content is 5%, the generated cementitious substances are maximal, the effects of filling pores and solidifying fine-grained soil are most pronounced, and the microstructure is the densest.
The results based on NMR fractal dimension show that, with the addition of RHA, the fractal dimensions of the capillary pores and macropores show a trend of first increasing and then decreasing. Under the influence of the alkali activator, the capillary pores exhibit an overall increasing trend, while the fractal dimension of the macropores fluctuates slightly. Compared to the SEM fractal dimension, the NMR fractal dimension has a better fit effect with compressive strength. The XRD results indicate that the addition of RHA and the alkali activator causes changes in the mineral composition, but no new phases are generated within the materials.
Author Contributions
Conceptualization, X.Z.; Methodology, K.C.; investigation, C.S.; data curation, L.G.; writing—original draft preparation, R.L.; writing—review and editing, H.H.; supervision, Q.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52109154); Henan Province Science and Technology Research Project (192102310224).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, X.; Zhang, Y.; Guo, X.; Zhang, X.; Li, F.; Zhang, T. Review on research progress of cemented sand and gravel dam. Sci. Eng. Compos. Mater. 2022, 29, 438–451. [Google Scholar] [CrossRef]
- Cai, X.; Wu, Y.; Guo, X.; Ming, Y. Research review of the cement sand and gravel (CSG) dam. Front. Struct. Civ. Eng. 2012, 6, 19–24. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Xu, L.; Wang, J. Study on the safety of cemented sand and gravel dams based on the profile form of model tests. In Structures; Elsevier: Amsterdam, The Netherlands, 2023; Volume 56, p. 104978. [Google Scholar]
- Haeri, S.M.; Hosseini, S.M.; Toll, D.G.; Yasrebi, S.S. The behaviour of an artificially cemented sandy gravel. Geotech. Geol. Eng. 2005, 23, 537–560. [Google Scholar] [CrossRef]
- Karimi, S.; Farshbaf Aghajani, H. The strength and microstructure of cemented sand-gravel (CSG) mixture containing fine-grained particles. Int. J. Geo-Eng. 2023, 14, 5. [Google Scholar] [CrossRef]
- Hao, N.; Li, X.; Li, Y.; Jia, J.; Gao, L. A novel reliability-based method of calibrating safety factor: Application to the cemented sand and gravel dams. Eng. Geol. 2022, 306, 106719. [Google Scholar]
- Ai-min, G.; Kang, Y.; Zhuo, J.; Huang, Y.-E.; Wang, F.-L.; Shao, S.-Q.; Luo, J.-H. Analysis of the Influence of Mud Content on the Mechanical Properties and Microstructure of Cemented Sand and Gravel. J. Chang. River Sci. Res. Inst. 2024. [Google Scholar]
- SL 678-2014; Technical Guideline for Cemented Granular Material Dams. Water & Power Press: Beijing, China, 2014.
- Kaur, K.; Singh, J.; Kaur, M. Compressive strength of rice husk ash based geopolymer: The effect of alkaline activator. Constr. Build. Mater. 2018, 169, 188–192. [Google Scholar]
- Hossain, S.S.; Roy, P.K.; Bae, C.J. Utilization of waste rice husk ash for sustainable geopolymer: A review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Somna, R.; Saowapun, T.; Somna, K.; Chindaprasirt, P. Rice husk ash and fly ash geopolymer hollow block based on NaOH activated. Case Stud. Constr. Mater. 2022, 16, e01092. [Google Scholar]
- Chen, R.; Congress, S.S.C.; Cai, G.; Duan, W.; Liu, S. Sustainable utilization of biomass waste-rice husk ash as a new solidified material of soil in geotechnical engineering: A review. Constr. Build. Mater. 2021, 292, 123219. [Google Scholar]
- Chen, R.; Cai, G.; Dong, X.; Pu, S.; Dai, X.; Duan, W. Green utilization of modified biomass by-product rice husk ash: A novel eco-friendly binder for stabilizing waste clay as road material. J. Clean. Prod. 2022, 376, 134303. [Google Scholar]
- Cai, D.; Ouyang, M.; Bao, X.; Zhang, Q.; Bi, Z.; Yan, H.; Li, S.; Shi, Y. Performance Evaluation of Stabilized Soils with Selected Common Waste Materials of Rice Husk Ash, Steel Slag and Iron Tailing Powder. Materials 2025, 18, 346. [Google Scholar] [CrossRef]
- Li, N.; Yu, S.; Wu, E.; Song, X.; Jiang, P.; Xu, H.; Wang, W. Study on small strain characteristics and microscopic mechanism of rice husk ash modified lime soil. Transp. Geotech. 2024, 45, 101209. [Google Scholar] [CrossRef]
- Li, L.H.; Han, Q.P.; Yang, X.; Xiao, H.; Li, W.T.; Huang, S.P. Mechanical properties and micro-mechanisms of RHA-cement solidified sludge. China Civ. Eng. J. 2022, 56, 166–176. [Google Scholar]
- Chen, R.; Congress, S.S.C.; Cai, G.; Zhou, R.; Xu, J.; Duan, W.; Liu, S. Evaluating the effect of active ions on the early performance of soft clay solidified by modified biomass waste-rice husk ash. Acta Geotech. 2023, 18, 1039–1056. [Google Scholar]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.; Setunge, S.; Dissanayake, R. Engineering properties of waste-based alkali activated concrete brick containing low calcium fly ash and rice husk ash: A comparison with traditional Portland cement concrete brick. J. Build. Eng. 2022, 46, 103810. [Google Scholar]
- Das, S.K.; Adediran, A.; Kaze, C.R.; Mustakim, S.M.; Leklou, N. Production, characteristics, and utilization of rice husk ash in alkali activated materials: An overview of fresh and hardened state properties. Constr. Build. Mater. 2022, 345, 128341. [Google Scholar]
- Fernando, S.; Nasvi, M.C.M.; Gunasekara, C.; Law, D.W.; Setunge, S.; Dissanayake, R. Systematic review on alkali-activated binders blended with rice husk ash. J. Mater. Civ. Eng. 2021, 33, 04021229. [Google Scholar]
- Zhao, W.; Ji, C.; Sun, Q.; Gu, Q. Preparation and microstructure of alkali-activated rice husk ash-granulated blast furnace slag tailing composite cemented paste backfill. Materials 2022, 15, 4397. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Li, W.; Xiao, H.; Xu, K.; Yang, J. Mechanical properties of soil reinforced by fiber and alkaline-activated rice husk ash, and rainfall erosion model tests. Sci. Total Environ. 2025, 958, 178099. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R.; Ismail, M.G.M.U. Investigation of the reaction mechanism of blended fly ash and rice husk ash alkali-activated binders. Arch. Civ. Mech. Eng. 2022, 22, 24. [Google Scholar]
- Zhang, X.; Qiu, Q.; Huang, H.; Cao, K.; Song, Y.; Guo, L.; Ge, H. Mesoscopic hysteretic model and parameter study on cemented sand and gravel material. Case Stud. Constr. Mater. 2023, 19, e02571. [Google Scholar]
- Sun, X.; She, D.; Fei, Y.; Wang, H.; Gao, L. Three-dimensional fractal characteristics of soil pore structure and their relationships with hydraulic parameters in biochar-amended saline soil. Soil Tillage Res. 2021, 205, 104809. [Google Scholar]
- Bai, Y.; Qin, Y.; Lu, X.; Zhang, J.; Chen, G.; Li, X. Fractal dimension of particle-size distribution and their relationships with alkalinity properties of soils in the western Songnen Plain, China. Sci. Rep. 2020, 10, 20603. [Google Scholar]
- Zhao, M.; Kang, R.; Chen, S.; Zhang, J.; Chen, G.; Li, X. Study on the scale effect and mechanical properties of gravel materials in riverbed cover layers. Sci. Rep. 2024, 14, 30698. [Google Scholar]
- Zhang, P.; Gao, Z.; Shi, Y.; Lin, Y.; Li, J. Effect of large broken stone content on properties of roller compacted concrete based on fractal theory. Constr. Build. Mater. 2020, 262, 120821. [Google Scholar] [CrossRef]
- Lü, Q.; Qiu, Q.; Zheng, J.; Wang, J.; Zeng, Q. Fractal dimension of concrete incorporating silica fume and its correlations to pore structure, strength and permeability. Constr. Build. Mater. 2019, 228, 116986. [Google Scholar]
- Yang, Y.; Wang, B.; Yuan, Q.; Huang, D.; Peng, H. Characterization, factors, and fractal dimension of pore structure of fly ash-based geopolymers. J. Mater. Res. Technol. 2023, 26, 3395–3407. [Google Scholar]
- GB 175-2007; Common Portland Cement. China Building Materials Research Institute: Beijing, China, 2007.
- GB/T 50123-2019; Ministry of Housing and Urban-Rural Development of the People′s Republic of China. Standard for Geotechnical Testing Method; China Planning Press: Beijing, China, 2019.
- Ferreira, F.A.; Desir, J.M.; De Lima, G.E.S.; Pedroti, L.G.; de Carvalho, J.M.F.; Lotero, A.; Consoli, N.C. Evaluation of mechanical and microstructural properties of eggshell lime/rice husk ash alkali-activated cement. Constr. Build. Mater. 2023, 364, 129931. [Google Scholar]
- Su, Q.; Xu, J. Mechanical properties of rice husk ash and glass powder concrete: Experimental and mesoscopic studies. J. Build. Eng. 2024, 95, 110278. [Google Scholar]
- Li, L.; Yue, Y.; Xiao, H.; Li, W.; Han, Q.; Cao, Y. Performance and influence mechanism of Cd-contaminated soil solidified by rice husk ash-cement. Chin. J. Geotech. Eng. 2023, 45, 252–261. [Google Scholar]
- Gong, F.; Zhang, D.; Sicat, E.; Ueda, T. Empirical estimation of pore size distribution in cement, mortar, and concrete. J. Mater. Civ. Eng. 2014, 26, 04014023. [Google Scholar]
- Ding, F.; Fan, X.; Xie, Y.; Jiang, S.; Qiu, C.; Sun, D.; Wu, R. Combined effect of rice husk ash and cementitious capillary crystalline waterproofing materials on the performance of mortar. J. Build. Eng. 2024, 84, 108479. [Google Scholar]
- Wang, J.; Xiao, J.; Zhang, Z.; Han, K.; Hu, X.; Jiang, F. Action mechanism of rice husk ash and the effect on main performances of cement-based materials: A review. Constr. Build. Mater. 2021, 288, 123068. [Google Scholar]
- Kunchariyakun, K.; Asavapisit, S.; Sinyoung, S. Influence of partial sand replacement by black rice husk ash and bagasse ash on properties of autoclaved aerated concrete under different temperatures and times. Constr. Build. Mater. 2018, 173, 220–227. [Google Scholar]
- Kong, B.; Dai, C.X.; Hu, H.; Xia, J.; He, S.-H. The fractal characteristics of soft soil under cyclic loading based on SEM. Fractal Fract. 2022, 6, 423. [Google Scholar] [CrossRef]
- Bai, Y.; Xie, Y.; Chen, Y. Alkali-activated rice husk ash-foamed composites: Correlation between pore structure, hydration, and hardening properties. Int. J. Appl. Ceram. Technol. 2025, 22, e14941. [Google Scholar]
- Wang, J.; Hu, X.; Jiang, F.; Chen, H. The Role and Mechanism of Rice Husk Ash Particle Characteristics in Cement Hydration Process. Materials 2024, 17, 5594. [Google Scholar] [CrossRef]
- Zhao, K.; Ma, C.; Yang, J.; Wu, J.; Yan, Y.; Lai, Y.; Ao, W.; Tian, Y. Pore fractal characteristics of fiber-reinforced backfill based on nuclear magnetic resonance. Powder Technol. 2023, 426, 118678. [Google Scholar] [CrossRef]
- Tang, S.; Huang, J.; Duan, L.; Yu, P.; Chen, E. A review on fractal footprint of cement-based materials. Powder Technol. 2020, 370, 237–250. [Google Scholar] [CrossRef]
- Guo, J.C.; Zhou, H.Y.; Zeng, J.; Wang, K.-J.; Lai, J.; Liu, Y.-X. Advances in low-field nuclear magnetic resonance (NMR) technologies applied for characterization of pore space inside rocks: A critical review. Pet. Sci. 2020, 17, 1281–1297. [Google Scholar] [CrossRef]
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese rice husk ash for the production of sodium silicate as the activator for alkali-activated binders. J. Clean. Prod. 2018, 201, 272–286. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Hwang, C.L.; Huynh, T.P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).