Cerofolini’s Model and the Fractal Adsorption Isotherms
Abstract
1. Introduction
2. Theory
2.1. Cerofolini’s Model
2.2. Geometric and Energetic Surface Heterogeneities. Partially Correlated Surfaces
3. Results and Discussions. Energetic Fractal Relations and Fractal Isotherms
3.1. A Simple Fractal Relation between Excess Energy and Adsorption Energy
3.2. A Generalized Fractal Relation between Excess Energy and Adsorption Energy
3.3. A Fractal Relation between Adsorption Energy and Pore Size
3.4. Energetic Fractal Dimension Computation: “A Simple Application”
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cerofolini, G.F. A model which allows for the Freundlich and the Dubinin-Radushkevich adsorption isotherms. Surf. Sci. 1975, 51, 333–335. [Google Scholar] [CrossRef]
- Cerofolini, G.F. Equilibrium surfaces. Surface Sci. 1976, 61, 678–682. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S. Adsorption, Surface Area and Porosity; Academic Press: New York, NY, USA, 1982; pp. 15–20. [Google Scholar]
- Avnir, D.; Farin, D.; Pfeifer, P. Surface geometric irregularity of particulate materials: The fractal approach. J. Colloid Interface Sci. 1985, 103, 112–123. [Google Scholar] [CrossRef]
- Cole, M.W.; Holter, N.S.; Pfeifer, P. Henry’s law of adsorption on a fractal surface. Phys. Rev. B 1986, 33, 8806–8809. [Google Scholar] [CrossRef]
- Fripiat, I.J.; Gatineau, L.; van Damme, H. Multilayer physical adsorption on fractal surface. Langmuir 1986, 2, 562–567. [Google Scholar] [CrossRef]
- Pfeifer, P.; Obert, M.; Cole, M.W. Fractal BET and FHH Theories of Adsorption: A Comparative Study. Proc. R. Soc. Lond. A 1989, 423, 169–188. [Google Scholar] [CrossRef]
- Pfeifer, P.; Wu, Y.J.; Cole, M.W.; Krim, J. Multilayer adsorption on a fractally rough surface. Phys. Rev. Lett. 1989, 62, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Avnir, D.; Jaroniec, M. An isotherm equation for adsorption on fractal surfaces of heterogeneous porous materials. Langmuir 1989, 5, 1431–1433. [Google Scholar] [CrossRef]
- Avnir, D.; Biham, O.; Lidar, D.; Malci, O. Is the geometry of nature fractal? Science 1998, 279, 39–40. [Google Scholar] [CrossRef]
- Pfeifer, P. Fractal dimension as working tool for surface-roughness problems. Appl. Surf. Sci. 1984, 18, 146–164. [Google Scholar] [CrossRef]
- Conner, W.; Bennett, C.O. Are the pore and surface morphologies of real catalysts fractal? J. Chem. Soc. Faraday Trans. 1993, 89, 4109–4114. [Google Scholar] [CrossRef]
- Rudzinski, W.; Lee, S.-L.; Yan, C.-C.; Panczyk, T. A Fractal Approach to Adsorption on Heterogeneous Solid Surfaces. 1. The Relationship between Geometric and Energetic Surface Heterogeneities. J. Phys. Chem. B 2001, 105, 10847–10856. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Gauden, P.A.; Kowalczyk, P. Fractal Geometry Concept in Physical Adsorption on Solids. Arab. J. Sci. Eng. 2003, 28, 133–167. [Google Scholar]
- Rudzinski, W.; Everett, D.H. Adsorption of Gases on Heterogeneous Surfaces; Academic Press: London, UK, 1992; pp. 180–190. [Google Scholar]
- Giona, M.; Giustiniani, M.; Ludlow, D. Influence of geometric and energetic heterogeneity on adsorption isotherms. Fractals 1995, 3, 235–250. [Google Scholar] [CrossRef]
- Ehrburger-Dolle, F. Analysis of the derived curves of adsorption isotherms. Langmuir 1997, 13, 1189–1198. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K.J. Method for the Calculation of Effective Pore Size Distribution in Molecular Sieve Carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H.C. Curvature and parametric sensitivity in models for adsorption in micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Everett, D.H.; Powl, J.C. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons. J. Chem. Soc. Faraday Trans. 1976, 72, 619–636. [Google Scholar] [CrossRef]
- Kaminsky, R.D.; Maglara, E.; Conner, W.C. A Direct Assessment of Mean-Field Methods of Determining Pore Size Distributions of Microporous Media from Adsorption Isotherm Data. Langmuir 1994, 10, 1556–1565. [Google Scholar] [CrossRef]
- Cheng, L.S.; Yang, R.T. Improved Horvath—Kawazoe equations including spherical pore models for calculating micropore size distribution. Chem. Eng. Sci. 1994, 16, 2599–2609. [Google Scholar] [CrossRef]
- Mariwala, R.K.; Foley, H.C. Calculation of Micropore Sizes in Carbogenic Materials from Methyl Chloride Adsorption Isotherms. Ind. End. Chem. Res. 1994, 33, 2314–2321. [Google Scholar] [CrossRef]
- Dubinin, M.M. Porous Structure of Adsorbents and Catalysts. Adv. Colloid Interface Sci. 1968, 2, 217–235. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the Concepts of Volume Filling of Micropores in the Adsorption of Gases and Vapors by Microporous Adsorbents. Russ. Chem. Bull. 1971, 20, 8–12. [Google Scholar] [CrossRef]
- Ionescu, N.I. Cerofolini’s Model and the Adsorption Isotherms. Rev. Roum. Chim. 1979, 24, 83–84. [Google Scholar]
- Hobson, J.P.; Armstrong, R.A. A study of physical adsorption at very low pressures using ultrahigh vacuum techniques. J. Phys. Chem. 1963, 67, 2000–2007. [Google Scholar] [CrossRef]
- Dobrescu, G.; Papa, F.; Atkinson, I.; Culita, D.; Balint, I. Correlation between the basicity and the fractal dimension of Rh-nanoparticles supported on Al2O3, TiO2 and WO3. IOSR-JAC 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Dobrescu, G.; Papa, F.; State, R.; Raciulete, M.; Berger, D.; Balint, I.; Ionescu, N.I. Modified Catalysts and Their Fractal Properties. Catalysts 2021, 11, 1518. [Google Scholar] [CrossRef]
- Family, F.; Vicsek, T. Scaling of the active zone in the Eden process on percolation networks and the ballistic deposition model. J. Phys. A Math. Gen. 1985, 18, L75–L81. [Google Scholar] [CrossRef]
- Chauvy, P.F.; Madore, C.; Landolt, D. Variable length scale analysis of surface topography: Characterization of titanium surfaces for biomedical applications. Surf. Coat. Technol. 1998, 110, 48–56. [Google Scholar] [CrossRef]
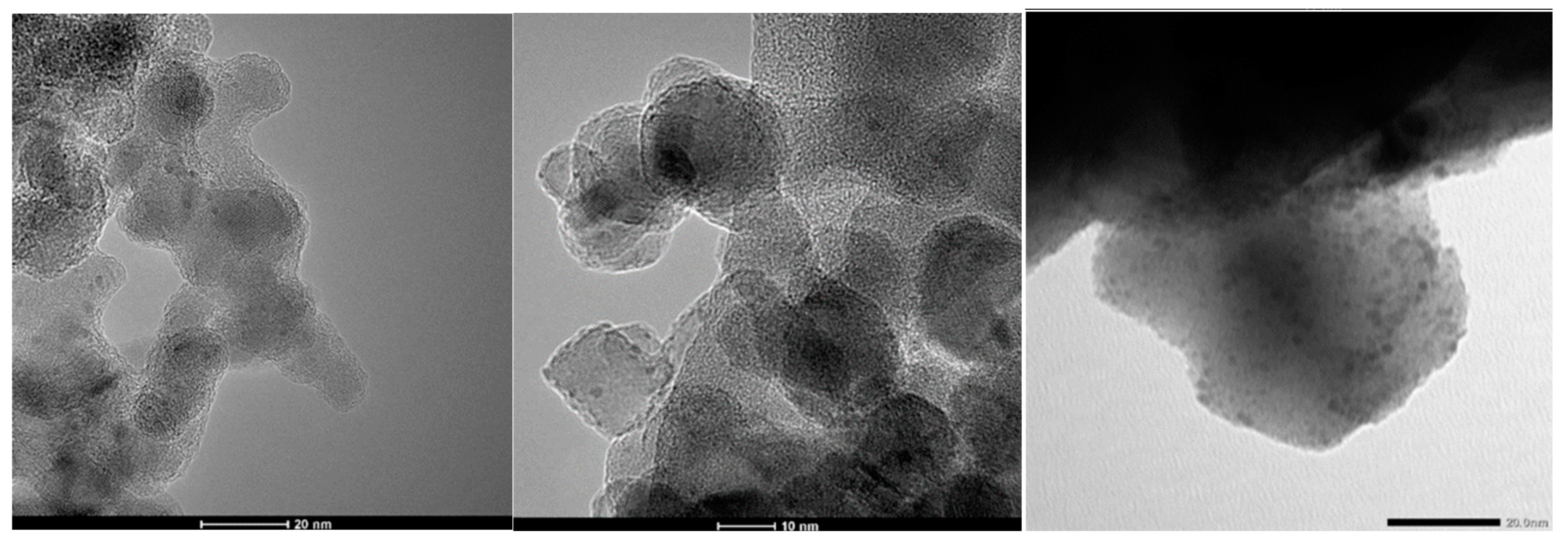
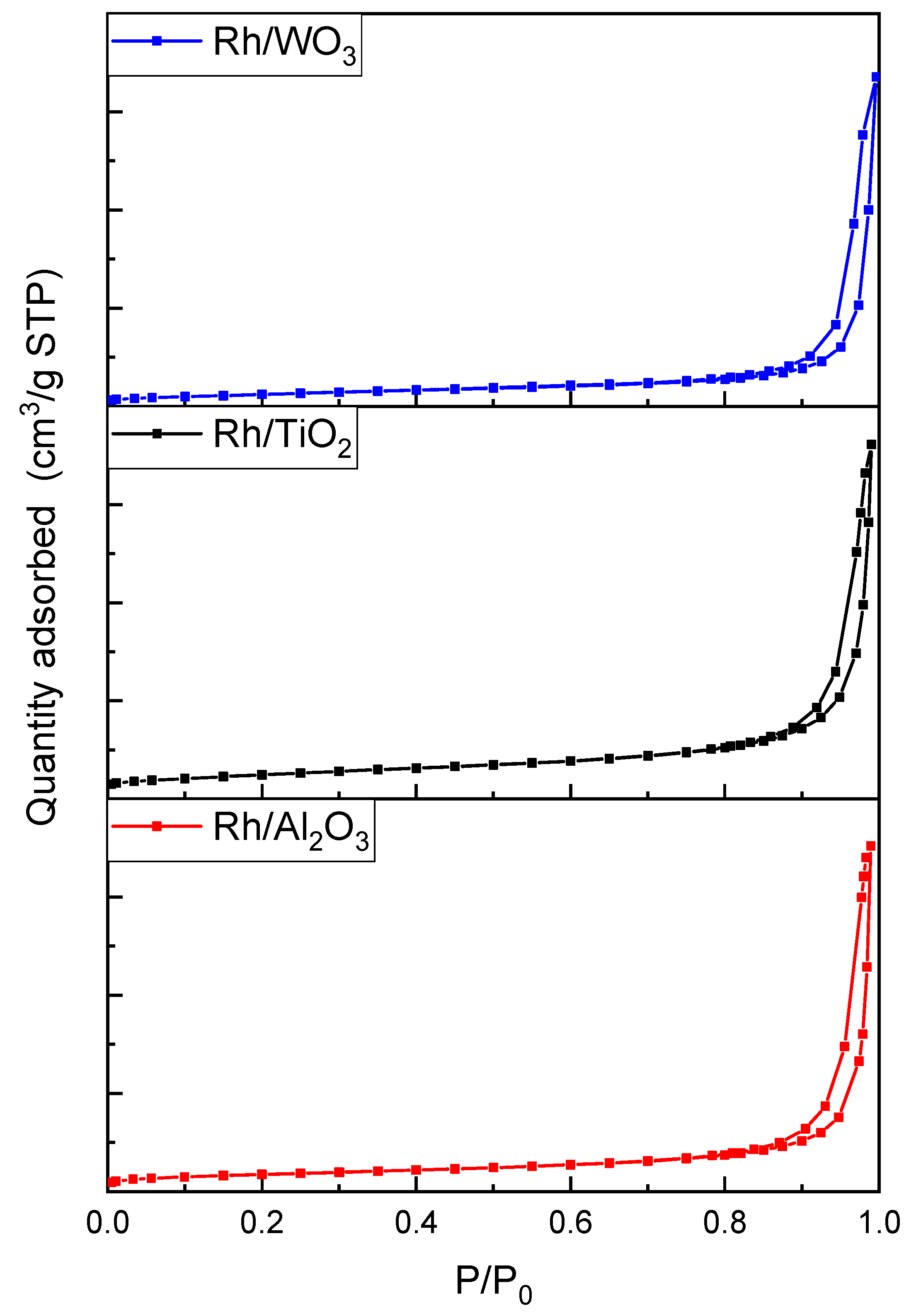
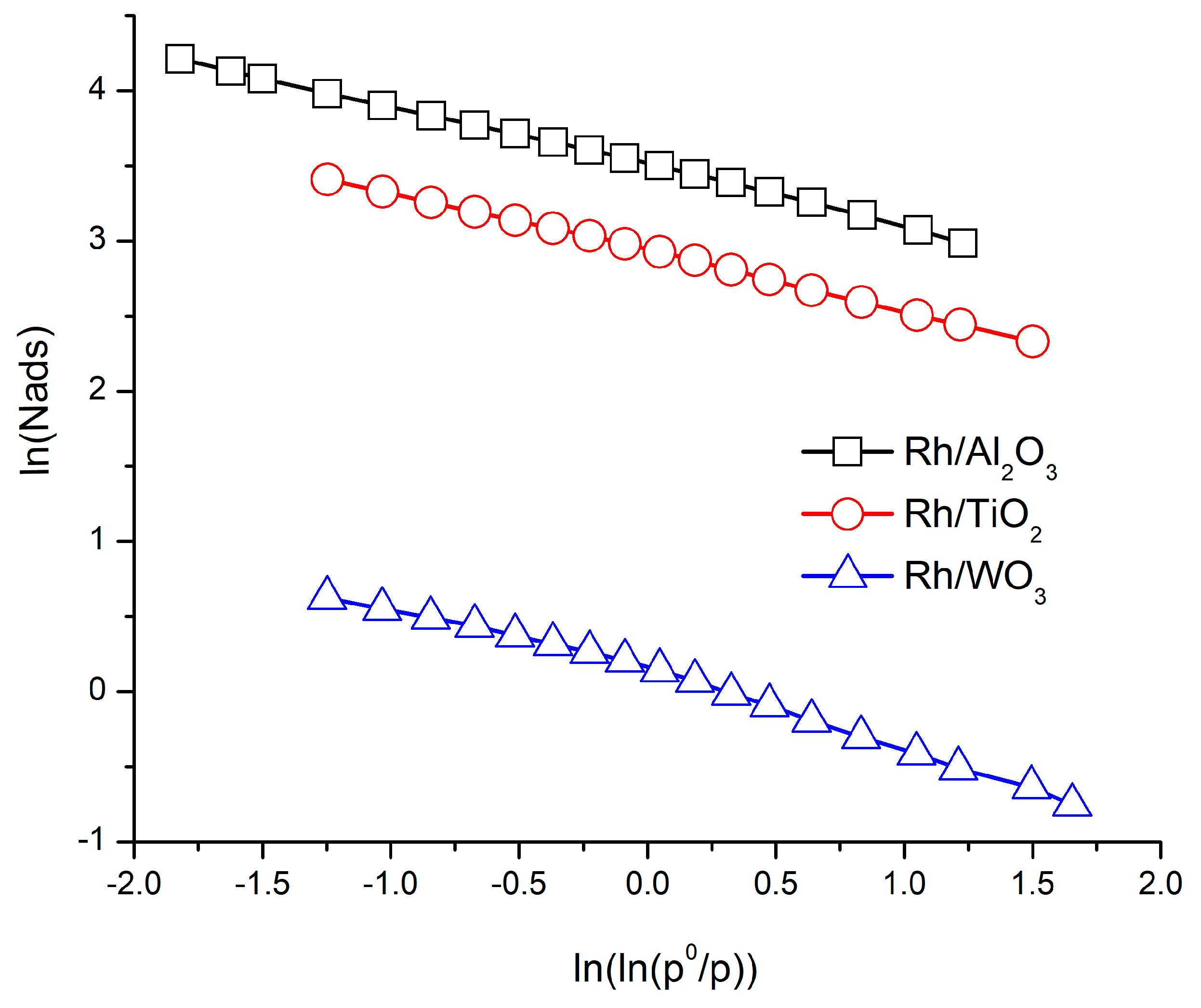
| Sample | TEM Fractal Dimension | Method | Self-Similarity Domain (nm) | DR Fractal Dimension Equation (22a) | Self-Similarity Domain (p/p0) |
|---|---|---|---|---|---|
| Rh/Al2O3 | 2.872 ± 0.001 2.784 ± 0.051 | C V | 4.4–14.4 7.2–11.8 | 2.607 ± 0.004 | 0.033–0.850 |
| Rh/TiO2 | 2.733 ± 0.001 2.832 ± 0.009 | C V | 4.9–14.4 5.4–17.3 | 2.604 ± 0.003 | 0.011–0.750 |
| Rh/WO3 | 2.490 ± 0.001 2.469 ± 0.035 2.330 ± 0.001 2.226 ± 0.047 | C C V V | 0.2–2.7 2.7–13.8 4.5–11 11–29.3 | 2.448 ± 0.012 2.589 ± 0.014 | 0.005–0.200 0.200–0.750 |
| Sample | Energetic Fractal Dimension De | Standard Errors |
|---|---|---|
| Rh/Al2O3 | 0.715 | 0.023 |
| Rh/TiO2 | 1.179 | 0.003 |
| Rh/WO3 | 1.939 | 0.038 |
| 2.430 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrescu, G.; Papa, F.; Culita, D.; Balint, I.; Ionescu, N.I. Cerofolini’s Model and the Fractal Adsorption Isotherms. Fractal Fract. 2023, 7, 262. https://doi.org/10.3390/fractalfract7030262
Dobrescu G, Papa F, Culita D, Balint I, Ionescu NI. Cerofolini’s Model and the Fractal Adsorption Isotherms. Fractal and Fractional. 2023; 7(3):262. https://doi.org/10.3390/fractalfract7030262
Chicago/Turabian StyleDobrescu, Gianina, Florica Papa, Daniela Culita, Ioan Balint, and Niculae I. Ionescu. 2023. "Cerofolini’s Model and the Fractal Adsorption Isotherms" Fractal and Fractional 7, no. 3: 262. https://doi.org/10.3390/fractalfract7030262
APA StyleDobrescu, G., Papa, F., Culita, D., Balint, I., & Ionescu, N. I. (2023). Cerofolini’s Model and the Fractal Adsorption Isotherms. Fractal and Fractional, 7(3), 262. https://doi.org/10.3390/fractalfract7030262









