Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times
Abstract
:1. Introduction
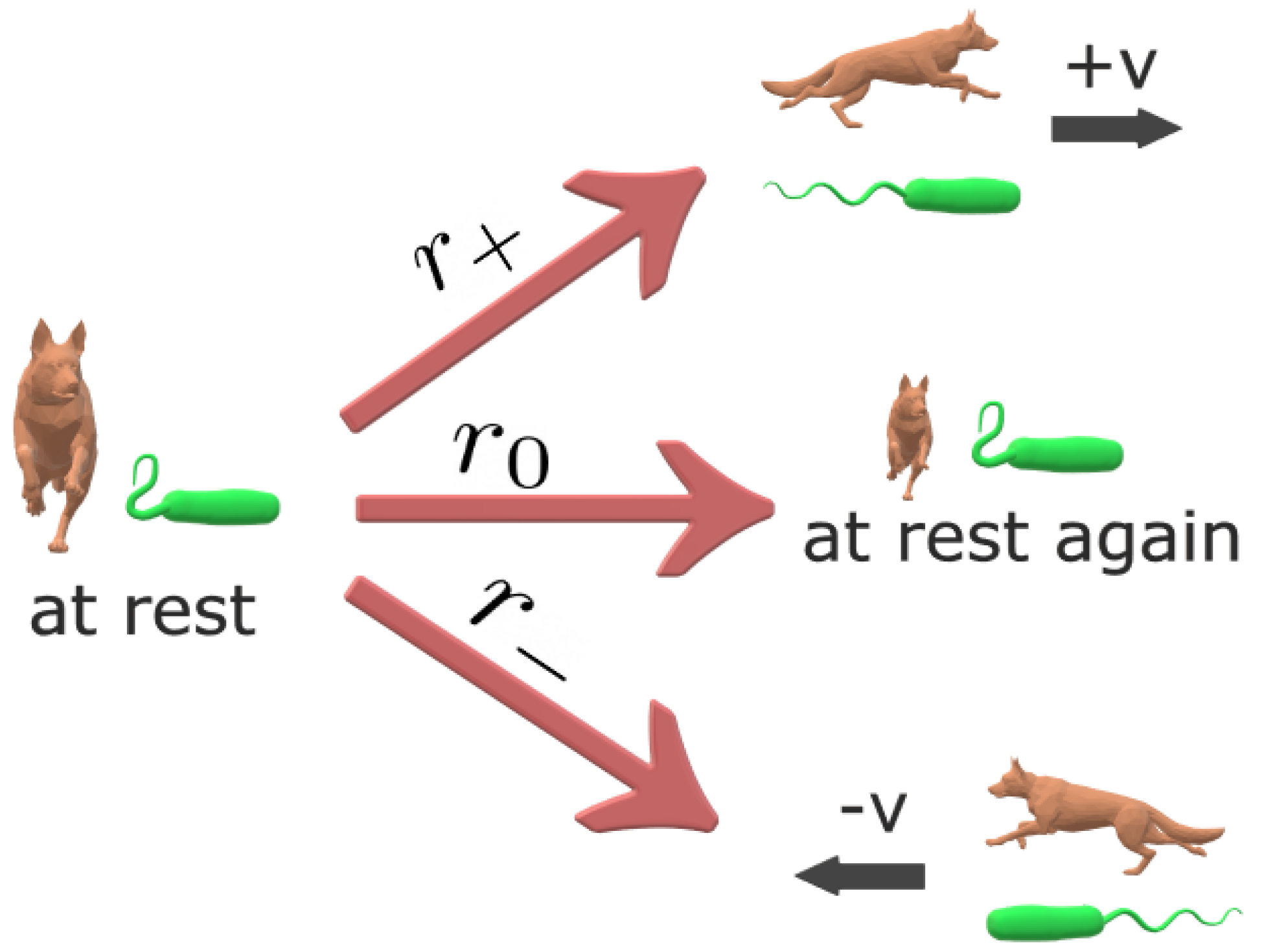
2. Stochastic Transport with Self-Reinforcement and Mittag–Leffler Distributed Rest Times
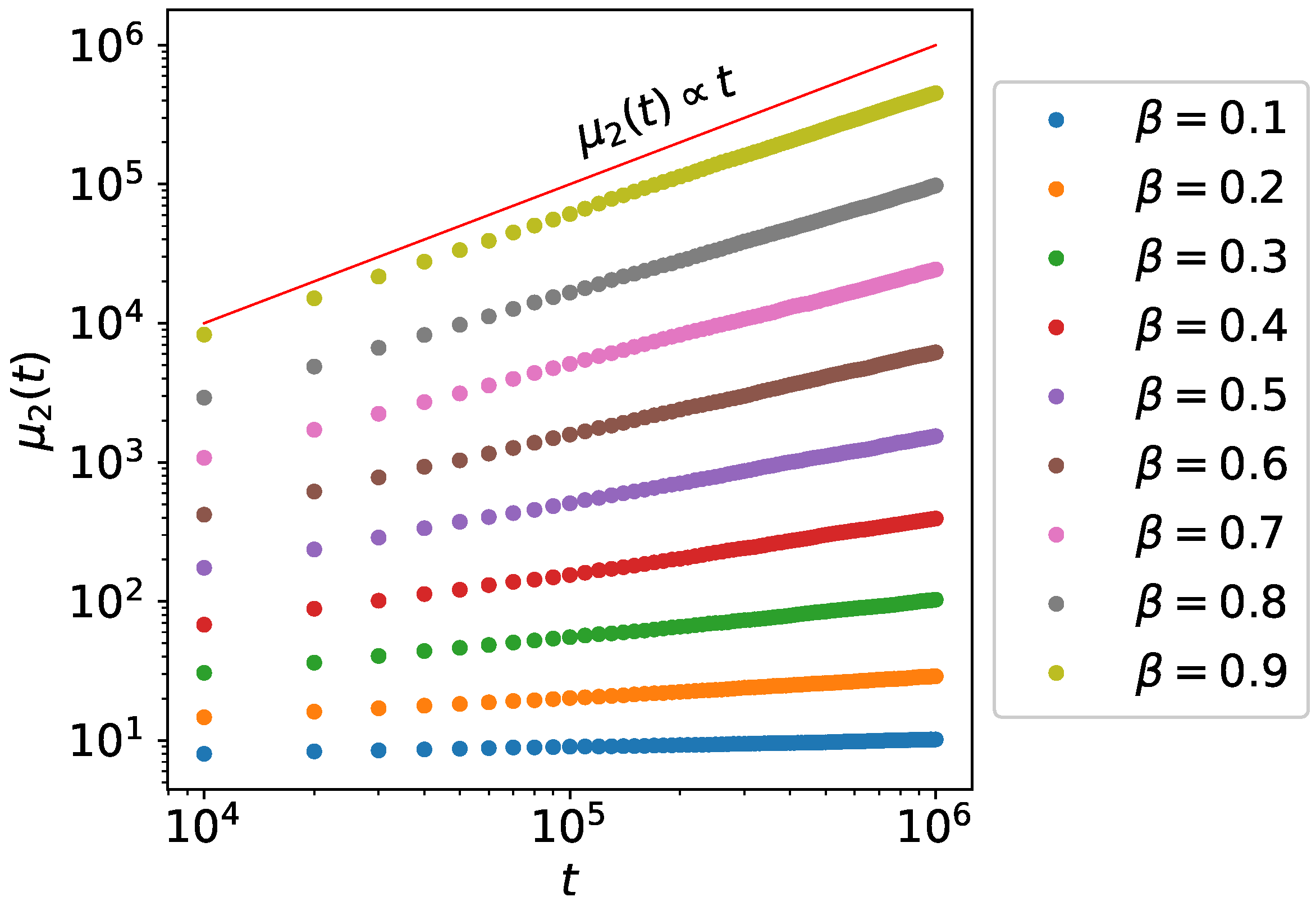
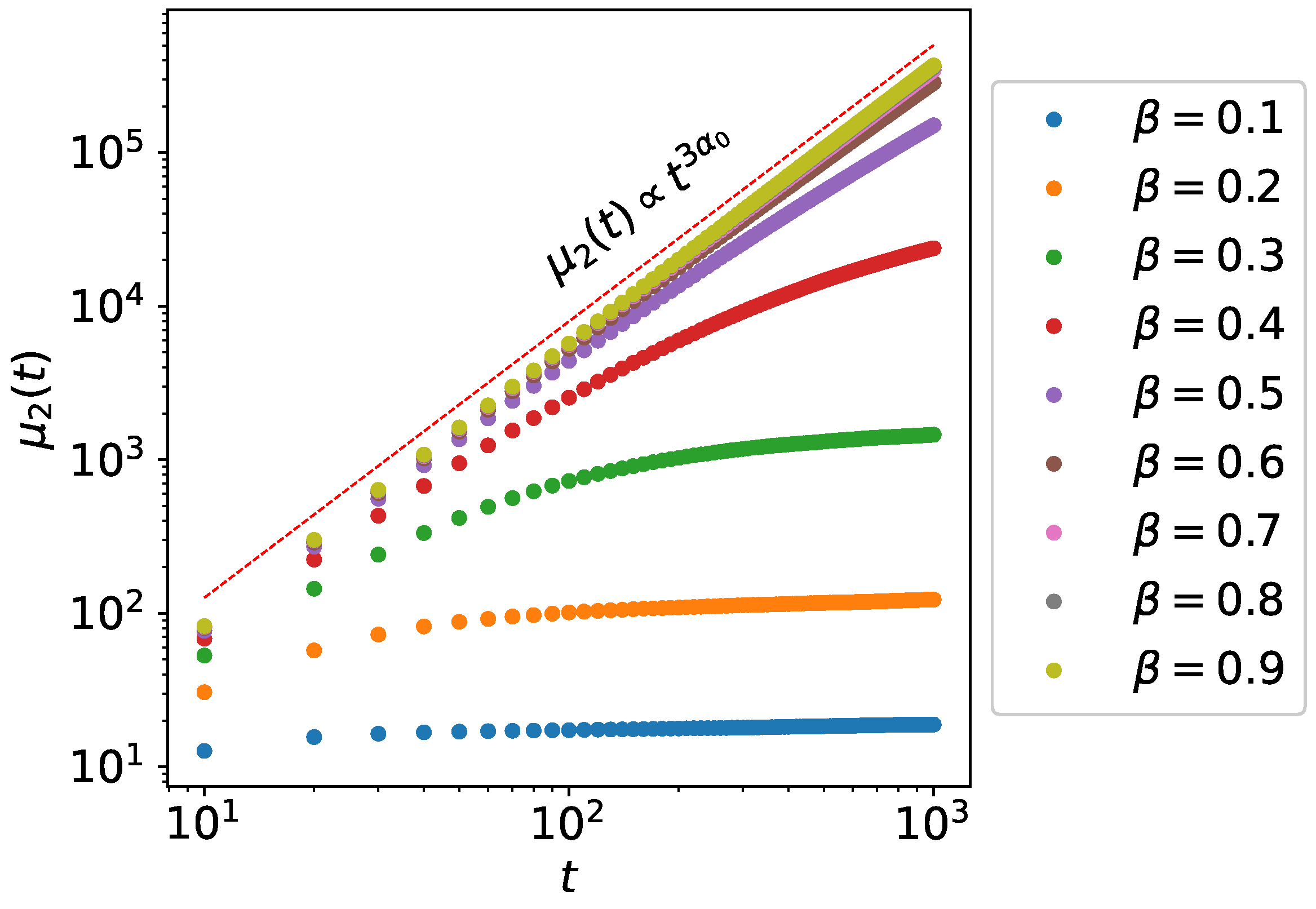
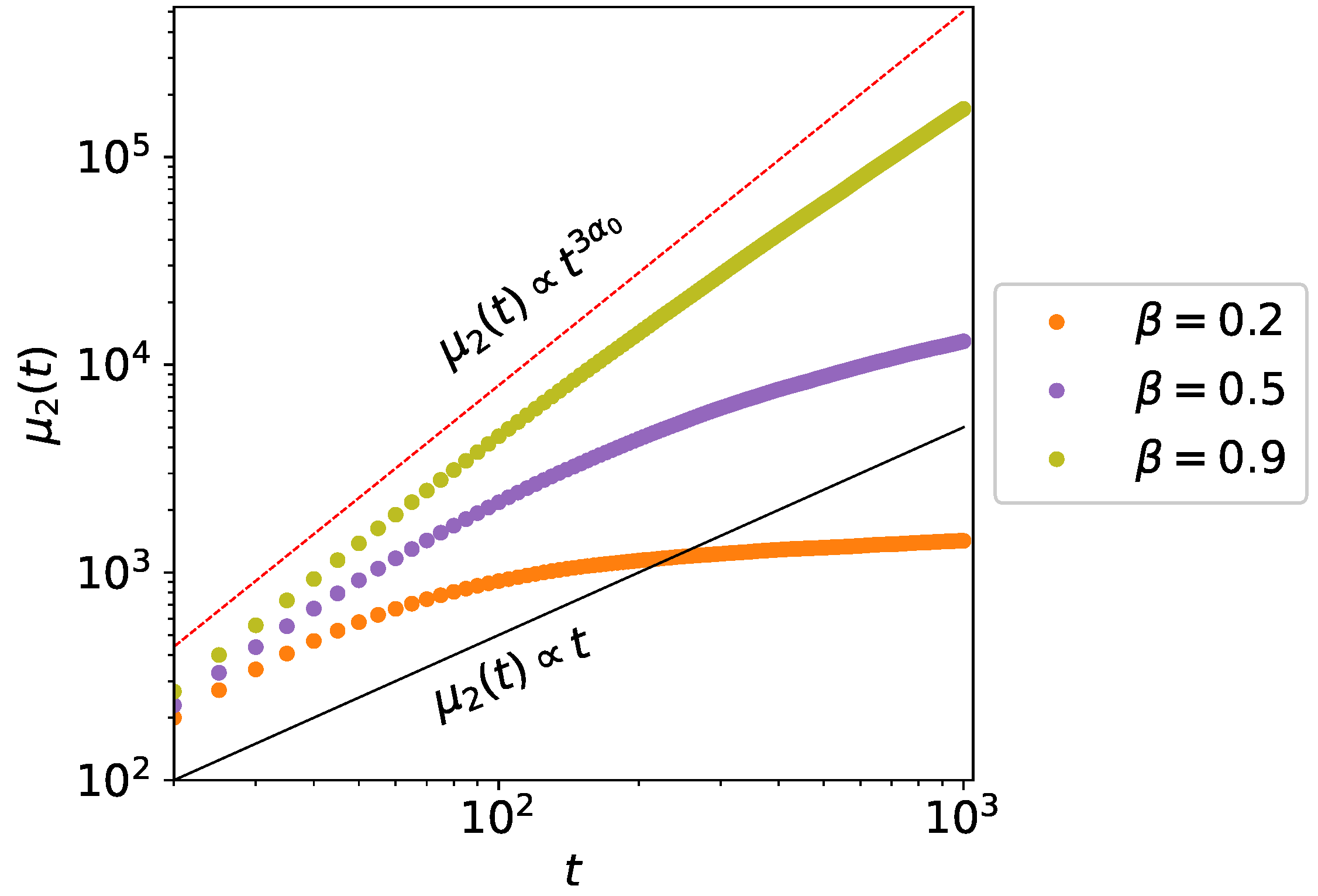
3. Second Moment Calculations
3.1. Single Active State Model
3.2. Bi-Directional Transport Model
4. Monte Carlo Simulations
- Initialize variables for current time , particle position and state . The possible values for are 0, 1 and corresponding to the rest, positive velocity and negative velocity states, respectively. For convenience, assume the random walk starts with .
- Set the constants: , , , , , and , the end time of the simulation.
- Increment simulation time and particle position .
- If , then set . Otherwise, do the following:
- -
- if , set ;
- -
- if , set ;
- -
- otherwise, set ;
where is a uniformly distributed random number and . - Iterate steps 3 to 5 until .
5. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTRW | Continuous Time Random Walk |
| PDE | Partial Differential Equation |
| Probability Density Function |
References
- Metzler, R.; Klafter, J. The random walk’s guide to anomalous diffusion: A fractional dynamics approach. Phys. Rep. 2000, 339, 1–77. [Google Scholar] [CrossRef]
- Méndez, V.; Fedotov, S.; Horsthemke, W. Reaction-Transport Systems: Mesoscopic Foundations, Fronts, and Spatial Instabilities; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Huda, S.; Weigelin, B.; Wolf, K.; Tretiakov, K.V.; Polev, K.; Wilk, G.; Iwasa, M.; Emami, F.S.; Narojczyk, J.W.; Banaszak, M.; et al. Lévy-like movement patterns of metastatic cancer cells revealed in microfabricated systems and implicated in vivo. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Rodriguez, G.; Perthame, B. Motility switching and front-back synchronisation in polarized cells. arXiv 2021, arXiv:2109.08981. [Google Scholar]
- Fedotov, S.; Korabel, N.; Waigh, T.A.; Han, D.; Allan, V.J. Memory effects and Lévy walk dynamics in intracellular transport of cargoes. Phys. Rev. E 2018, 98, 042136. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.M. Current status and future directions of Lévy walk research. Biol. Open 2018, 7, bio030106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenwright, D.A.; Harrison, A.W.; Waigh, T.A.; Woodman, P.G.; Allan, V.J. First-passage-probability analysis of active transport in live cells. Phys. Rev. E 2012, 86, 031910. [Google Scholar] [CrossRef]
- Han, D.; Korabel, N.; Chen, R.; Johnston, M.; Gavrilova, A.; Allan, V.J.; Fedotov, S.; Waigh, T.A. Deciphering anomalous heterogeneous intracellular transport with neural networks. eLife 2020, 9, e52224. [Google Scholar] [CrossRef]
- Chen, K.; Wang, B.; Granick, S. Memoryless self-reinforcing directionality in endosomal active transport within living cells. Nat. Mater. 2015, 14, 589. [Google Scholar] [CrossRef]
- Han, D.; da Silva, M.A.; Korabel, N.; Fedotov, S. Self-reinforcing directionality generates truncated Lévy walks without the power-law assumption. Phys. Rev. E 2021, 103, 022132. [Google Scholar] [CrossRef]
- Portillo, I.G.; Campos, D.; Méndez, V. Intermittent random walks: Transport regimes and implications on search strategies. J. Stat. Mech. Theory Exp. 2011, 2011, P02033. [Google Scholar] [CrossRef]
- Zaburdaev, V.; Denisov, S.; Klafter, J. Lévy walks. Rev. Mod. Phys. 2015, 87, 483. [Google Scholar] [CrossRef] [Green Version]
- Klafter, J.; Sokolov, I.M. First Steps in Random Walks: From Tools to Applications; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Schütz, G.M.; Trimper, S. Elephants can always remember: Exact long-range memory effects in a non-Markovian random walk. Phys. Rev. E 2004, 70, 045101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Harbola, U.; Lindenberg, K. Memory-induced anomalous dynamics: Emergence of diffusion, subdiffusion, and superdiffusion from a single random walk model. Phys. Rev. E 2010, 82, 021101. [Google Scholar] [CrossRef] [Green Version]
- Paraan, F.N.C.; Esguerra, J.P. Exact moments in a continuous time random walk with complete memory of its history. Phys. Rev. E 2006, 74, 032101. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.; Viswanathan, G.; Cressoni, J. Ultraslow diffusion in an exactly solvable non-Markovian random walk. Phys. Rev. E 2014, 89, 052110. [Google Scholar] [CrossRef] [Green Version]
- Boyer, D.; Romo-Cruz, J. Solvable random—Walk model with memory and its relations with Markovian models of anomalous diffusion. Phys. Rev. E 2014, 90, 042136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, E.; Bertoin, J. Elephant random walks and their connection to Pólya-type urns. Phys. Rev. E 2016, 94, 052134. [Google Scholar] [CrossRef] [Green Version]
- Bercu, B.; Chabanol, M.L.; Ruch, J.J. Hypergeometric identities arising from the elephant random walk. J. Math. Anal. Appl. 2019, 480, 123360. [Google Scholar] [CrossRef] [Green Version]
- Bercu, B.; Laulin, L. On the multi-dimensional elephant random walk. J. Stat. Phys. 2019, 175, 1146–1163. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.; Rocha, E.; Cressoni, J.; da Silva, L.; Viswanathan, G. Non-Lévy stable random walk propagators for a non-Markovian walk with both superdiffusive and subdiffusive regimes. Phys. A Stat. Mech. Its Appl. 2020, 538, 122793. [Google Scholar] [CrossRef]
- Goldstein, S. On diffusion by discontinuous movements, and on the telegraph equation. Q. J. Mech. Appl. Math. 1951, 4, 129–156. [Google Scholar] [CrossRef]
- Rossetto, V. The one-dimensional asymmetric persistent random walk. J. Stat. Mech. Theory Exp. 2018, 2018, 043204. [Google Scholar] [CrossRef] [Green Version]
- Othmer, H.G.; Dunbar, S.R.; Alt, W. Models of dispersal in biological systems. J. Math. Biol. 1988, 26, 263–298. [Google Scholar] [CrossRef] [PubMed]
- Hillen, T. Hyperbolic models for chemosensitive movement. Math. Model. Methods Appl. Sci. 2002, 12, 1007–1034. [Google Scholar] [CrossRef]
- Fort, J.; Méndez, V. Wavefronts in time-delayed reaction-diffusion systems. Theory and comparison to experiment. Rep. Prog. Phys. 2002, 65, 895. [Google Scholar] [CrossRef]
- Filbet, F.; Laurençot, P.; Perthame, B. Derivation of hyperbolic models for chemosensitive movement. J. Math. Biol. 2005, 50, 189–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetecau, R.C.; Eftimie, R. An investigation of a nonlocal hyperbolic model for self-organization of biological groups. J. Math. Biol. 2010, 61, 545–579. [Google Scholar] [CrossRef]
- Bouin, E.; Calvez, V.; Nadin, G. Hyperbolic traveling waves driven by growth. Math. Model. Methods Appl. Sci. 2014, 24, 1165–1195. [Google Scholar] [CrossRef] [Green Version]
- Perthame, B.; Tang, M.; Vauchelet, N. Derivation of the bacterial run-and-tumble kinetic equation from a model with biochemical pathway. J. Math. Biol. 2016, 73, 1161–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvez, V. Chemotactic waves of bacteria at the mesoscale. J. Eur. Math. Soc. 2019, 22, 593–668. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Li, J.; Surulescu, C. Multiscale modeling of glioma pseudopalisades: Contributions from the tumor microenvironment. J. Math. Biol. 2021, 82, 1–45. [Google Scholar] [CrossRef]
- Angstmann, C.N.; Donnelly, I.C.; Henry, B.I. Continuous time random walks with reactions forcing and trapping. Math. Model. Nat. Phenom. 2013, 8, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Fedotov, S. Nonlinear subdiffusive fractional equations and the aggregation phenomenon. Phys. Rev. E 2013, 88, 032104. [Google Scholar] [CrossRef] [Green Version]
- Angstmann, C.N.; Erickson, A.M.; Henry, B.I.; McGann, A.V.; Murray, J.M.; Nichols, J.A. A General Framework for Fractional Order Compartment Models. SIAM Rev. 2021, 63, 375–392. [Google Scholar] [CrossRef]
- Fedotov, S.; Iomin, A.; Ryashko, L. Non-Markovian models for migration-proliferation dichotomy of cancer cells: Anomalous switching and spreading rate. Phys. Rev. E 2011, 84, 061131. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Ashwin, P.; Steinberg, G. Modelling the motion of organelles in an elongated cell via the coordination of heterogeneous drift–diffusion and long-range transport. Eur. Phys. J. E 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Mainardi, F.; Raberto, M.; Gorenflo, R.; Scalas, E. Fractional calculus and continuous-time finance II: The waiting-time distribution. Phys. A Stat. Mech. Its Appl. 2000, 287, 468–481. [Google Scholar] [CrossRef] [Green Version]
- Clauset, A.; Shalizi, C.R.; Newman, M.E. Power-law distributions in empirical data. SIAM Rev. 2009, 51, 661–703. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, L.; Keating, S.; Dudley, J.; Richmond, P. Waiting time distributions in financial markets. Eur. Phys. J. B-Condens. Matter Complex Syst. 2002, 27, 273–275. [Google Scholar] [CrossRef]
- Liebovitch, L.S.; Schwartz, I.B. Information flow dynamics and timing patterns in the arrival of email viruses. Phys. Rev. E 2003, 68, 017101. [Google Scholar] [CrossRef] [Green Version]
- Suki, B.; Barabási, A.L.; Hantos, Z.; Peták, F.; Stanley, H.E. Avalanches and power-law behaviour in lung inflation. Nature 1994, 368, 615–618. [Google Scholar] [CrossRef]
- Henderson, T.; Bhatti, S. Modelling user behaviour in networked games. In Proceedings of the Ninth ACM international Conference on Multimedia, Ottawa, ON, Canada, 30 September–5 October 2001; pp. 212–220. [Google Scholar]
- Fedotov, S.; Stage, H. Anomalous metapopulation dynamics on scale-free networks. Phys. Rev. Lett. 2017, 118, 098301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabasi, A.L. The origin of bursts and heavy tails in human dynamics. Nature 2005, 435, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Masuda, N.; Kume, S.; Kume, K. Dopamine modulates the rest period length without perturbation of its power law distribution in Drosophila melanogaster. PLoS ONE 2012, 7, e32007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korabel, N.; Waigh, T.A.; Fedotov, S.; Allan, V.J. Non-Markovian intracellular transport with sub-diffusion and run-length dependent detachment rate. PLoS ONE 2018, 13, e0207436. [Google Scholar] [CrossRef] [Green Version]
- Laskin, N. Fractional poisson process. Commun. Nonlinear Sci. Numer. Simul. 2003, 8, 201–213. [Google Scholar] [CrossRef]
- Mainardi, F.; Gorenflo, R.; Scalas, E. A fractional generalization of the Poisson processes. arXiv 2007, arXiv:math/0701454. [Google Scholar]
- Mainardi, F.; Gorenflo, R.; Vivoli, A. Beyond the Poisson renewal process: A tutorial survey. J. Comput. Appl. Math. 2007, 205, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Beghin, L.; Orsingher, E. Poisson-type processes governed by fractional and higher-order recursive differential equations. Electron. J. Probab. 2010, 15, 684–709. [Google Scholar] [CrossRef]
- Cahoy, D.O.; Uchaikin, V.V.; Woyczynski, W.A. Parameter estimation for fractional Poisson processes. J. Stat. Plan. Inference 2010, 140, 3106–3120. [Google Scholar] [CrossRef] [Green Version]
- Meerschaert, M.; Nane, E.; Vellaisamy, P. The fractional Poisson process and the inverse stable subordinator. Electron. J. Probab. 2011, 16, 1600–1620. [Google Scholar] [CrossRef]
- Politi, M.; Kaizoji, T.; Scalas, E. Full characterization of the fractional Poisson process. EPL (Europhys. Lett.) 2011, 96, 20004. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.A.; Simmons, R.M. Models of motor-assisted transport of intracellular particles. Biophys. J. 2001, 80, 45–68. [Google Scholar] [CrossRef] [Green Version]
- Fulger, D.; Scalas, E.; Germano, G. Monte Carlo simulation of uncoupled continuous-time random walks yielding a stochastic solution of the space-time fractional diffusion equation. Phys. Rev. E 2008, 77, 021122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Garcia, D.; Sandev, T.; Safdari, H.; Pagnini, G.; Chechkin, A.; Metzler, R. Crossover from anomalous to normal diffusion: Truncated power-law noise correlations and applications to dynamics in lipid bilayers. New J. Phys. 2018, 20, 103027. [Google Scholar] [CrossRef] [Green Version]
- Hartich, D.; Godec, A. Thermodynamic Uncertainty Relation Bounds the Extent of Anomalous Diffusion. Phys. Rev. Lett. 2021, 127, 080601. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Alexandrov, D.V.; Gavrilova, A.; Fedotov, S. Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times. Fractal Fract. 2021, 5, 221. https://doi.org/10.3390/fractalfract5040221
Han D, Alexandrov DV, Gavrilova A, Fedotov S. Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times. Fractal and Fractional. 2021; 5(4):221. https://doi.org/10.3390/fractalfract5040221
Chicago/Turabian StyleHan, Daniel, Dmitri V. Alexandrov, Anna Gavrilova, and Sergei Fedotov. 2021. "Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times" Fractal and Fractional 5, no. 4: 221. https://doi.org/10.3390/fractalfract5040221
APA StyleHan, D., Alexandrov, D. V., Gavrilova, A., & Fedotov, S. (2021). Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times. Fractal and Fractional, 5(4), 221. https://doi.org/10.3390/fractalfract5040221






