Wearable Prophylaxis Tool for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers
Abstract
:1. Introduction
1.1. Pressure Ulcers Prevalence and Health Care Cost
1.2. Pathogenesis of Pressure Ulcers
1.3. Pressure Ulcer Classification
1.4. Current Approaches for Pressure Ulcer Prevention
1.5. Tissue Blood Oxygenation—Measurement Methods
1.6. Research Hypothesis
2. Materials and Methods
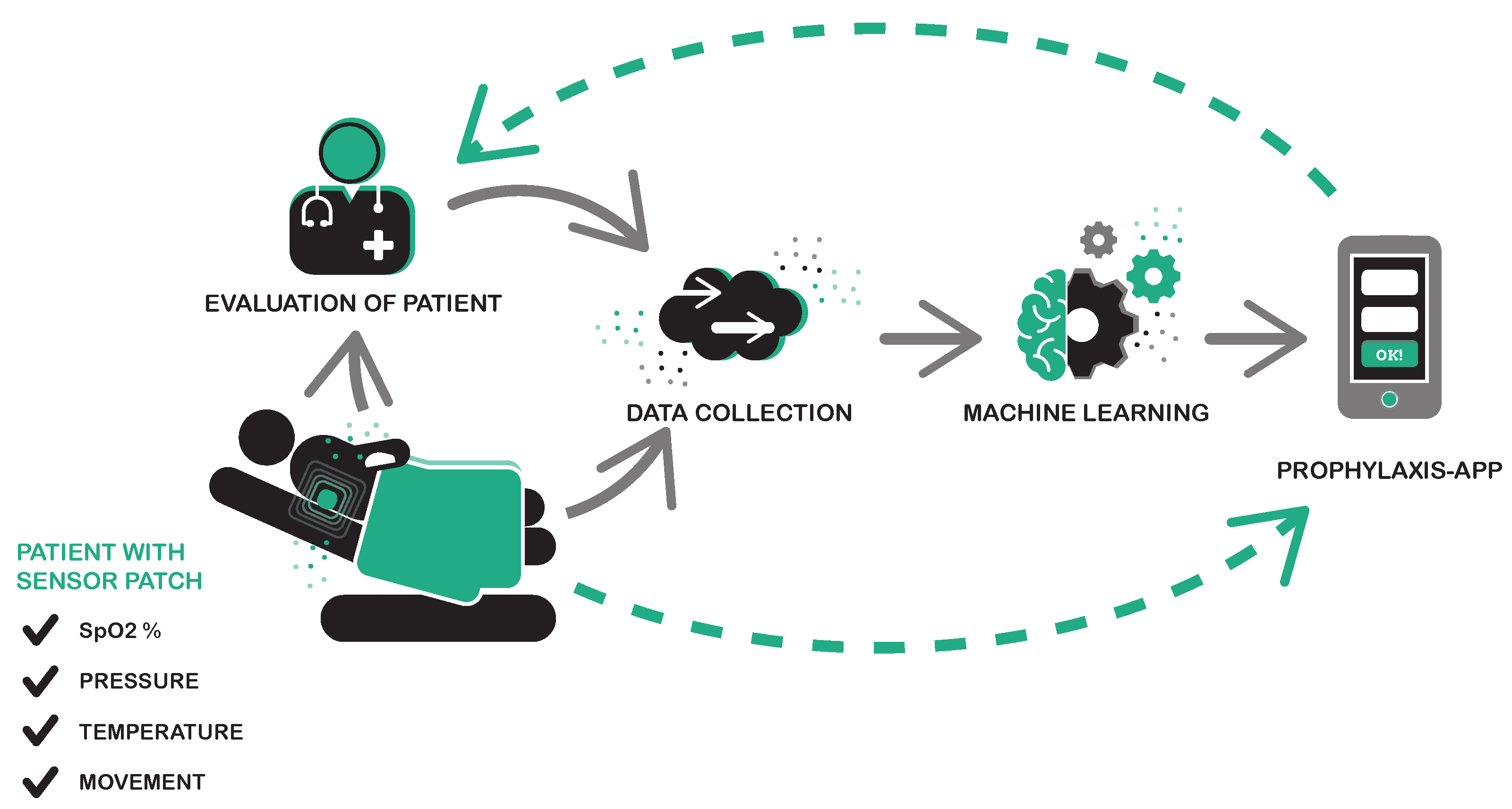
2.1. Sensor System and Electronics
2.2. Data Pipeline
2.3. Medical Data Acquisiton
2.4. Study Population
2.5. Data Analysis
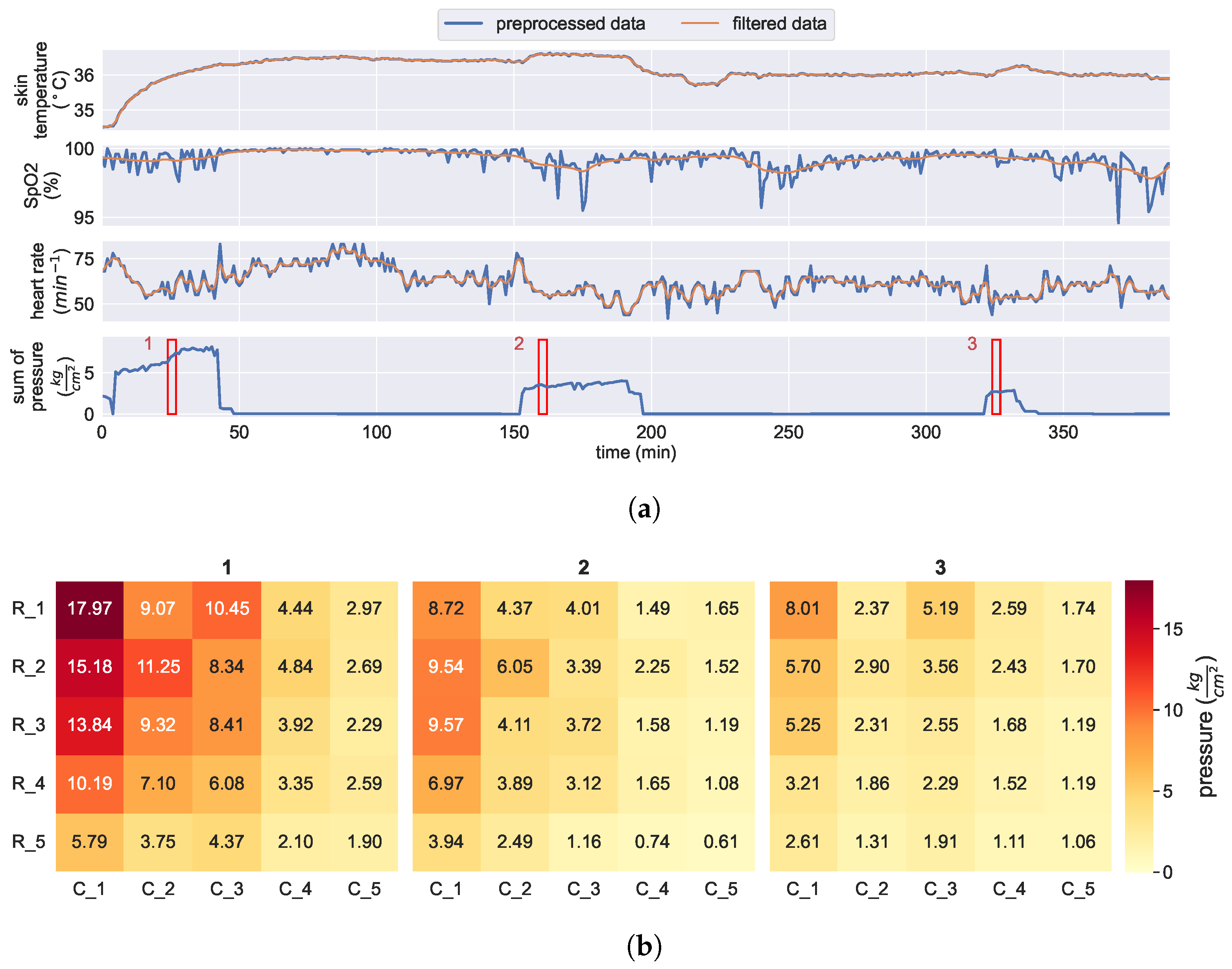
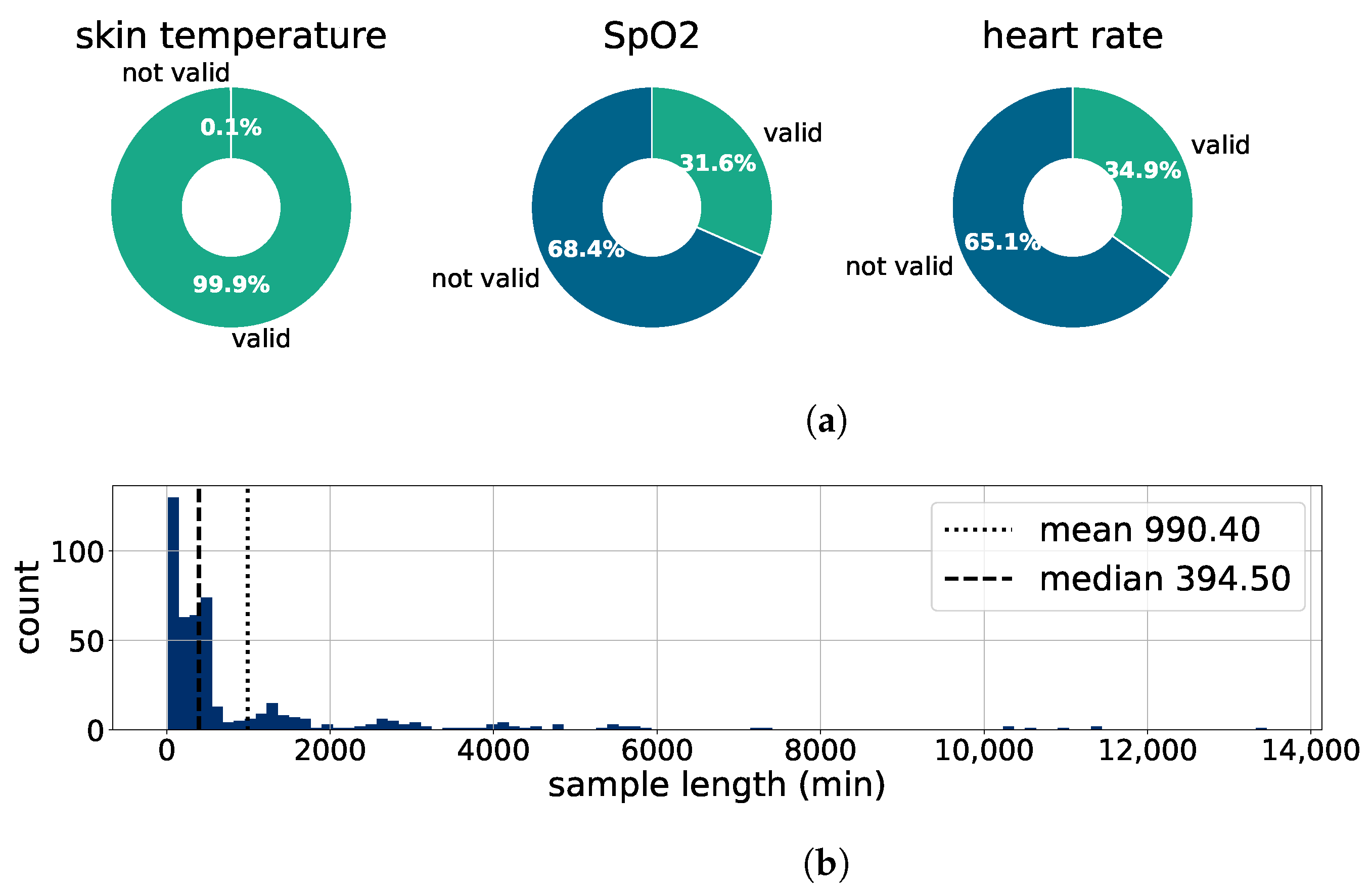
2.5.1. Preprocessing
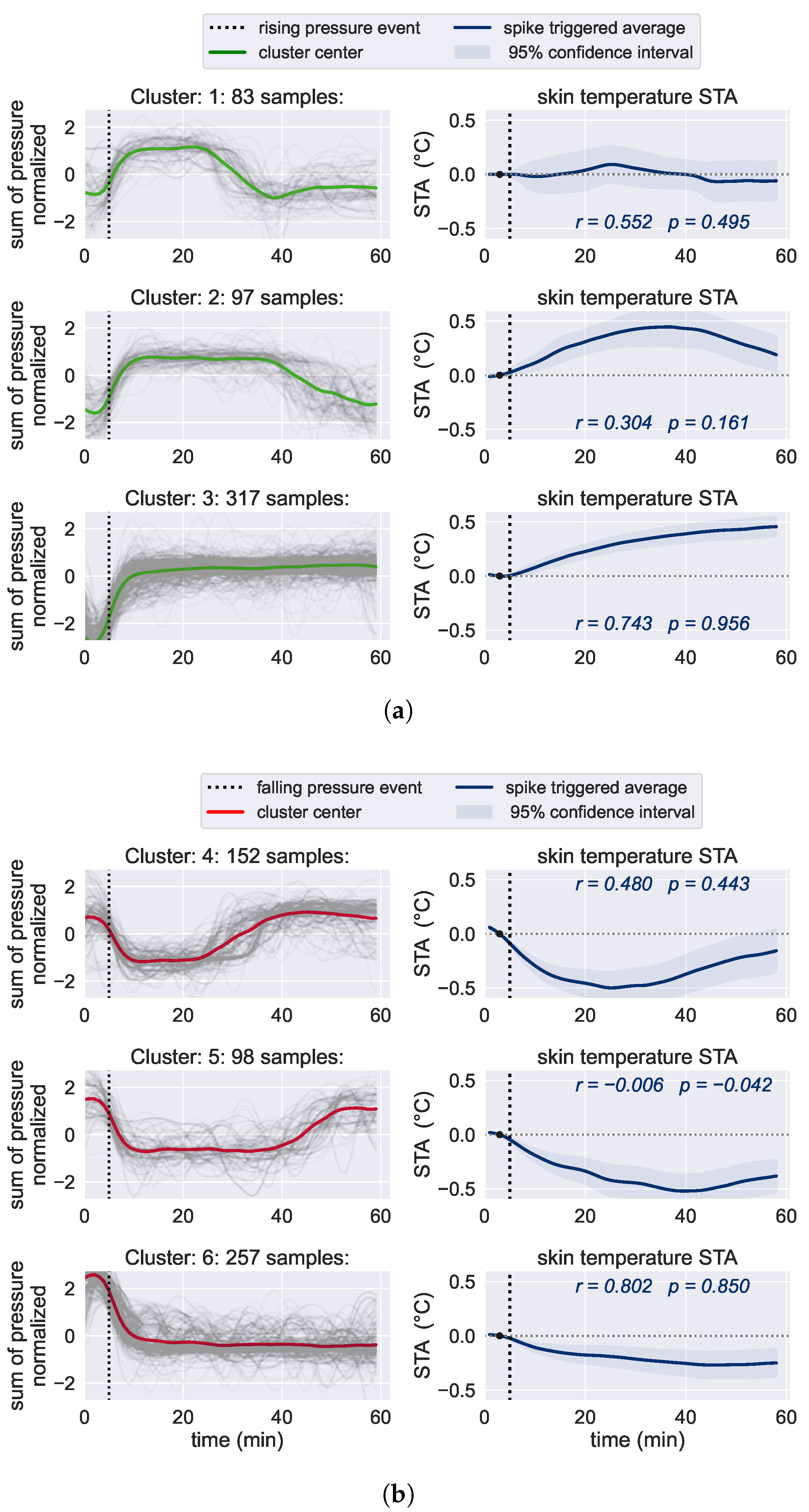
2.5.2. Relationship between Skin Temperature and Pressure Loading
3. Results
3.1. Verification of Kiprode Sensor System
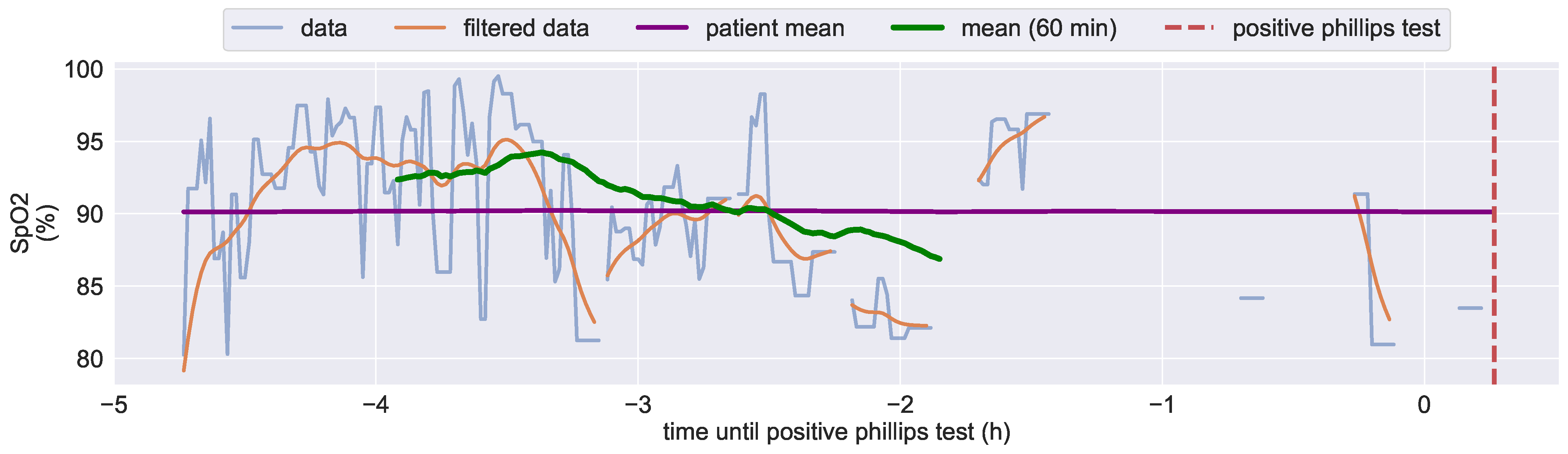
3.1.1. Kiprode Sensor Data vs. Medical Reference
3.1.2. Vital Sign Analysis of Patients and Control
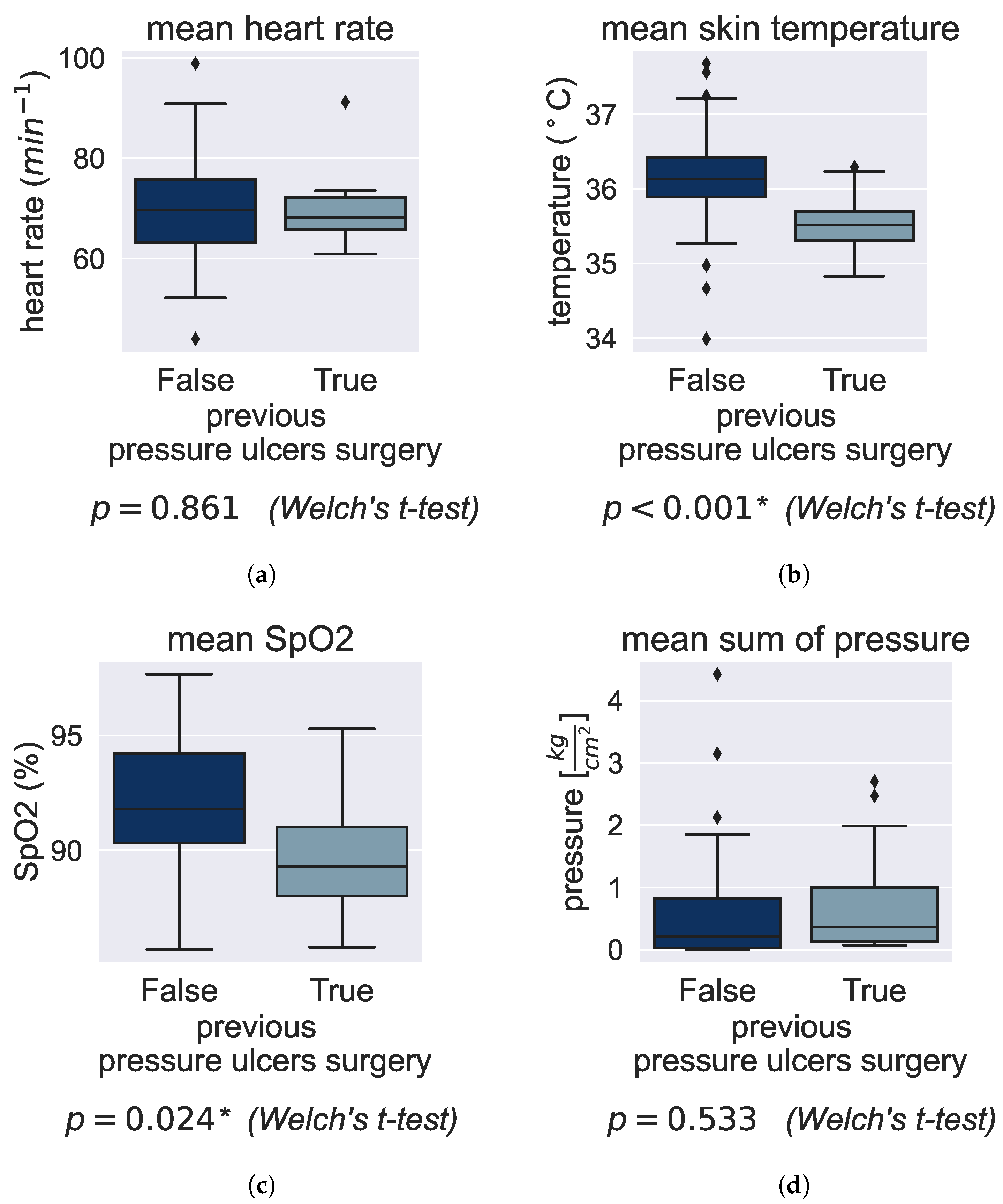
3.2. Effect of Preexisting Conditions on Vital Signs
3.3. Relationship between Skin Temperature and Pressure Loading
3.4. Development of a Pressure Ulcers Predictor
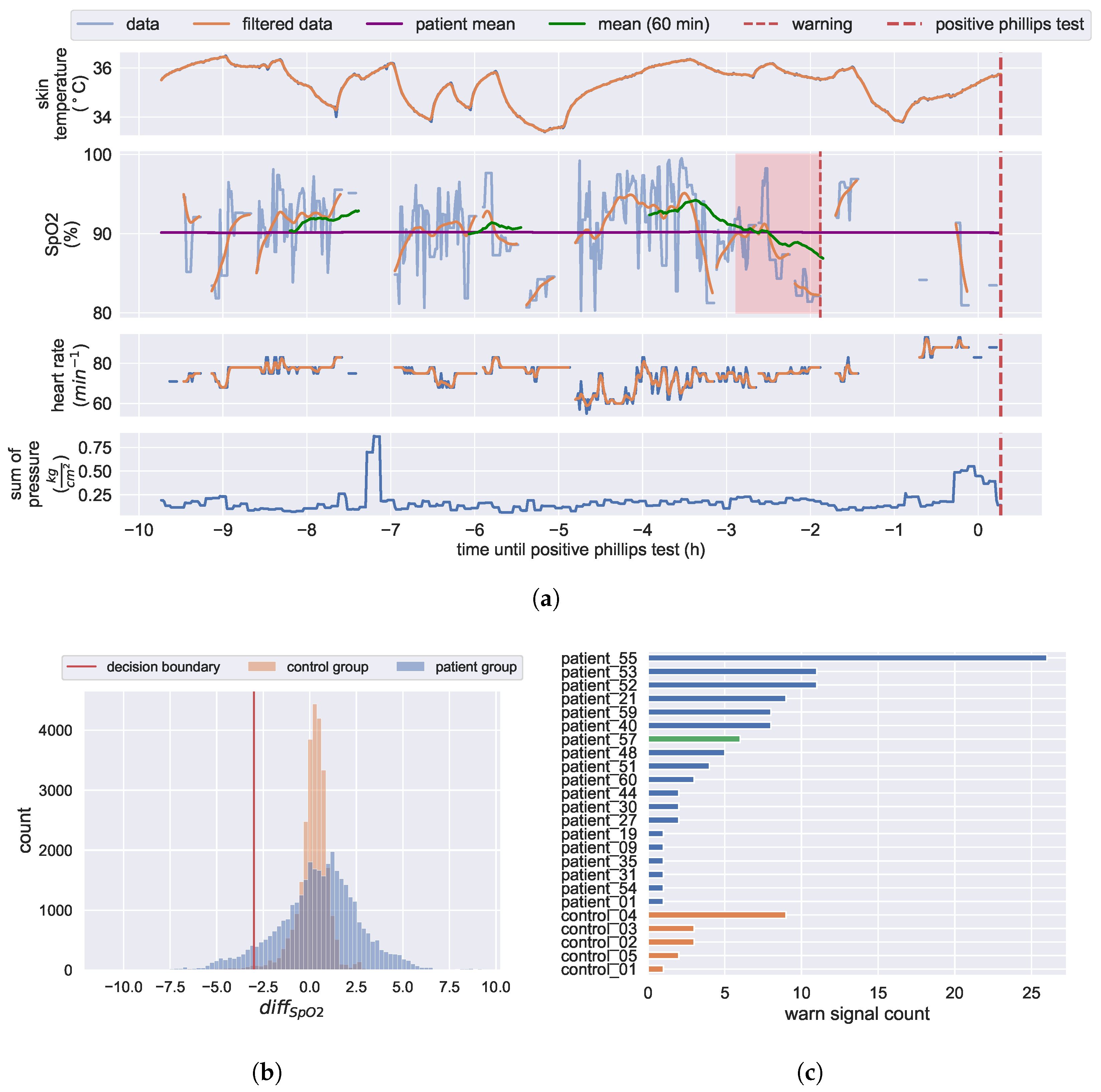
3.4.1. Analysis of Diagnosed Pressure Ulcers Development
3.4.2. KIPRODE Pressure Ulcers Predictor
3.5. Pressure Ulcers at Patients’ Feet
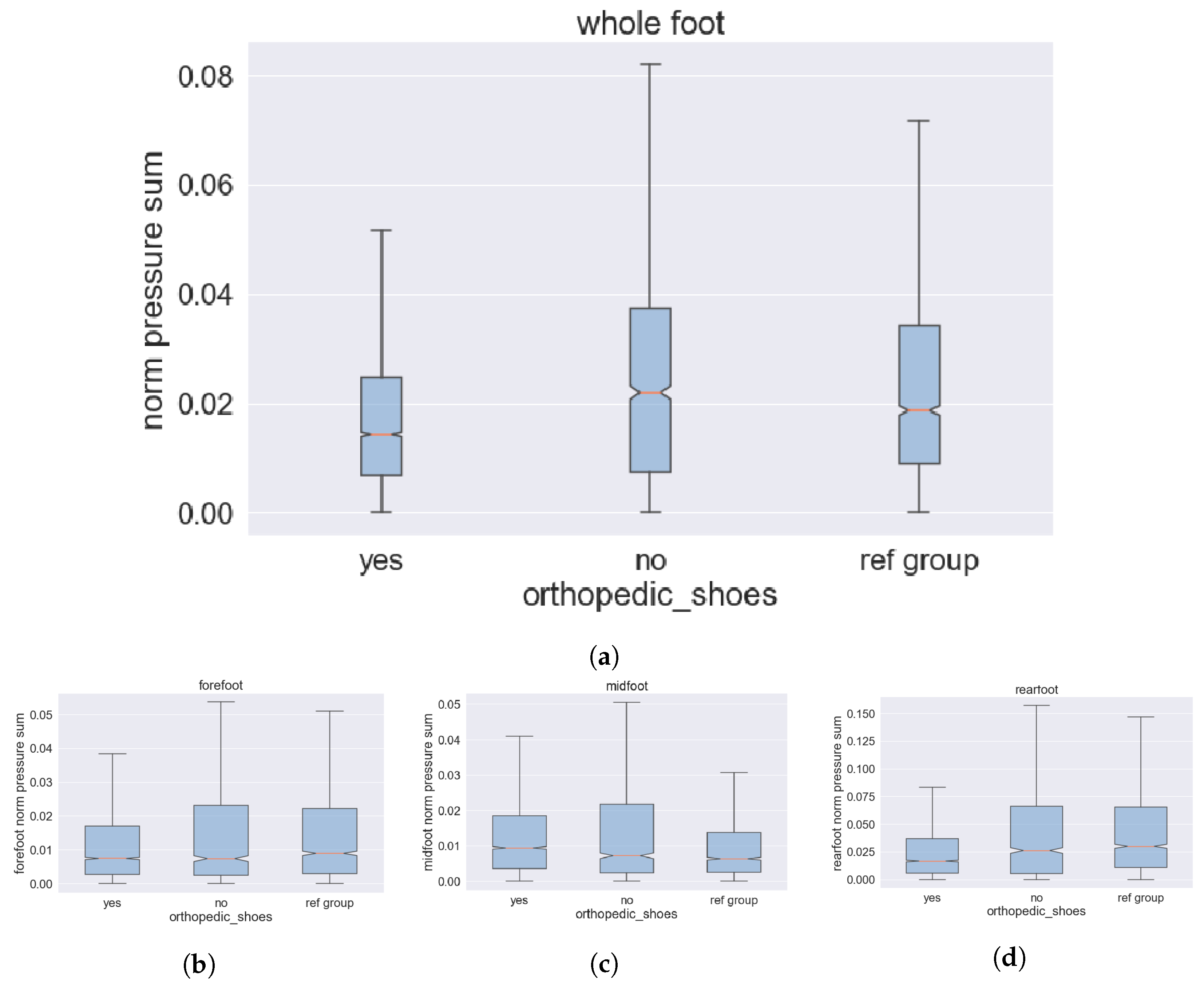
3.5.1. System Adjustment for Prophylaxis of Feet Pressure Ulcers
3.5.2. PU-Risk Mitigation by Individual Shoes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMFT | Fraunhofer Institute for Electronic Microsystems and Solid State Technologies |
| FNB | Fußnetz Bayern |
| KIPRODE | KI (AI) for prophylaxis of decubitus [70] |
| ML | Machine Learning |
| MONKS | Monks GmbH: Kiprode Partner for IT and telemedicine |
| MRI | Rechts der Isar Hospital of TU Munich |
| SpO | Blood oxygenation |
| STA | Spike triggered average |
Appendix A
Appendix A.1
Appendix A.2
- 1.
- Initialize STA with window size T, reference point and the dataset S, produced by the pressure event detection, containing N pressure rise events. All the segments are aligned, meaning in every segment the pressure rise happens exactly at (after 5 min). The reference point, to which we compare all the other temperature values is therefore the measurement at . The window size T is set to 60 min, because we want to inspect the temperature in a one hour window, from 5 min before until 55 min after the pressure fall.
- 2.
- Iterate over all the timepoints t in range :
- (a)
- For each t, iterate over all the segments in S:
- i.
- Compute the difference between the temperature at the current timepoint and the temperature at the reference timepoint for sample according to:
- ii.
- Store the temperature differences in a list.
- (b)
- Calculate the mean of the temperature differences over all the segments S at the current timepoint t:
- (c)
- Calculate the corresponding 95%-confidence interval at timepoint t, according to:With being the standard deviation of the s and z taken from the standard normal distribution at , knowing that our measurements are normally distributed.
- 3.
- Combine all the temperature differences into one series and plot it including the confidence interval, and highlight the reference point and the timepoint where the pressure event happens.
- 4.
- Repeat for the pressure fall events.
Appendix A.3

References
- Grey, J.E.; Harding, K.G.; Enoch, S. Pressure ulcers. BMJ (Clin. Res. Ed.) 2006, 332, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Gruenerbel, L. Project KIPRODE: AI-Assisted Prophylaxis of Pressure Ulcers. 2020. Available online: https://www.emft.fraunhofer.de/en/projects-fraunhofer-emft/AI-assisted-prophylaxis-pressure-ulcers.html (accessed on 4 May 2023).
- MONKS-Aerzte im Netz. Available online: https://www.monks.de/monks-aerzte-im-netz/ (accessed on 1 November 2021).
- Destatis—Statistisches Bundesamt Germany. 5 Million People in Need of Long-Term Care at the End of 2021. 2022. Available online: https://www.destatis.de/EN/Press/2022/12/PE22_554_224.html (accessed on 17 July 2023).
- IQTIG. DeQS-RL Dekubitusprophylaxe EJ 2021 Jahresbericht. 2022. Available online: https://iqtig.org/downloads/auswertung/2021/dek/DeQS_DEK_2021_BUAW_V01_2022-10-21.pdf (accessed on 17 July 2023).
- Zhang, X.; Zhu, N.; Li, Z.; Xie, X.; Liu, T.; Ouyang, G. The global burden of decubitus ulcers from 1990 to 2019. Sci. Rep. 2021, 11, 21750. [Google Scholar] [CrossRef]
- Demarré, L.; van Lancker, A.; van Hecke, A.; Verhaeghe, S.; Grypdonck, M.; Lemey, J.; Annemans, L.; Beeckman, D. The cost of prevention and treatment of pressure ulcers: A systematic review. Int. J. Nurs. Stud. 2015, 52, 1754–1774. [Google Scholar] [CrossRef]
- Anthony, D.; Alosoumi, D.; Safari, R. Prevalence of pressure ulcers in long-term care: A global review. J. Wound Care 2019, 28, 702–709. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Tschannen, D.; Anderson, C. The pressure injury predictive model: A framework for hospital–acquired pressure injuries. J. Clin. Nurs. 2020, 29, 1398–1421. [Google Scholar] [CrossRef]
- Lustig, A.; Margi, R.; Orlov, A.; Orlova, D.; Azaria, L.; Gefen, A. The mechanobiology theory of the development of medical device-related pressure ulcers revealed through a cell-scale computational modeling framework. Biomech. Model. Mechanobiol. 2021, 20, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Jaul, E. Assessment and Management of Pressure Ulcers in the Elderly. Drugs Aging 2010, 27, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Andrews, K.L.; Chutka, D.S.; Fleming, K.C.; Garness, S.L. Pressure ulcers: Prevention and management. Mayo Clin. Proc. 1995, 70, 789–799. [Google Scholar] [CrossRef]
- Aloweni, F.; Yuh Ang, S.; Fook-Chong, S.; Agus, N.; Yong, P.; Meh Goh, M.; Tucker-Kellogg, L.; Soh, R.C. A Prediction Tool for Hospital Acquired Pressure Ulcer Among Surgical Patients: Surgical Pressure Ulcer Risk Score. Int. Wound J. 2019, 2019, 164–175. [Google Scholar] [CrossRef]
- Paker, N.; Buğdaycı, D.; Gökşenoğlu, G.; Akbaş, D.; Korkut, T. Recurrence rate after pressure ulcer reconstruction in patients with spinal cord injury in patients under control by a plastic surgery and physical medicine and rehabilitation team. Turk. J. Phys. Med. Rehabil. 2018, 64, 322–327. [Google Scholar] [CrossRef] [PubMed]
- European Pressure Ulcer Advisory Panel-EPUAP. Available online: https://www.epuap.org/pu-guidelines/ (accessed on 14 July 2023).
- National Pressure Injury Advisory Panel-NPIAP. Available online: https://npiap.com/ (accessed on 14 July 2023).
- Pan Pacific Pressure Injury Alliance-PPPIA. Available online: https://pppia.org/ (accessed on 14 July 2023).
- Kottner, J.; Cuddigan, J.; Carville, K.; Balzer, K.; Berlowitz, D.; Law, S.; Litchford, M.; Mitchell, P.; Moore, Z.; Pittman, J.; et al. Pressure ulcer/injury classification today: An international perspective. J. Tissue Viability 2020, 29, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.; Scott, R.; Stewart, D.; Cockerell, C.J. Decubitus ulcers: A review of the literature. Int. J. Dermatol. 2005, 44, 805–810. [Google Scholar] [CrossRef]
- Bergstrom, N.; Braden, B.J.; Laguzza, A.; Holman, V. The Braden Scale for Predicting Pressure Sore Risk. Nurs. Res. 1987, 36, 205–210. [Google Scholar] [CrossRef]
- Lechner, A.; Kottner, J.; Coleman, S.; Muir, D.; Beeckman, D.; Chaboyer, W.; Cuddigan, J.; Moore, Z.; Rutherford, C.; Schmitt, J.; et al. Outcomes for Pressure Ulcer Trials (OUTPUTs) project: Review and classification of outcomes reported in pressure ulcer prevention research. Br. J. Dermatol. 2021, 184, 617–626. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.M.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET handbook: Version 1.0. Trials 2017, 18, 1–50. [Google Scholar] [CrossRef]
- Roaf, R. The causation and prevention of bed sores. J. Tissue Viability 2006, 16, 6–8. [Google Scholar] [CrossRef]
- Moio.Care. Available online: https://moio.care/moio-app/ (accessed on 11 November 2021).
- Wong, G.; Gabison, S.; Dolatabadi, E.; Evans, G.; Kajaks, T.; Holliday, P.; Alshaer, H.; Fernie, G.; Dutta, T. Toward mitigating pressure injuries: Detecting patient orientation from vertical bed reaction forces. J. Rehabil. Assist. Technol. Eng. 2020, 7, 2055668320912168. [Google Scholar] [CrossRef]
- Matar, G.; Lina, J.M.; Kaddoum, G. Artificial neural network for in-bed posture classification using bed-sheet pressure sensors. IEEE J. Biomed. Health Inform. 2019, 24, 101–110. [Google Scholar] [CrossRef]
- Nagarajapandian, M.; Geetha, M.; Sharmista, P. Risk Assessment System for Prevention of Decubitus Ulcer. In Sustainable Communication Networks and Application; Springer: Singapore, 2021; pp. 607–618. [Google Scholar] [CrossRef]
- Kosuge, M.; Ishihara, Y.; Takahashi, M. Body pressure prediction for pressure ulcer prevention in a bed head elevation operation. Adv. Robot. 2021, 35, 181–193. [Google Scholar] [CrossRef]
- Barsocchi, P. Position recognition to support bedsores prevention. IEEE J. Biomed. Health Inform. 2013, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cicceri, G.; De Vita, F.; Bruneo, D.; Merlino, G.; Puliafito, A. A deep learning approach for pressure ulcer prevention using wearable computing. Hum.-Centric Comput. Inf. Sci. 2020, 10, 5. [Google Scholar] [CrossRef]
- Monroy, E.B.; Romero, D.Z.; Estévez, M.E.; Cruciani, F.; Cleland, I.; Nugent, C.; Medina-Quero, J. Intelligent System for the Prevention of Pressure Ulcers by Monitoring Postural Changes with Wearable Inertial Sensors. Proceedings 2019, 31, 79. [Google Scholar]
- Silva, A.; Metrôlho, J.; Ribeiro, F.; Fidalgo, F.; Santos, O.; Dionisio, R. A Review of Intelligent Sensor-Based Systems for Pressure Ulcer Prevention. Computers 2022, 11, 6. [Google Scholar] [CrossRef]
- Panahi, M.; Masihi, S.; Hanson, A.; Rodriguez-Labra, J.; Masihi, A.; Maddipatla, D.; Narakathu, B.; Lawson, D.; Atashbar, M. Development of a Flexible Smart Wearable Oximeter Insole for Monitoring SpO2 Levels of Diabetics’ Foot Ulcer. IEEE J. Flex. Electron. 2022, 2, 61–70. [Google Scholar] [CrossRef]
- Niwayama, M.; Unno, N. Tissue Oximeter with Selectable Measurement Depth Using Spatially Resolved Near-Infrared Spectroscopy. Sensors 2021, 21, 5573. [Google Scholar] [CrossRef]
- Comerota, A.J.; Throm, R.C.; Kelly, P.; Jaff, M. Tissue (muscle) oxygen saturation (StO2): A new measure of symptomatic lower-extremity arterial disease. J. Vasc. Surg. 2003, 38, 724–729. [Google Scholar] [CrossRef]
- Lauterbach, C.J.; Romano, P.A.; Greisler, L.A.; Brindle, R.A.; Ford, K.R.; Kuennen, M.R. Accuracy and reliability of commercial wrist-worn pulse oximeter during normobaric hypoxia exposure under resting conditions. Res. Q. Exerc. Sport 2021, 92, 549–558. [Google Scholar] [CrossRef]
- Kang, B.J.; Kim, M.; Bang, J.Y.; Lee, E.K.; Choi, B.M.; Noh, G.J. Quantitative analysis of the effect of fraction of inspired oxygen on peripheral oxygen saturation in healthy volunteers. J. Dent. Anesth. Pain Med. 2020, 20, 73–81. [Google Scholar] [CrossRef]
- Schramm, W.M.; Bartunek, A.; Gilly, H. Effect of local limb temperature on pulse oximetry and the plethysmographic pulse wave. Int. J. Clin. Monit. Comput. 1997, 14, 17–22. [Google Scholar] [CrossRef]
- Grünerbel, L.; Heinrich, F.; Diebolder, D.; Richter, M. Wearable Decubitus Prophylaxis Tool Based on Machine Learning Methods. In Proceedings of the 2022 IEEE International Conference on Pervasive Computing and Communications Workshops and Other Affiliated Events (PerCom Workshops), Pisa, Italy, 21–25 March 2022; pp. 730–734. [Google Scholar] [CrossRef]
- Gruenerbel, L.; Heinrich, F.; Böhlhoff-Martin, J.; Steinbacher, L.; Röper, L.; Gruenerbel, A.; Schillinger, M.; Kist, A.; Wenninger, F.; Richter, M.; et al. Time Series Dataset for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers. 2023. [Google Scholar]
- MLS/Heinrich/KIPRODE/KIRPRODE-Public. GitLab. 2023. Available online: https://gitlab.cc-asp.fraunhofer.de/mls/heinrich/kiprode/kirprode-public (accessed on 5 August 2023).
- Pulseoximeter MAX30102, Electronic Component. Available online: https://www.analog.com/en/products/max30102.html (accessed on 3 July 2023).
- Ahmad, R.; Kaidi, H.M.; Nordin, M.N.; Ramli, A.F.; Abu, M.A.; Kadase, Y. Development of Blood Oxygen Level, Heart Rate and Temperature Monitoring System by Using ESP32. In Proceedings of the 2022 4th International Conference on Smart Sensors and Application (ICSSA), Kuala Lumpur, Malaysia, 26–28 July 2022; pp. 167–172. [Google Scholar] [CrossRef]
- Infrared Thermometer MLX90632, Electronic Component. Available online: https://www.melexis.com/en/product/MLX90632/Miniature-SMD-Infrared-Thermometer-IC (accessed on 3 July 2023).
- Shi, C.; Goodall, M.; Dumville, J.; Hill, J.; Norman, G.; Hamer, O.; Clegg, A.; Watkins, C.L.; Georgiou, G.; Hodkinson, A.; et al. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: A systematic review and meta-analysis. BMC Med. 2022, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, A.M.; Fuentes-Guajardo, M.; Latorre, K.; León, D.; Martín-Escudero, P. Skin pigmentation influence on pulse oximetry accuracy: A systematic review and bibliometric analysis. Sensors 2022, 22, 3402. [Google Scholar] [CrossRef]
- Löning, M.; Bagnall, A.; Ganesh, S.; Kazakov, V.; Lines, J.; Király, F.J. sktime: A Unified Interface for Machine Learning with Time Series. arXiv 2019, arXiv:1909.07872. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Lloyd, S. Least squares quantization in PCM. IEEE Trans. Inf. Theory 1982, 28, 129–137. [Google Scholar] [CrossRef]
- Tavenard, R.; Faouzi, J.; Vandewiele, G.; Divo, F.; Androz, G.; Holtz, C.; Payne, M.; Yurchak, R.; Rußwurm, M.; Kolar, K.; et al. Tslearn, A Machine Learning Toolkit for Time Series Data. J. Mach. Learn. Res. 2020, 21, 1–6. [Google Scholar]
- Yuan, C.; Yang, H. Research on K-Value Selection Method of K-Means Clustering Algorithm. J 2019, 2, 226–235. [Google Scholar] [CrossRef]
- Schwartz, O.; Pillow, J.W.; Rust, N.C.; Simoncelli, E.P. Spike-triggered neural characterization. J. Vis. 2006, 6, 484–507. [Google Scholar] [CrossRef]
- Schillinger, M. Characterizing the Relationship between Pressure Loading and Skin Temperature in Pressure Ulcer Risk Patients. Master’s Thesis, Friedrich-Alexander-Universität, Erlangen, Germany, 2022. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Dong, N.; Deng, H.; Wang, Y.; Ling, X.; Guo, H.; Zhang, L.; Cai, F. Skin temperature and vascular attributes as early warning signs of pressure injury. J. Tissue Viability 2020, 29, 258–263. [Google Scholar] [CrossRef]
- Rapp, M.P.; Bergstrom, N.; Padhye, N.S. Contribution of skin temperature regularity to the risk of developing pressure ulcers in nursing facility residents. Adv. Skin Wound Care 2009, 22, 506–513. [Google Scholar] [CrossRef]
- Lützkendorf, S.; Grünerbel, A.; Dietlein, M.; Lüdemann, C.; Becker, E.; Möller, U.; Thomassin, L.; Bohbot, S.; Dissemond, J. TLC-Ag dressings: A prospective, multicentre study on 728 patients with wounds at risk of or with local infection. J. Wound Care 2022, 31, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Allman, R.M. Pressure Ulcer Prevalence, Incidence, Risk Factors, and Impact. Clin. Geriatr. Med. 1997, 13, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Ndosi, M.; Wright-Hughes, A.; Brown, S.; Backhouse, M.; Lipsky, B.A.; Bhogal, M.; Reynolds, C.; Vowden, P.; Jude, E.B.; Nixon, J.; et al. Prognosis of the infected diabetic foot ulcer: A 12–month prospective observational study. Diabet. Med. 2017, 35, 78–88. [Google Scholar] [CrossRef]
- Deutsche Diabetes Gesellschaft (DDG) und DiabetesDE—Deutsche Diabetes-Hilfe; Deutscher Gesundheitsbericht Diabetes: Berlin, Germany, 2018.
- Zhang, Y.; Lazzarini, P.A.; McPhail, S.M.; van Netten, J.J.; Armstrong, D.G.; Pacella, R.E. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care 2020, 43, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Bavarian Foot Network. Available online: https://www.fussnetz-bayern.de/ (accessed on 1 November 2021).
- Jasiewicz, B.; Klimiec, E.; Młotek, M.; Guzdek, P.; Duda, S.; Adamczyk, J.; Potaczek, T.; Piekarski, J.; Kołaszczyński, G. Quantitative analysis of foot plantar pressure during walking. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4916. [Google Scholar] [CrossRef]
- Gómez-González, A.J.; Morilla-Herrera, J.C.; Lupiáñez-Pérez, I.; Morales-Asencio, J.M.; García-Mayor, S.; León-Campos, Á.; Marfil-Gómez, R.; Aranda-Gallardo, M.; Moya-Suárez, A.B.; Kaknani-Uttumchandani, S. Perfusion, tissue oxygenation and peripheral temperature in the skin of heels of healthy participants exposed to pressure: A quasi-experimental study. J. Adv. Nurs. 2020, 76, 654–663. [Google Scholar] [CrossRef]
- Yafi, A.; Muakkassa, F.K.; Pasupneti, T.; Fulton, J.; Cuccia, D.J.; Mazhar, A.; Blasiole, K.N.; Mostow, E.N. Quantitative skin assessment using spatial frequency domain imaging (SFDI) in patients with or at high risk for pressure ulcers. Lasers Surg. Med. 2017, 49, 827–834. [Google Scholar] [CrossRef]
- Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. [Google Scholar] [CrossRef]
- Poorzargar, K.; Pham, C.; Ariaratnam, J.; Lee, K.; Parotto, M.; Englesakis, M.; Chung, F.; Nagappa, M. Accuracy of pulse oximeters in measuring oxygen saturation in patients with poor peripheral perfusion: A systematic review. J. Clin. Monit. Comput. 2022, 36, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Leiderer, R.; Peer, F.; Messmer, K. Skeletal muscle microvascular and tissue injury after varying durations of ischemia. Am. J. Physiol.-Heart Circ. Physiol. 1996, 271, H2388–H2398. [Google Scholar] [CrossRef] [PubMed]
- Germany Federal Ministry of Health. Künstliche Intelligenz zur Vorbeugung von Wundliegegeschwüren (KIPRODE); Federal Ministry of Health: Bonn, Germany, 2020. Available online: https://www.bundesgesundheitsministerium.de/ministerium/ressortforschung-1/handlungsfelder/forschungsschwerpunkte/digitale-innovation/modul-1-smarte-sensorik/kiprode.html (accessed on 4 May 2023).






| Study Duration | Patient Group | Control Group |
|---|---|---|
| Total | 60 | 10 |
| More than 10 days | 12 | 10 |
| More than 5 days | 29 | 10 |
| More than 3 days | 44 | 10 |
| Research | Reference | Methods | Findings | Analogy |
|---|---|---|---|---|
| Tissue SpO during long-lasting pressure load | Gómez-González et al. [65] | Doppler laser devices and infrared beams for determining the degree of oxygenation | Healthy subjects do not experience a reduction in tissue SpO | Agrees well with Section 3.1.2 |
| Assess skin status in care | Yafi et al. [66] | Near-infrared Spatial Frequency Domain Imaging (SFDI) | Decrease in tissue oxygen saturation due to PUs | Aligns well with Section 3.4.1 |
| Quality of pulse oximeter in wearables | Poorzagar et al. [68] | Literature review | Oximeters, particularly newer models, are accurate in poorly perfused patients | Confirms the KIPRODE measurement method |
| Effects of skin pigmentation on the accuracy of SpO measurements | Shi et al. [46] | Literature review | Pulse oximetry overestimates SpO of dark-skinned people | Must be considered in future design |
| Effects of skin pigmentation on the accuracy of SpO measurements | Cabanas et al. [47] | Literature review | Pulse oximeters are less accurate in dark-skinned individuals at lower SpO | Must be considered in future design |
| Validate the skin temperature on sacral region as early warning signs of PU | Jiang et al. [55] | Relative skin temperature at sacral area measured daily | Differences in skin temperature for patients with high/low PU risk | Aligns well with Section 3.2 |
| Influence of skin temperature regulation on PU risk | Rapp et al. [56] | Skin temperature monitors | PU risk depends on multiscale entropy for skin temperature | Aligns well with Section 3.2 |
| Effect of varying duration of ischemia on tissue damage | Harris et al. [69] | Tissue blood analysis in animals | Changes in blood composition after lasting ischemia | Aligns well with Section 3.4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruenerbel, L.; Heinrich, F.; Böhlhoff-Martin, J.; Röper, L.; Machens, H.-G.; Gruenerbel, A.; Schillinger, M.; Kist, A.; Wenninger, F.; Richter, M.; et al. Wearable Prophylaxis Tool for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers. Bioengineering 2023, 10, 1125. https://doi.org/10.3390/bioengineering10101125
Gruenerbel L, Heinrich F, Böhlhoff-Martin J, Röper L, Machens H-G, Gruenerbel A, Schillinger M, Kist A, Wenninger F, Richter M, et al. Wearable Prophylaxis Tool for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers. Bioengineering. 2023; 10(10):1125. https://doi.org/10.3390/bioengineering10101125
Chicago/Turabian StyleGruenerbel, Lorenz, Ferdinand Heinrich, Jonathan Böhlhoff-Martin, Lynn Röper, Hans-Günther Machens, Arthur Gruenerbel, Moritz Schillinger, Andreas Kist, Franz Wenninger, Martin Richter, and et al. 2023. "Wearable Prophylaxis Tool for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers" Bioengineering 10, no. 10: 1125. https://doi.org/10.3390/bioengineering10101125
APA StyleGruenerbel, L., Heinrich, F., Böhlhoff-Martin, J., Röper, L., Machens, H.-G., Gruenerbel, A., Schillinger, M., Kist, A., Wenninger, F., Richter, M., & Steinbacher, L. (2023). Wearable Prophylaxis Tool for AI-Driven Identification of Early Warning Patterns of Pressure Ulcers. Bioengineering, 10(10), 1125. https://doi.org/10.3390/bioengineering10101125






