Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Topiramate
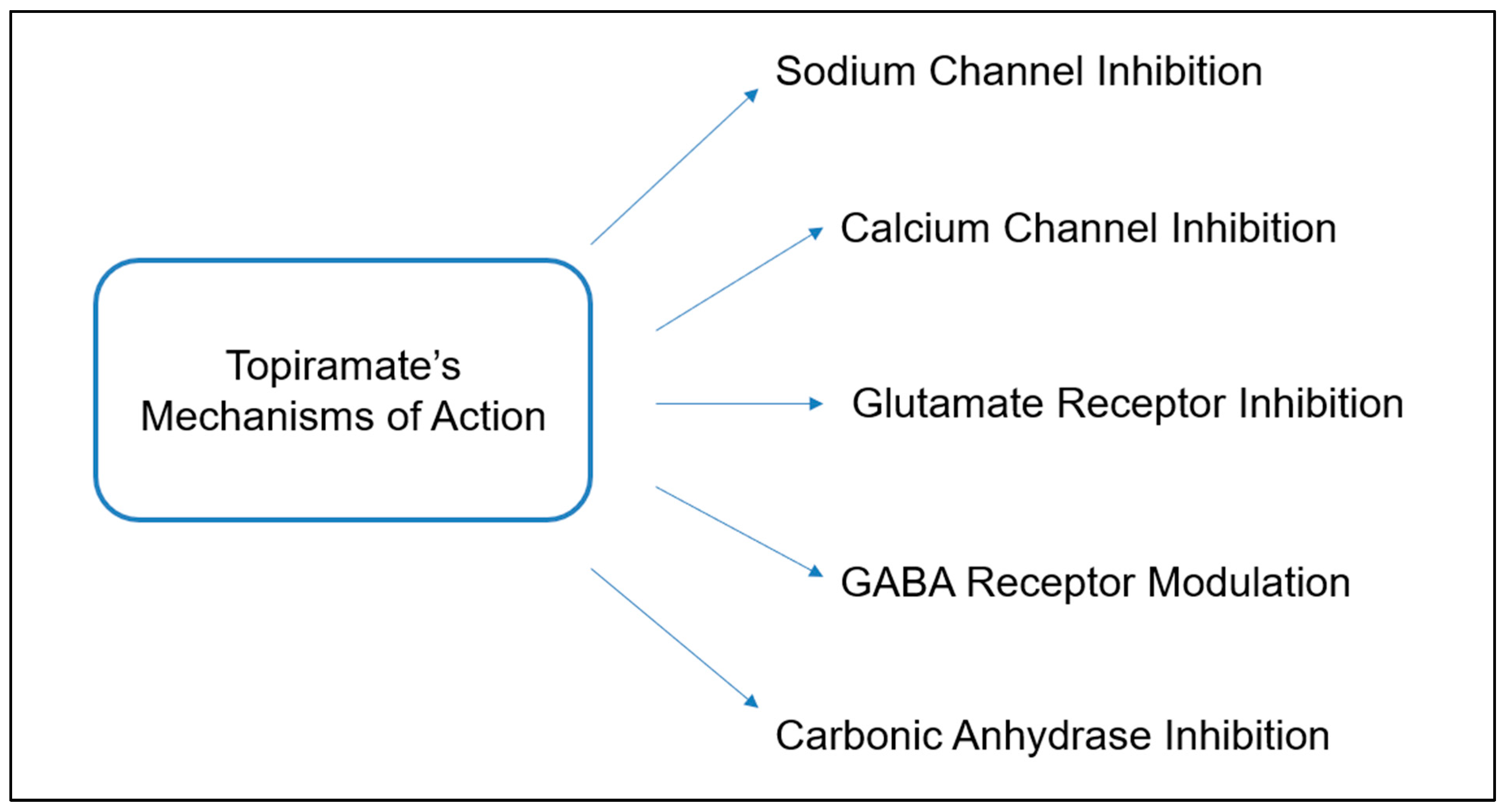
3.1. General Mechanism of Action
3.2. Mechanism of Action in Relation to Weight Loss
4. Topiramate Common Usage
5. Clinical Studies
5.1. Absorption
5.2. Distribution
5.3. Metabolism
5.4. Excretion
5.5. Side Effects
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Toubro, S. Topiramate: A New Potential Pharmacological Treatment for Obesity. Obes. Res. 2004, 12, 167S–173S. [Google Scholar] [CrossRef] [PubMed]
- Fariba, K.A.; Saadabadi, A. Topiramate; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://ncbi.nlm.nih.gov/books/NBK554530/ (accessed on 12 July 2023).
- Faught, E. Topiramate in the treatment of partial and generalized epilepsy. Neuropsychiatr. Dis. Treat. 2007, 3, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Suppes, T. Review of the Use of Topiramate for Treatment of Bipolar Disorders. J. Clin. Psychopharmacol. 2002, 22, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, S.J.; Shank, R.P.; Maryanoff, B.E. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 2000, 41, 35–39. [Google Scholar] [CrossRef]
- Pearl, N.Z.; Babin, C.P.; Catalano, N.T.; Blake, J.C.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Narrative Review of Topiramate: Clinical Uses and Pharmacological Considerations. Adv. Ther. 2023. [Google Scholar] [CrossRef]
- Liang, Y.; She, P.; Wang, X.; Demarest, K. The messenger RNA profiles in liver, hypothalamus, white adipose tissue, and skeletal muscle of female Zucker diabetic fatty rats after topiramate treatment. Metabolism 2006, 55, 1411–1419. [Google Scholar] [CrossRef]
- Verrotti, A.; Scaparrotta, A.; Agostinelli, S.; Di Pillo, S.; Chiarelli, F.; Grosso, S. Topiramate-induced weight loss: A review. Epilepsy Res. 2011, 95, 189–199. [Google Scholar] [CrossRef]
- Richard, D.; Ferland, J.; Lalonde, J.; Samson, P.; Deshaies, Y. Influence of topiramate in the regulation of energy balance. Nutrition 2000, 16, 961–966. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Axelsen, M.; Johanson, E.H. Predictors-of-Weight-Loss-in-Patients-with-Epilepsy-Treated-with-Topiramate. 2001. Available online: https://www.aesnet.org/abstractslisting/predictors-of-weight-loss-in-patients-with-epilepsy-treated-with-topiramate (accessed on 12 July 2022).
- Tremblay, A.; Chaput, J.-P.; Bérubé-Parent, S.; Prud’homme, D.; Leblanc, C.; Alméras, N.; Després, J.-P. The effect of topiramate on energy balance in obese men: A 6-month double-blind randomized placebo-controlled study with a 6-month open-label extension. Eur. J. Clin. Pharmacol. 2007, 63, 123–134. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Caricilli, A.M.; Penteado, E.; de Abreu, L.L.; Quaresma, P.G.F.; Santos, A.C.; Guadagnini, D.; Razolli, D.; Mittestainer, F.C.; Carvalheira, J.B.; Velloso, L.A.; et al. Topiramate treatment improves hypothalamic insulin and leptin signaling and action and reduces obesity in mice. Endocrinology 2012, 153, 4401–4411. [Google Scholar] [CrossRef] [PubMed]
- Narula, P.K.; Rehan, H.S.; Unni, K.E.S.; Gupta, N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: A double-blind, placebo-controlled trial. Schizophr. Res. 2010, 118, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, B.; Gudbjörnsdottir, S.; Cederholm, J.; Liang, Y.; Vercruysse, F.; Smith, U. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: Randomized double-blind placebo-controlled trial. Int. J. Obes. 2007, 31, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.J.; Nguyen, M.T.A.; Bandyopadhyay, G.K.; Nelson, E.; Olefsky, J.M. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E1015–E1022. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elmatty, D.M.; Zaitone, S.A. Topiramate induces weight loss and improves insulin sensitivity in dietary obese rats: Comparison to sibutramine. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1187–1195. [Google Scholar] [PubMed]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Domecq, J.P.; Prutsky, G.; Leppin, A.; Sonbol, M.B.; Altayar, O.; Undavalli, C.; Wang, Z.; Elraiyah, T.; Brito, J.P.; Mauck, K.F.; et al. Clinical review: Drugs commonly associated with weight change: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 363–370. [Google Scholar] [CrossRef]
- Marson, A.G.; Al-Kharusi, A.M.; Alwaidh, M.; Appleton, R.; Baker, G.A.; Chadwick, D.W.; Cramp, C.; Cockerell, O.C.; Cooper, P.N.; Doughty, J.; et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: An unblinded randomised controlled trial. Lancet 2007, 369, 1000–1015. [Google Scholar] [CrossRef]
- Marson, A.G.; Al-Kharusi, A.M.; Alwaidh, M.; Appleton, R.; Baker, G.A.; Chadwick, D.W.; Cramp, C.; Cockerell, O.C.; Cooper, P.N.; Doughty, J.; et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: An unblinded randomised controlled trial. Lancet 2007, 369, 1016–1026. [Google Scholar] [CrossRef]
- Biton, V. Topiramate in Patients With Juvenile Myoclonic Epilepsy. Arch. Neurol. 2005, 62, 1705. [Google Scholar] [CrossRef]
- Diener, H.-C.; Tfelt-Hansen, P.; Dahlöf, C.; Láinez, M.J.A.; Sandrini, G.; Wang, S.-J.; Neto, W.; Vijapurkar, U.; Doyle, A.; Jacobs, D.; et al. Topiramate in migraine prophylaxis: Results from a placebo-controlled trial with propranolol as an active control. J. Neurol. 2004, 251, 943–950. Available online: http://link.springer.com/10.1007/s00415-004-0464-6 (accessed on 12 July 2022). [CrossRef]
- Silberstein, S.; Neto, W.; Schmitt, J.; Jacobs, D. Topiramate in Migraine Prevention: Results of a Large Controlled Trial. Arch. Neurol. 2004, 61, 490–495. [Google Scholar] [CrossRef]
- Chang, K.-H.; Wang, S.-H.; Chi, C.-C. Efficacy and Safety of Topiramate for Essential Tremor: A Meta-Analysis of Randomized Controlled Trials. Medicine 2015, 94, e1809. [Google Scholar] [CrossRef]
- Guglielmo, R.; Martinotti, G.; Quatrale, M.; Ioime, L.; Kadilli, I.; Di Nicola, M.; Janiri, L. Topiramate in Alcohol Use Disorders: Review and Update. CNS Drugs 2015, 29, 383–395. [Google Scholar] [CrossRef]
- Silberstein, S.D. Topiramate in Migraine Prevention: A 2016 Perspective. Headache J. Head Face Pain 2017, 57, 165–178. [Google Scholar] [CrossRef]
- Bray, G.A.; Hollander, P.; Klein, S.; Kushner, R.; Levy, B.; Fitchet, M.; Perry, B.H. A 6-Month Randomized, Placebo-Controlled, Dose-Ranging Trial of Topiramate for Weight Loss in Obesity. Obes. Res. 2003, 11, 722–733. [Google Scholar] [CrossRef]
- Moradi, S.; Kerman, S.R.J.; Mollabashi, M. The effect of topiramate on weight loss in patients with type 2 diabetes. J. Res. Med. Sci. 2013, 18, 297–302. [Google Scholar]
- Toplak, H.; Hamann, A.; Moore, R.; Masson, E.; Gorska, M.; Vercruysse, F.; Sun, X.; Fitchet, M. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Int. J. Obes. 2007, 31, 138–146. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Axelsen, M.; Johanson, E.H.; Stagge, A.; Smith, U. Predictors of weight loss in adults with topiramate-treated epilepsy. Obes. Res. 2003, 11, 556–562. [Google Scholar] [CrossRef]
- Astrup, A.; Caterson, I.; Zelissen, P.; Guy-Grand, B.; Carruba, M.; Levy, B.; Sun, X.; Fitchet, M.; OBES-004 Study Group. Topiramate: Long-Term Maintenance of Weight Loss Induced by a Low-Calorie Diet in Obese Subjects. Obes. Res. 2004, 12, 1658–1669. [Google Scholar] [CrossRef]
- Tonstad, S.; Tykarski, A.; Weissgarten, J.; Ivleva, A.; Levy, B.; Kumar, A.; Fitchet, M. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am. J. Cardiol. 2005, 96, 243–251. [Google Scholar] [CrossRef]
- Wilding, J.; Van Gaal, L.; Rissanen, A.; Vercruysse, F.; Fitchet, M.; OBES-002 Study Group. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int. J. Obes. 2004, 28, 1399–1410. [Google Scholar] [CrossRef]
- Lonneman, D.J., Jr.; Rey, J.A.; McKee, B.D. Phentermine/Topiramate extended-release capsules (qsymia) for weight loss. Pharm. Ther. 2013, 38, 446–452. [Google Scholar]
- Shin, J.H.; Gadde, K.M. Clinical utility of phentermine/topiramate (Qsymia™) combination for the treatment of obesity. Diabetes Metab. Syndr. Obes. 2013, 6, 131–139. [Google Scholar] [CrossRef]
- Steffen, K.J.; Kolotkin, R.L. A Review of the Combination of Phentermine and Topiramate Extended-Release for Weight Loss. Comb. Prod. Ther. 2012, 2, 3. [Google Scholar] [CrossRef]
- Kramer, C.K.; Leitão, C.B.; Pinto, L.C.; Canani, L.H.; Azevedo, M.J.; Gross, J.L. Efficacy and safety of topiramate on weight loss: A meta-analysis of randomized controlled trials. Obes Rev. 2011, 12, e338–e347. [Google Scholar] [CrossRef]
| Author (Year) | Year | Groups Studied and Intervention | Results and Findings | Side Effects | Conclusions |
|---|---|---|---|---|---|
| Bray et al. [29] | 2003 | Randomized, double-blind, placebo-controlled, dose-ranging trial on the efficacy of TPM for weight loss in subjects aged 18–75 years with BMI ≥ 30 to <50 kg/m2 (n = 385). The subjects received either placebo, 64, 96, 192, or 384 mg of TPM daily. | All doses of TPM produced significant weight loss in comparison with placebo. Higher doses of TPM correspond to greater weight loss. | Side effects were most often CNS- and PNS-related. The most common side effects were paresthesia, memory difficulty, and fatigue. Side effect incidence was generally correlated to higher doses of TPM. | All studied TPM doses effectively reduced subjects’ body weights compared to placebo. Lower doses (64 mg and 96 mg) were better tolerated while still producing statistically significant reductions in the weight of the subjects. |
| Moradi et al. [30] | 2013 | Randomized, double-blind, placebo-controlled, parallel-group study on the efficacy of TPM for weight loss in subjects with documented DM2, a BMI between 27 and 50 kg/m2, and aged 18–75 years (n = 69). Subjects were treated with either a placebo or 150 mg of TPM per day. | The TPM treatment group achieved statistically significant decreases in weight compared to the placebo group. The treatment group’s systolic blood pressure and HbA1c significantly decreased compared to the placebo group, and diastolic blood pressure showed no significant difference. | Twenty-four of the thirty-nine subjects in the treatment group reported side effects, the most common being paresthesia and memory problems. | TPM 150 mg/day can effectively reduce weight loss by around 5% in obese patients with DM2. Measurements of metabolic parameters related to obesity and DM2 also show improvement when treating subjects with TPM. |
| Toplak et al. [31] | 2007 | Randomized, double-blind, placebo-controlled multicenter trial on the efficacy of TPM for weight loss and HbA1c reduction in subjects with DM2 on metformin monotherapy, a BMI ≥ 27 to <50 kg/m2, and aged 18–75 years (n = 646). Subjects were treated with a placebo, 96 mg/day of TPM, or 192 mg/day of TPM. | The 96 mg/day and 192 mg/day TPM treatment groups had statistically significant reductions in baseline bodyweight (4.5% and 6.5%, respectively) compared to placebo. TPM groups also had significant reductions in HbA1c compared to the placebo. | Side effects were significantly more common in the treatment group. A total of 32% of subjects treated with TPM experienced paresthesia. | The addition of TPM to subjects with DM2 treated with metformin is effective in reducing body weight. Improved glycemic control was also observed in subjects taking TPM; side effects are common. |
| Ben-Menachem et al. [32] | 2003 | Uncontrolled, prospective trial of 38 in which topiramate was added to patient medication regimens who were also taking anticonvulsants for partial-onset seizure and monitored for 1 year. | Patients’ baseline weights and mean body weights were reduced. After 1 year for all patients, 3 months correlated to stronger reductions in caloric intake. | This study reported only fatigue and anxiousness—concentration/attention trouble, depression, paresthesia, language impairments, and dizziness. The study had no major adverse events or deaths. Seven patients discontinued topiramate due to treatment-emergent adverse effects. Nervousness, fatigue, and depression were the most common symptoms after topiramate discontinuation. | Topiramate caused significant weight loss and was sustainable for 1 year with treatment. |
| Astrup et al. [33] | 2004 | Randomized, double-blind, placebo-controlled, parallel-group study on the efficacy of TPM on maintaining weight loss after 8 weeks of a low-calorie diet. Obese subjects aged 18–75 years who lost ≥8% of their body weight received a placebo, 96 mg/day of TPM, or 192 mg/day of TPM (n = 701). | After weight loss of ≥8% across 8 weeks from diet, subjects treated with 96 mg/day or 192 mg/day of TPM had further weight decreases of 5.2 and 6.4%, respectively. These results were statistically significant compared to the weight gain of 1.8% experienced by the placebo group. | Paresthesia and fatigue were common side effects, but the effects were mainly mild to moderate. | TPM was effective for weight loss maintenance achieved previously by dieting in obese subjects. TPM also produced significant weight loss, while the placebo group experienced weight gain. |
| Tonstad et al. [34] | 2005 | Randomized, placebo-controlled study on TPM’s efficacy for weight and blood pressure reduction in obese subjects with documented hypertension (n = 531). Subjects were treated with a placebo, 96 mg/day of TPM, or 192 mg/day of TPM. | The 96 mg/day and 192 mg/day treatment groups had significant weight decreases compared to placebo. Diastolic blood pressure decreased in both treatment groups, but systolic blood pressure did not in comparison to the placebo. | Common adverse effects included paresthesia, fatigue, and concentration difficulty. | TPM 96 mg/day and 192 mg/day were effective for reducing weight in obese subjects compared to the placebo. |
| Wilding J. et al. [35] | 2004 | Randomized, double-blind, placebo-controlled, parallel-group study on the efficacy of TPM for weight loss in obese subjects without comorbidities. Subjects with hypertension and/or dyslipidemia were included. Subjects were treated with a placebo, 96 mg/day of TPM, 192 mg/day of TPM, or 256 mg/day of TPM (n = 1289) for 1 year. | Subjects in the groups treated with 96 mg/day, 192 mg/day, and 256 mg/day achieved a mean weight decrease of 7.0, 9.1, and 9.7%, respectively. These results were statistically significant compared to the placebo group. | The most common side effects are related to CNS, PNS, and psychiatric disorders. Paresthesia was reported by most subjects treated with TPM; however, it was generally well-tolerated. | TPM produced significant weight loss in obese subjects. Weight loss continued to week 60. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajid, I.; Vega, A.; Thornhill, K.; Jenkins, J.; Merriman, C.; Chandler, D.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review. Life 2023, 13, 1845. https://doi.org/10.3390/life13091845
Wajid I, Vega A, Thornhill K, Jenkins J, Merriman C, Chandler D, Shekoohi S, Cornett EM, Kaye AD. Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review. Life. 2023; 13(9):1845. https://doi.org/10.3390/life13091845
Chicago/Turabian StyleWajid, Irza, Alexis Vega, Katherine Thornhill, Jack Jenkins, Chandler Merriman, Debbie Chandler, Sahar Shekoohi, Elyse M. Cornett, and Alan D. Kaye. 2023. "Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review" Life 13, no. 9: 1845. https://doi.org/10.3390/life13091845
APA StyleWajid, I., Vega, A., Thornhill, K., Jenkins, J., Merriman, C., Chandler, D., Shekoohi, S., Cornett, E. M., & Kaye, A. D. (2023). Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review. Life, 13(9), 1845. https://doi.org/10.3390/life13091845







