Abstract
There are a wide variety of interbody devices available for use in transforaminal lumbar interbody fusion (TLIF). While traditionally these interbodies are bullet-shaped, crescent-shaped cages have become increasingly common. There is a paucity of literature comparing the effect of cage geometry with substratification for surgical approach (minimally invasive (MIS) vs. open). The aim of this study was to determine the effect of implant geometry, positioning, and surgical approach on the correction of different spinal alignment parameters in patients undergoing TLIF. A retrospective chart and imaging review was performed on 103 patients with a total of 131 instrumented segments performed by a single surgeon. Preoperative, initial postoperative, and final postoperative standing lateral lumbar radiographs were evaluated for lumbar lordosis (LL), segmental lordosis (SL), anterior disc height (ADH), and posterior disc height (PDH). Anterior-posterior implant positioning was recorded for initial and final postoperative radiographs. These measurements were compared among four groups: open bullet (OB), MIS bullet (MB), open crescent (OC), and MIS crescent (MC). SL increased in all groups by a mean of 2.9° at initial imaging and 2.2° at final imaging. The OC group had greater initial improvement in SL compared to the MB group (p = 0.02), though this effect was lost at final follow-up (p = 0.11). The OB and OC groups conferred greater initial improvement in ADH (p = 0.02; p = 0.04), while the OC group had greater final improvement in ADH compared to the MB and MC groups (p = 0.01; p = 0.01). The OC group had less initial improvement in PDH compared with the other groups (p = 0.03, p = 0.02, p < 0.01). The MB group provided greater final improvement in PDH compared with the MC and OC groups (p = 0.04, p = 0.01). Cage geometry, surgical approach, and implant position all demonstrated a statistically significant but clinically minor impact on segmental alignment for TLIF procedures.
1. Introduction
Transforaminal lumbar interbody fusion (TLIF) is a commonly used procedure for the management of degenerative spine pathologies such as broad-based disc prolapses, degenerative disc diseases, recurrent disc herniations, pseudoarthrosis, instability, and symptomatic spondylosis [1]. The TLIF procedure offers an improved fusion surface area, extensive fusion blood supply through cancellous vertebral body bone, and greater access for medial and lateral decompression [2]. One of the key advantages of a TLIF as compared to a posterolateral fusion is the ability to restore the intervertebral disc height and segmental lordosis [3]. Disc height restoration provides the indirect decompression of the neuroforamen by increasing the space for the exiting nerve root [4,5]. Additionally, an interbody device can be used to restore segmental lordosis with the goal of maintaining or improving the overall sagittal alignment of the lumbar spine. Ultimately, sagittal spine malalignment has been associated with a decreased quality of life [6,7,8].
TLIFs can be performed via a conventional open posterior approach or minimally invasive (MIS) techniques. The traditional open approach requires greater soft tissue dissection to obtain suitable disc space access [1]. This is associated with long-term complications like persistent pathologic changes in the paraspinous muscles and decreased trunk muscle strength [1,3]. MIS TLIF utilizes a smaller incision than the open approach and aims to reduce muscular dissection, minimize bleeding, and improve postoperative outcomes [1]. Some surgeons are hesitant to use MIS techniques for fear of inadequate segmental lordosis restoration (i.e., inducing kyphosis), the risk of anatomic disorientation due to limited exposure, unfamiliarity with specialized instruments and equipment, and increased dependence on fluoroscopy [3,9]. There has been ample research comparing MIS versus open TLIFs, showing similar outcomes [10,11,12].
A variety of interbody devices are available for TLIF, varying in shape (bullet vs. crescent), mechanism (static vs. expandable), and material (titanium vs. PEEK). Bullet cages are straight and are the traditional shape of an interbody device (Figure 1). Crescent-shaped cages better match the curvature of the anterior endplate, which is thought to enhance surface contact. There have been many studies aiming to determining which implant is more effective at restoring segmental lordosis and intervertebral height without increasing the risk of complications such as subsidence [13,14,15]. Crescent-shaped cages have become an increasingly popular choice given the notion that their geometry allows for more anterior placement in the intervertebral space and thus aligns with the geometry of the anterior vertebral body endplate. Given that the interbody rests on strong apophyseal bone, it is thought that more anterior positioning may allow for greater cage stability and the restoration of segmental lordosis.
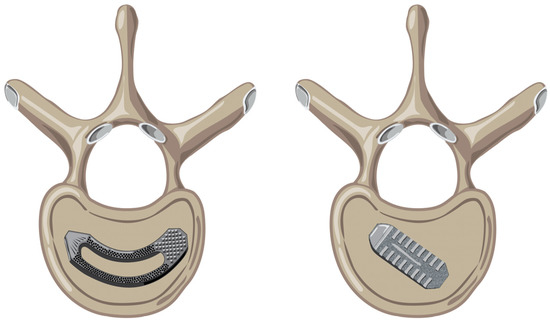
Figure 1.
(Left): Crescent-shaped cage sitting on an endplate of a vertebral body. (Right): Bullet-shaped cage sitting on an endplate of a vertebral body.
There is a relative paucity of research comparing the effectiveness of various cage types at restoring different radiographic parameters of sagittal alignment with substratification for approach (MIS vs. conventional open). The primary goal of this study was to report the impact of cage geometry through either the open or MIS approach on the restoration of sagittal radiographic parameters including lumbar lordosis, segmental lordosis, and disc height. The secondary goal was to determine the effects of implant positioning on the restoration of segmental lordosis substratified by the four study groups. The authors hypothesized that crescent-shaped expandable implants would result in the greater restoration of sagittal radiographic parameters in comparison to the bullet-shaped implants regardless of surgical approach due to the ability of crescent-shaped cages to be placed more anteriorly on the apophyseal ring.
2. Materials and Methods
The study was approved by our institutional review board prior to initiation. A retrospective chart review was performed for patients undergoing 1- or 2-level TLIF with titanium cages between June 2016 and June 2021 performed by a single surgeon with concomitant posterior stabilization using rod and pedicle screw constructs. Patients with prior fusions, TLIF instrumentation at >2 levels, unavailable pre- or postoperative lateral lumbar radiographs, or patients undergoing treatment for cancer or trauma were excluded. Additionally, patients treated with expandable cages or cages composed of PEEK were excluded. Demographic information such as age and BMI at the time of surgery was collected for each patient. The geometry of the TLIF cage and the approach (MIS versus open) used for each procedure were recorded. Patients were divided into one of four categories: open bullet (OB), open crescent (OC), MIS bullet (MB), and MIS crescent (MC).
2.1. Surgical Technique
All surgeries were performed with the patient in a supine position. For open procedures, the levels intended for instrumentation were identified via intraoperative fluoroscopy. A midline dissection was carried through skin and fascia; then, the paraspinal musculature was dissected off the spinous processes, lamina, facet joints, and transverse processes of the intended levels. A laminectomy and facetectomy were then performed to decompress the neural elements and to allow access to the disc space. Pedicle screws were placed into the levels above and below the intervertebral space before instrumentation and a distractor was used to distract the disc space. The dura was gently retracted, and an annulotomy was performed with a surgical blade. The disc space was then prepared using a combination of curettes, shavers, and pituitaries without violating the endplates. An implant size was then chosen via trial implants with the goal of using the largest implant possible without compromising the integrity of the endplates. The implants and the disc spaces were packed with autogenous bone graft. Bone allograft was additionally used if the autograft available was deemed insufficient by the surgeon (Figure 2).
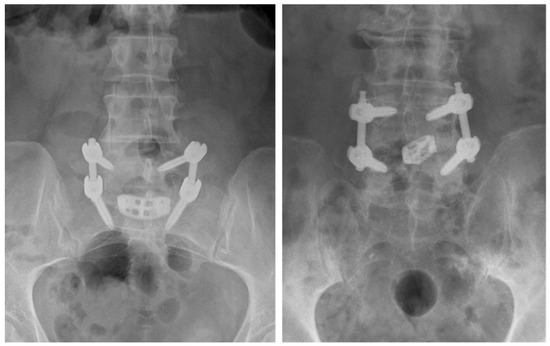
Figure 2.
Anterior-posterior lumbar radiographs of a crescent-shaped interbody device placed at the L5-S1 level (left) and a bullet-shaped interbody device placed at the L4-L5 interbody level (right).
For MIS surgeries, the intended levels for instrumentation were similarly identified using intraoperative fluoroscopy. Modular pedicle screws were placed percutaneously using either fluoroscopic guidance or navigation technology. The initial dilator for a tubular retractor system was docked under fluoroscopic guidance over the facet joint of the level intended for instrumentation. Using a microscope, a lateral facetectomy was performed to allow access to the disc space. An annulotomy was performed, and the disc space was prepared in a similar fashion to the open procedure with care to avoid violating the endplates. Similarly, trial implants were used to determine the largest possible implant that would not risk damaging the surrounding endplates. The implant was then inserted under fluoroscopic guidance with similar grafting to that used in the open procedures.
Regardless of the approach, when inserting the bullet-shaped implants, a straight inserter was utilized for final implant placement. For the crescent-shaped implants, the final implants were inserted on a straight inserter; however, once the implant was contained in the intervertebral space, the inserter was allowed to articulate with the implant so that the implant could rotate 90 degrees from its initial position, allowing the curve of the implant to lie parallel to the anterior curvature of the endplates.
2.2. Radiographic Measurements
All measurements were performed for each patient at three separate time points by two research assistants as well as a senior fellowship-trained spine surgeon (Figure 3). The first set of measurements were obtained from preoperative lateral radiographs in standing neutral alignment obtained just before surgery. The following two sets of measurements were obtained from standing neutral alignment lateral radiographs obtained within 2 days of surgery and at the patients’ most recent follow-up visit. The measurements obtained were lumbar lordosis, segmental lordosis, anterior disc height, and posterior disc height at the levels of instrumentation. Lumbar lordosis was measured as the angle between the superior endplate of L1 and the inferior endplate of L5. Segmental lordosis was defined as the angle between the inferior endplate of the superiorly instrumented vertebrae and the superior endplate of the inferior vertebrae. Anterior disc height was defined as the distance from the anterior-most aspects of the endplates at the instrumented level, perpendicular to the disc space. Posterior disc height was obtained from the posterior aspects of the instrumented endplates.
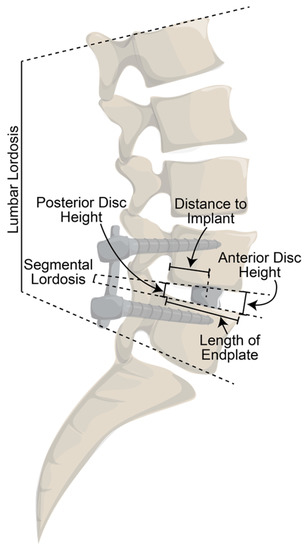
Figure 3.
Diagram of measurements obtained from lateral lumbar radiographs.
Additionally, on both sets of postoperative radiographs, the length of the superior endplate of the inferiorly instrumented vertebra was measured (“length of endplate”). The distance between the posterior point of the inferior vertebrae’s superior endplate and the center of the implant was measured (“distance to implant”). To determine the degree of anterior positioning of the implant, the “distance to implant” was divided by the “length of endplate”. For example, a patient with a “distance to implant” of 25 mm and a “length of endplate” of 50 mm would have a value of 0.50, indicating an implant that was positioned in the exact center of the endplate. A value > 0.50 indicated a more anterior implant, while a value < 0.50 indicated a more posterior implant.
All measurements from the initial postoperative and final postoperative radiographs were compared to the preoperative radiographs and amongst the four groups. Additionally, as segmental lordosis was the primary outcome measure, correlation analysis was performed to determine if there was an effect of either implant positioning or preoperative anterior disc height on the improvement in segmental lordosis, and if this effect was different amongst the four groups.
2.3. Statistical Analysis
Categorical and continuous demographic data were compared amongst the four groups using chi-square and analysis of variance (ANOVA) testing, respectively. Mixed-effects modeling was used to compare the different radiographic measurements of the patients between the three time points and amongst the four groups. ANOVA testing was used to assess anterior-posterior implant positioning amongst the four groups. Additionally, correlation analysis was used to determine the impact of implant positioning, as well as preoperative anterior disc height, on the restoration of segmental lordosis. To validate the radiographic analysis, interclass correlation coefficients (ICCs) were obtained by having each of the three investigators evaluate a subset of 10 patients. ICCs were calculated for each of the measurement modalities. An ICC value > 0.800 was deemed acceptable.
3. Results
There were 131 instrumented levels from a total of 103 patients included in this study. Of the instrumented levels, 23 were in the MB group, 34 in the MC group, 30 in the OB group, and 44 in the OC group. There were no differences between groups based on gender (p = 0.09), smoking status (p = 0.99), BMI (p = 0.900), or the level of instrumentation (p = 0.63) (Table 1). There was a significant difference in age between the groups (p < 0.001), with MIS approaches being performed in younger patients.

Table 1.
Patient demographics.
There was a significant difference in follow-up time between the MIS bullet (81 ± 53 weeks), MIS crescent (58 ± 40 weeks), open bullet (83 ± 62 weeks), and open crescent (45 ± 34 weeks) groups (p = 0.002). There was no difference in follow-up time when stratified by approach (p = 0.44); however, there was a significant difference when stratified by implant shape (p < 0.001).
ICC values for all measurements were greater than 0.80, with values of 0.939 for lumbar lordosis, 0.923 for segmental lordosis, 0.803 for anterior disc height, 0.842 for posterior disc height, 0.974 for length of endplate, and 0.942 for distance to implant.
The raw values for the changes in the different measurement parameters for the initial and final postoperative radiographs compared to the preoperative radiographs are shown in Table 2. On average, lumbar lordosis decreased at the initial postoperative imaging for each group; however, it improved at the final radiograph for all groups except for MIS bullet. Segmental lordosis improved for each group at the initial and final postoperative radiographs, as did anterior disc height and posterior disc height (Table 2). On average, implant positioning was more anterior for the crescent groups compared to the bullet groups at both initial and final postoperative radiographs.

Table 2.
Postoperative changes in measurements.
A comparison of the changes in the measurement parameters at the initial and final postoperative radiographs between the four groups is shown in Table 3. There was no difference in changes in lumbar lordosis at either timepoint amongst the groups. Initial segmental lordosis improved significantly more for patients in the open crescent group compared with the MIS bullet group (p = 0.015); however, this effect was lost at final imaging. Anterior disc height improved more at initial imaging for both the open bullet (p = 0.017) and open crescent (p = 0.042) groups when compared to the MIS crescent group. At final imaging, the improvement in anterior disc height was greater in the open crescent group compared to both the MIS bullet (p = 0.013) and MIS crescent groups (p = 0.009). Posterior disc height improved at initial imaging for the MIS bullet (p = 0.025), MIS crescent (p = 0.019), and open bullet (p < 0.001) groups when compared to the open crescent group. At final imaging, only the MIS bullet group maintained a greater improvement in posterior disc height than the open crescent group (p = 0.012). The open crescent group had a more anterior implant positioning than all three groups at both initial (MB p < 0.001; OB p < 0.001; MC p < 0.001) and final imaging (MB p < 0.001; OB p < 0.001; and MC p < 0.001). The MIS crescent group had more anterior implant positioning than the MIS bullet group at initial (p = 0.020) but not final (p = 0.178) radiographs; however, it had a more anterior positioning compared to the open bullet group at both the initial (p < 0.001) and final (p = 0.018) radiographs.

Table 3.
Comparison of postoperative changes in measurements by group.
More anterior implant positioning was associated with a greater improvement in initial segmental lordosis in both the MIS crescent (r = 0.566; p < 0.001) and the open bullet groups (r = 0.475; p = 0.009); however, this effect was not seen at the final radiographs (Table 4). Additionally, a greater preoperative anterior disc height was associated with a diminished improvement in segmental lordosis in the open bullet group at both initial (r = −0.052; p = 0.004) and final (r = −0.490; p = 0.007) radiographs. This effect was also seen in the MIS crescent group (r = −0.525; p = 0.001) at final imaging; however, this was not seen at initial postoperative imaging.

Table 4.
Correlation analysis of values influencing segmental lordosis.
4. Discussion
Our study found that significant differences existed in the ability to correct sagittal profile parameters between the four study groups. Segmental lordosis was better restored with the open crescent versus the MIS bullet technique. The open crescent method was most useful in restoring anterior disc height at final follow-up compared to both MIS procedures, while the MIS bullet method was most useful in restoring posterior disc height compared to both crescent techniques. Anterior implant placement was correlated with a greater improvement in segmental lordosis with both MIS crescent and MIS open bullet techniques but was unremarkable in the other groups. Preoperative anterior disc height was inversely proportional to the restoration of segmental lordosis for the open bullet group in the initial postoperative period and in the MIS crescent and open crescent groups at final follow-up.
In addition to achieving a fusion, an interbody graft allows for indirect foraminal decompression, disc height restoration, and the correction of radiographic spinopelvic alignment, especially lumbar lordosis. MIS-TLIF offers several potential advantages over open TLIF, including decreased blood loss [16], decreased hospital length of stay [17], decreased narcotic consumption [18], earlier ambulation [16,19,20] and return to work, and lower infection rates [10,12,17,20,21]. The disadvantages include a significant learning curve for minimally invasive procedures as complications, operative time, and radiation exposure may be higher until procedural proficiency is achieved [19,22].
Previous studies have compared the effectiveness of crescent- and bullet-shaped cages. Tassemeier et al. compared clinical and radiological results of bullet versus crescent cages in open TLIF. While this study demonstrated similar improvements in the restoration of segmental global lordosis between the groups, the crescent group showed the superior restoration of intervertebral disc height and a lower rate of subsidence [14]. Choi et al. evaluated the fusion rates and sagittal realignment of crescent versus bullet cages in MIS TLIF and found a greater improvement in postoperative segmental lordosis and disc height in the crescent group [13]. Truckenmueller et al. compared open crescent TLIF with the MIS bullet technique and found similar rates of the postoperative correction of segmental lordosis between the groups [15].
Importantly, while some of the differences in radiographic outcomes between the groups did reach statistical significance, such as for segmental lordosis between the open crescent and the MIS bullet groups, they may not be clinically significant. It is unclear if a 3° difference in segmental lordosis would confer any significant clinical benefit. Furthermore, the error level for the measurement of intervertebral angles has been reported to be between 8.2 and 11.1°, while differences in height measurements range from 4.5 to 6.5 mm [23]. The small differences observed in this study may then have been due to measurement errors, which further suggests that all four treatment options are likely equally efficacious with minimal clinically important differences amongst them.
A concern frequently addressed in the recent literature is the notion that MIS TLIF may be a fundamentally kyphosing procedure compared to its open counterpart. The published evidence supporting the restoration of lumbar lordosis and segmental lordosis with MIS TLIF is relatively sparse and presents variable results [24]. There is significant heterogeneity in these studies’ designs, the number of patients studied, the comparison groups, and the types of interbody devices used [24,25,26,27]. Although our study did show differences in the degree of segmental kyphosis conferred by each approach and implant, all groups regardless of the approach showed an overall increase in segmental lordosis. Lumbar lordosis did decrease at initial postoperative imaging in all groups, which may have been due to postoperative pain and guarding. At final imaging, lumbar lordosis did improve in all groups except for the MB group, which saw a decrease in lumbar lordosis of 0.2° on average.
Pre- and postoperative anterior disc height, posterior disc height, and implant position are speculated to impact the restoration of lordosis. Carlson et al. found that postoperative segmental lordosis was indirectly related to the position of the cage relative to the anterior cortex of the vertebral body, though this correlation was statistically insignificant (R2 = 0.02, p = 0.067) [24]. In our study, more anterior implant positioning was associated with a greater improvement in initial segmental lordosis in both the MIS crescent (R2 = 0.566; p < 0.001) and the open bullet groups (R2 = 0.475; p = 0.009), but this effect was not seen at final radiographs (Table 4). While the open crescent group had a more anterior implant positioning than all three groups at both initial (MB p < 0.001; OB p < 0.001; MC p < 0.001) and final postoperative radiographs (MB p < 0.001; OB p < 0.001; and MC p < 0.001), and the open crescent group had the greatest improvement in segmental lordosis, the more anterior positioning in this group was not correlated with an increased postoperative improvement in segmental lordosis.
There were several limitations to this study. The measurement method for lumbar lordosis was based on Polly et al. This method does not account for lordosis from L5/S1, which contributes significantly to lumbar lordosis; it was utilized in this study to ensure that the methods remained comparable to prior studies, and it was applied consistently among the study groups [28]. The follow-up time for bullet implants was significantly longer than for crescents, which was a reflection of the primary surgeon’s preference over time. While our results demonstrated adequate inter-rater reliability, there was some inter-rater variation and, therefore, potential for error. As surgeon learning curves are inherent with the adoption of various surgical approaches and implant types, it is possible that compounded improvement in surgical technique over time affected the outcome differences between implant types, particularly with MIS crescents. Moreover, the relatively small sample size of each cohort and data from only one surgeon may have increased the margin of error and decreased the generalizability. However, limiting the number of surgeons enabled us to ensure the consistency of surgical technique and postoperative care.
5. Conclusions
Ultimately, these data suggest that surgeons can use the differences we found to guide implant and approach selection for TLIF based on the aspects of alignment that are most in need of restoration. Additionally, we demonstrated that all four techniques were able to increase segmental lordosis equally well at final follow-up. Our data showed that a larger anterior disc height was correlated with less improvement in segmental lordosis in the open bullet and MIS crescent groups, suggesting that these may not be optimal treatment methods in patients with larger preoperative anterior disc heights. Finally, while a more anterior implant positioning was associated with a greater improvement in segmental lordosis in the MIS crescent and open bullet groups, these effects were not significant at final follow-up.
Author Contributions
Conceptualization, I.L.M.; methodology, I.J.W. and M.P.C.; formal analysis, M.P.C. and C.R.K.; investigation, I.J.W. and C.R.K.; resources, I.L.M., S.S.M. and H.S.; data curation, E.C. and C.L.A.; writing—original draft preparation, I.J.W., C.R.K., C.L.A. and E.C.; writing—review and editing, I.L.M., M.P.C., S.S.M. and H.S.; supervision, H.S., S.S.M. and I.L.M.; project administration, M.P.C. and I.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Connecticut (protocol #22X-001-2 on 22/7/21).
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data for this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to acknowledge Bernard Cook and Geneva Hargis for creating the illustration used in this study.
Conflicts of Interest
H.S. serves as a consultant for Stryker and AlphaTec. I.L.M. receives royalties from Spineart and AlphaTec; owns stock in Spinal Simplicity and Orthozon; and is a consultant for Styker, Biedermann Motech, AlphaTec, and Nuvasive.
References
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar] [CrossRef]
- Cole, C.D.; McCall, T.D.; Schmidt, M.H.; Dailey, A.T. Comparison of low back fusion techniques: Transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr. Rev. Musculoskelet. Med. 2009, 2, 118–126. [Google Scholar] [CrossRef]
- Schwender, J.D.; Holly, L.T.; Rouben, D.P.; Foley, K.T. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. Clin. Spine Surg. 2005, 18, S1–S6. [Google Scholar] [CrossRef]
- Lin, G.X.; Akbary, K.; Kotheeranurak, V.; Quillo-Olvera, J.; Jo, H.J.; Yang, X.W.; Mahatthanatrakul, A.; Kim, J.S. Clinical and Radiologic Outcomes of Direct Versus Indirect Decompression with Lumbar Interbody Fusion: A Matched-Pair Comparison Analysis. World Neurosurg. 2018, 119, e898–e909. [Google Scholar] [CrossRef]
- Yoshihara, H. Indirect decompression in spinal surgery. J. Clin. Neurosci. 2017, 44, 63–68. [Google Scholar] [CrossRef]
- Schwab, F.J.; Blondel, B.; Bess, S.; Hostin, R.; Shaffrey, C.I.; Smith, J.S.; Boachie-Adjei, O.; Burton, D.C.; Akbarnia, B.A.; International Spine Study Group; et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine 2013, 38, E803–E812. [Google Scholar] [CrossRef] [PubMed]
- Protopsaltis, T.; Schwab, F.; Bronsard, N.; Smith, J.S.; Klineberg, E.; Mundis, G.; Ryan, D.J.; Hostin, R.; Hart, R.; Burton, D.; et al. The T1 pelvic angle, a novel radiographic measure of global sagittal deformity, accounts for both spinal inclination and pelvic tilt and correlates with health-related quality of life. JBJS 2014, 96, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Berven, S.; Bridwell, K.; Horton, W.; Dimar, J.R. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine 2005, 30, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, P.; Mummaneni, P.V.; Xie, R.; Li, B.; Dong, Y.; Berven, S.; Chou, D. Does transforaminal lumbar interbody fusion induce lordosis or kyphosis? Radiographic evaluation with a minimum 2-year follow-up. J. Neurosurg. Spine 2021, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Yue, W.M.; Yeo, W.; Soeharno, H.; Tan, S.B. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur. Spine J. 2012, 21, 2265–2270. [Google Scholar] [CrossRef]
- Burneikiene, S.; Roeca, C.; Nelson, L.; Mason, A.; Villavicencio, A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg. Neurol. Int. 2010, 1, 12. [Google Scholar] [CrossRef]
- Peng, C.W.B.; Yue, W.M.; Poh, S.Y.; Yeo, W.; Tan, S.B. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine 2009, 34, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Kim, J.S.; Hur, J.W.; Seong, J.H. Minimally invasive transforaminal lumbar interbody fusion using banana-shaped and straight cages: Radiological and clinical results from a prospective randomized clinical trial. Clin. Neurosurg. 2018, 82, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tassemeier, T.; Haversath, M.; Jäger, M. Transforaminal lumbar interbody fusion with expandable cages: Radiological and clinical results of banana-shaped and straight implants. J. Craniovertebral Junction Spine 2018, 9, 196. [Google Scholar] [CrossRef]
- Truckenmueller, P.; Czabanka, M.; Bayerl, S.H.; Mertens, R.; Vajkoczy, P. Oblique insertion of a straight cage during single level TLIF procedure proves to be non-inferior in terms of restoring segmental lordosis. Brain Spine 2021, 1, 100302. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Mendenhall, S.K.; Shau, D.N.; Zuckerman, S.L.; Godil, S.S.; Cheng, J.S.; McGirt, M.J. Minimally Invasive versus Open Transforaminal Lumbar Interbody Fusion for Degenerative Spondylolisthesis: Comparative Effectiveness and Cost-Utility Analysis. World Neurosurg. 2014, 82, 230–238. [Google Scholar] [CrossRef]
- Karikari, I.O.; Isaacs, R.E. Minimally invasive transforaminal lumbar interbody fusion: A review of techniques and outcomes. Spine 2010, 35, S294–S301. [Google Scholar] [CrossRef]
- Adogwa, O.; Parker, S.L.; Bydon, A.; Cheng, J.; McGirt, M.J. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J. Spinal Disord. Tech. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Jin-tao, Q.; Yu, T.; Mei, W.; Xu-dong, T.; Tian-jian, Z.; Guo-hua, S.; Lei, C.; Yue, H.; Zi-tian, W.; Yue, Z. Comparison of MIS vs. open PLIF/TLIF with regard to clinical improvement, fusion rate, and incidence of major complication: A meta-analysis. Eur. Spine J. 2015, 24, 1058–1065. [Google Scholar] [CrossRef]
- Goldstein, C.L.; Phillips, F.M.; Rampersaud, Y.R. Comparative Effectiveness and Economic Evaluations of Open Versus Minimally Invasive Posterior or Transforaminal Lumbar Interbody Fusion. Spine 2016, 41, s74–s89. [Google Scholar] [CrossRef]
- Parker, S.L.; Adogwa, O.; Paul, A.R.; Anderson, W.N.; Aaronson, O.; Cheng, J.S.; McGirt, M.J. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis: Clinical article. J. Neurosurg. Spine 2011, 14, 598–604. [Google Scholar] [CrossRef]
- Lee, K.H.; Yeo, W.; Soeharno, H.; Yue, W.M. Learning Curve of a Complex Surgical Technique: Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS TLIF). J. Spinal Disord. Tech. 2014, 27, E234–E240. [Google Scholar] [CrossRef] [PubMed]
- Alanay, A.; Pekmezci, M.; Karaeminogullari, O.; Acaroglu, E.; Yazici, M.; Cil, A.; Pijnenburg, B.; Genç, Y.; Oner, F.C. Radiographic measurement of the sagittal plane deformity in patients with osteoporotic spinal fractures evaluation of intrinsic error. Eur. Spine J. 2007, 16, 2126–2132. [Google Scholar] [CrossRef][Green Version]
- Carlson, B.B.; Saville, P.; Dowdell, J.; Goto, R.; Vaishnav, A.; Gang, C.H.; McAnany, S.; Albert, T.J.; Qureshi, S. Restoration of lumbar lordosis after minimally invasive transforaminal lumbar interbody fusion: A systematic review. Spine J. 2019, 19, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, J.; Chen, J.; Wu, Y.; Chen, X.; Liu, Y.; Chu, Z.; Sheng, L.; Qin, R.; Chen, M. Three-year postoperative outcomes between MIS and conventional TLIF in1-segment lumbar disc herniation. Minim. Invasive Ther. Allied Technol. 2017, 26, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, L.; Zhang, H.; Gu, X.; Gu, G.; He, S. Radiographic Analysis of One-level Minimally Invasive Transforaminal Lumbar Interbody Fusion (MI-TLIF) With Unilateral Pedicle Screw Fixation for Lumbar Degenerative Diseases. J. Spinal Disord. Tech. 2016, 29, E1–E8. [Google Scholar] [CrossRef]
- Isaacs, R.E.; Sembrano, J.N.; Tohmeh, A.G. Two-Year Comparative Outcomes of MIS Lateral and MIS Transforaminal Interbody Fusion in the Treatment of Degenerative Spondylolisthesis. Spine 2016, 41, s133–s144. [Google Scholar] [CrossRef]
- Polly, D.W., Jr.; Kilkelly, F.X.; McHale, K.A.; Asplund, L.M.; Mulligan, M.; Chang, A.S. Measurement of lumbar lordosis: Evaluation of intraobserver, interobserver, and technique variability. Spine 1996, 21, 1530–1535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).