Numerical Assessment of Interspinous Spacers for Lumbar Spine
Abstract
1. Introduction
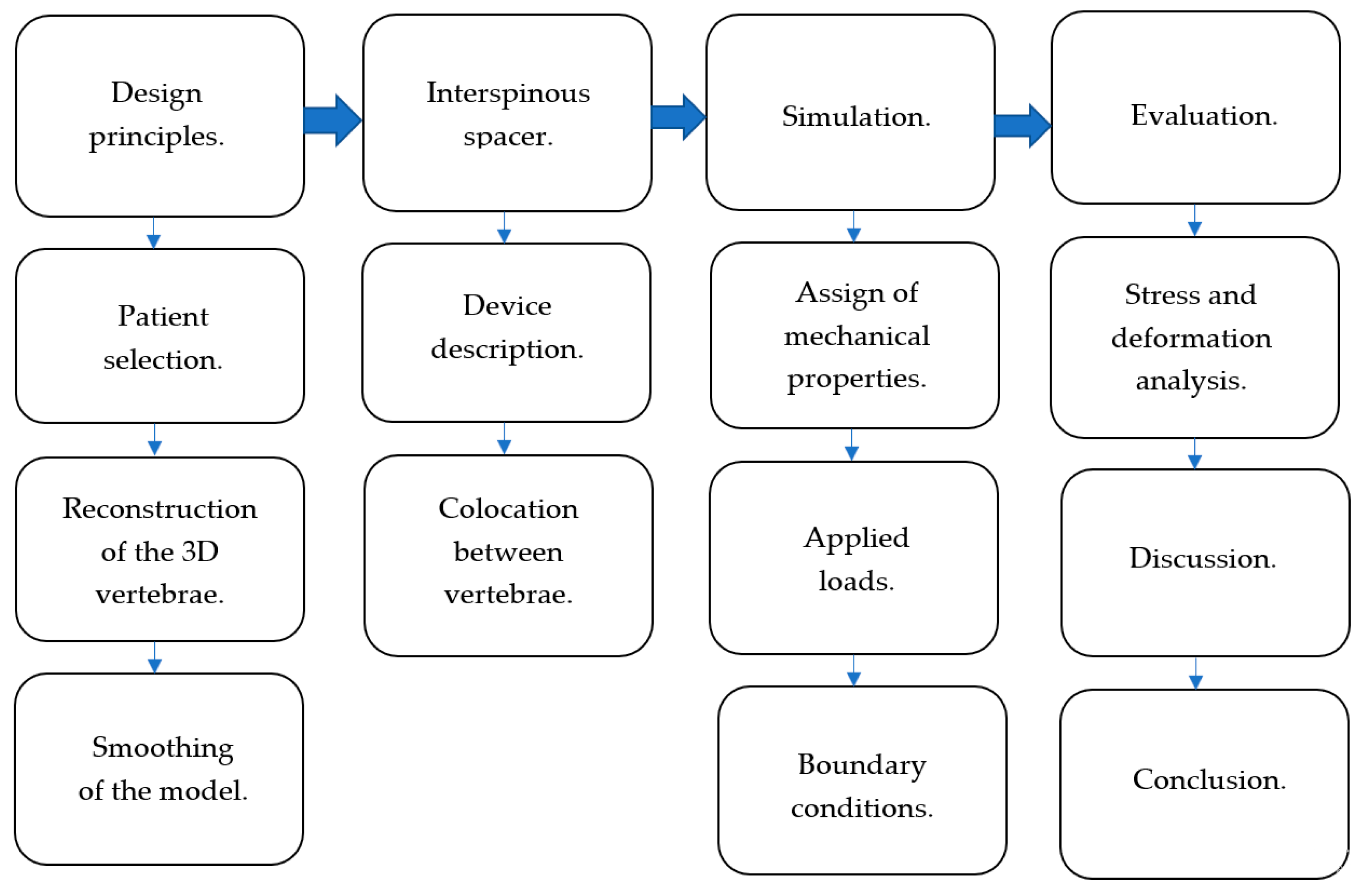
2. Materials and Methods
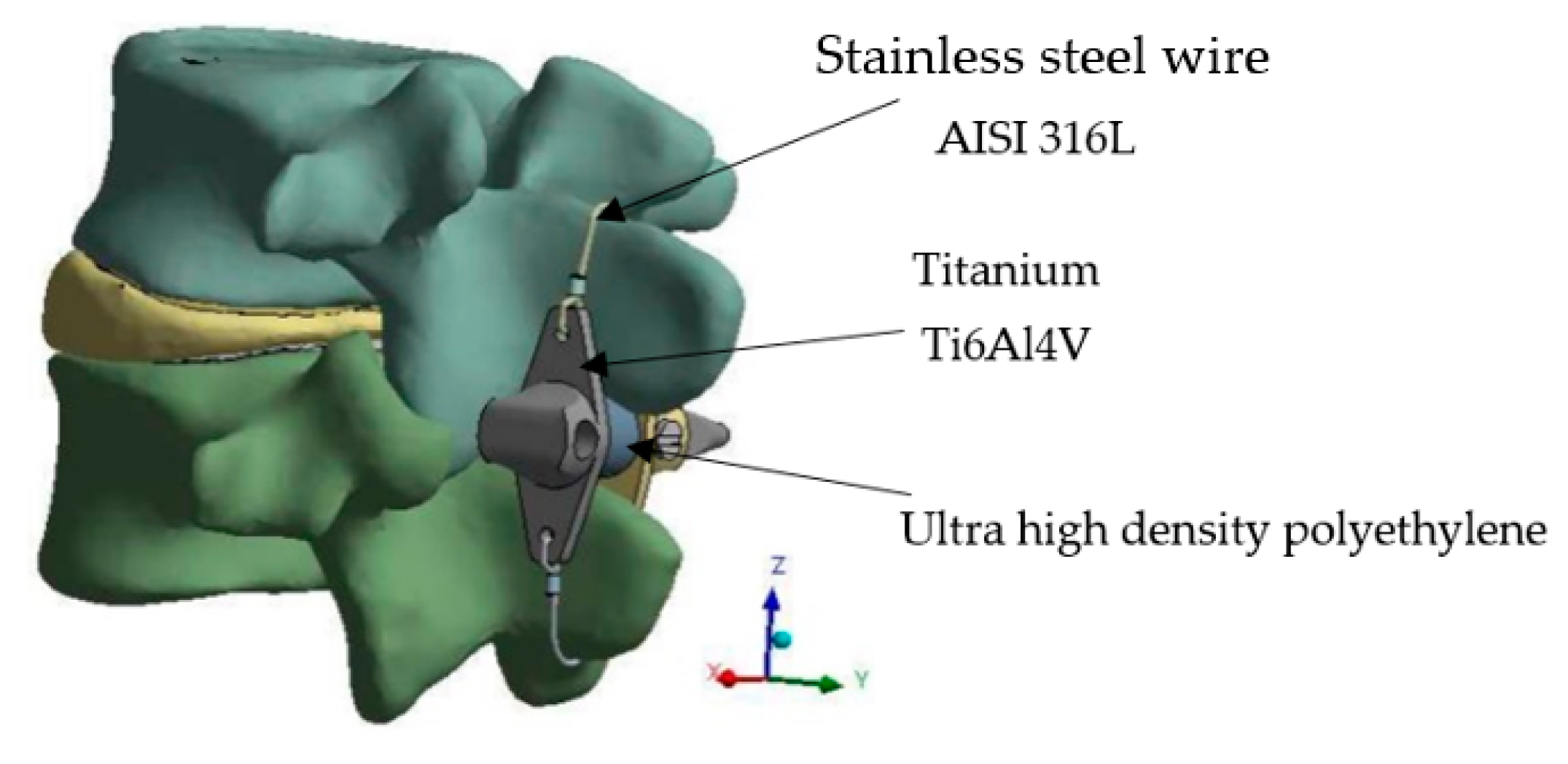
2.1. Device Description
2.2. General Conditions
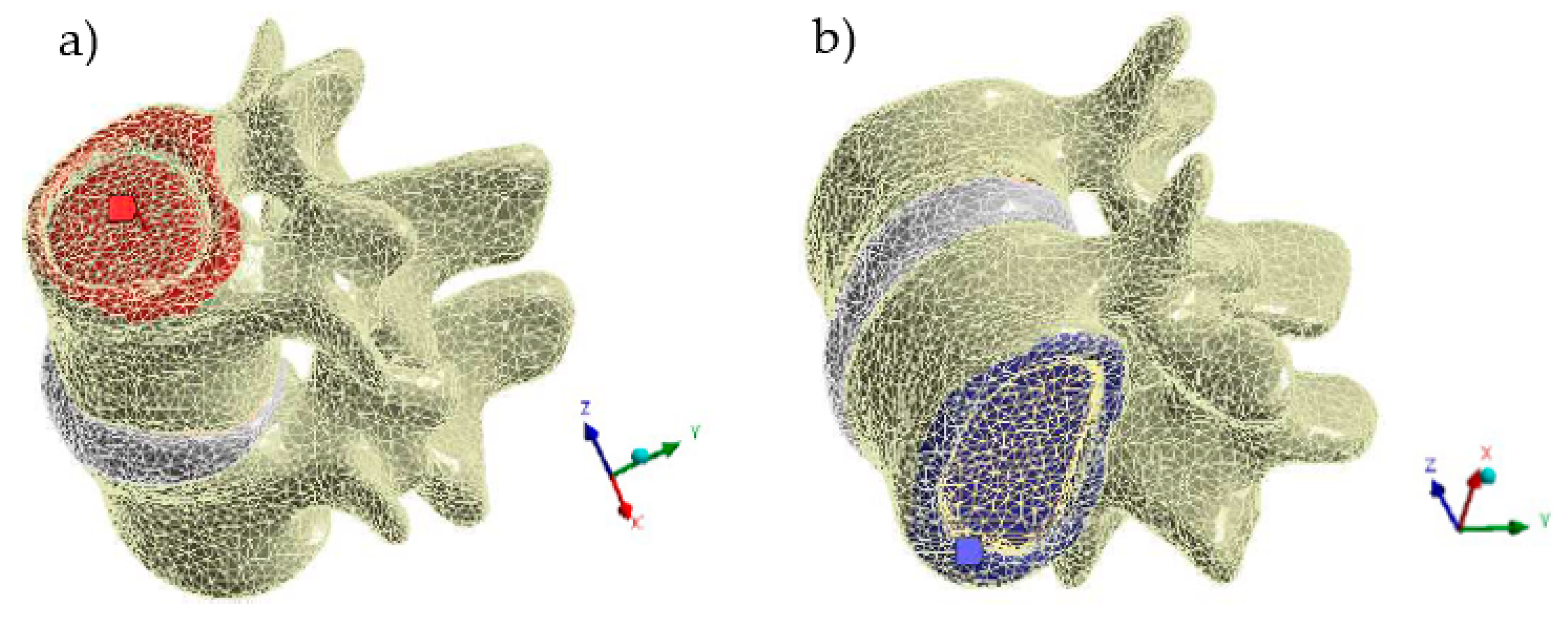
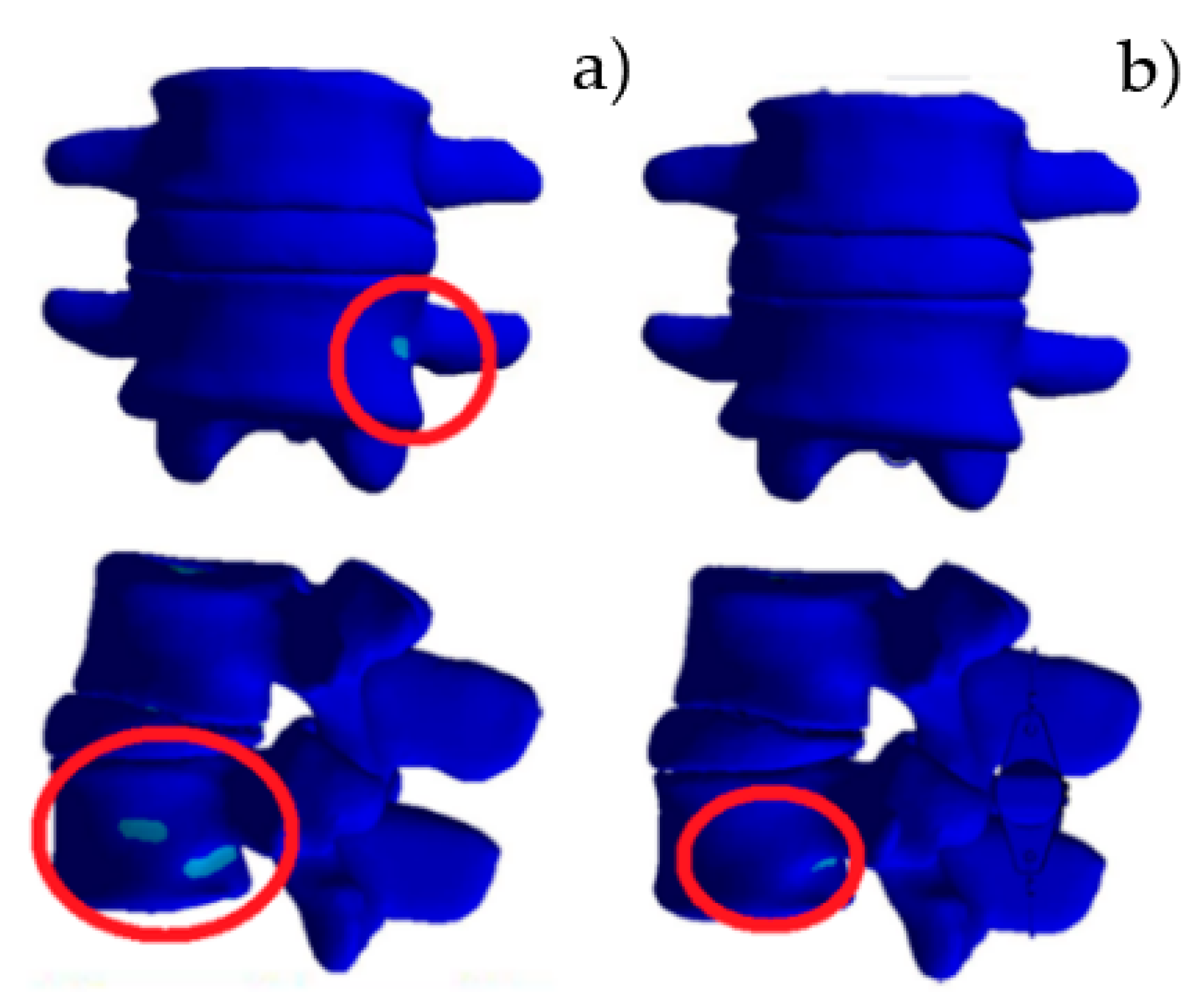
3. Results
3.1. Model Validation
3.2. Relaxed Standing
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Francisco, M.; Ortega, H. Numerical Analysis of an Interspinous Spacer; National Polytechnic Institute UPIS ESIME Zacatenco: Ciudad de México, México, 2019. [Google Scholar]
- Neal, C. The assessment of knowledge and application of proper body mechanics in the workplace. Orthop. Nurs. 1997, 16, 66–67, 69. [Google Scholar] [CrossRef] [PubMed]
- Social Communication. In the IMSS, More than 300 Thousand Consultations for Low Back Pain in 2017. Available online: http://www.imss.gob.mx/prensa/archivo/201810/246 (accessed on 3 October 2018).
- McGill, S.M.; Hughson, R.L.; Parks, K. Changes in lumbar lordosis modify the role of the extensor muscles. Clin. Biomech. 2000, 15, 777–780. [Google Scholar] [CrossRef]
- Behennah, J.; Conway, R.; Fisher, J.; Osborne, N.; Steele, J. The relationship between balance performance, lumbar extension strength, trunk extension endurance, and pain in participants with chronic low back pain, and those without. Clin. Biomech. 2018, 53, 22–30. [Google Scholar] [CrossRef]
- Mork, R.; Falkenberg, H.K.; Fostervold, K.I.; Thorud, H.-M.S. Discomfort glare and psychological stress during computer work: Subjective responses and associations between neck pain and trapezius muscle blood flow. Int. Arch. Occup. Environ. Health 2019, 93, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-J.; Chen, J. Disc degeneration implies low back pain. Theor. Biol. Med Model. 2015, 12, 24. [Google Scholar] [CrossRef]
- Allegri, M.; Montella, S.; Salici, F.; Valente, A.; Marchesini, M.; Compagnone, C.; Baciarello, M.; Manferdini, M.E.; Fanelli, G. Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Research 2016, 5, 1530. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.-J.; Rohlmann, F.; Neidlinger-Wilke, C.; Werner, K.; Claes, L.; Kettler, A. Validity and interobserver agreement of a new radiographic grading system for intervertebral disc degeneration: Part I. Lumbar spine. Eur. Spine J. 2006, 15, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lucas, P.; Leirós-Rodríguez, R.; Lopez-Barreiro, J.; García-Soidán, J.L. Is the combination of exercise therapy and health education more effective than usual medical care in the prevention of non-specific back pain? A systematic review with meta-analysis. Ann. Med. 2022, 54, 3106–3115. [Google Scholar] [CrossRef] [PubMed]
- Jecko, V.; Loit, M.-P.; Houston, D.; Champeaux-Depond, C. Functional Outcome after Spinal Meningioma Surgery. Asian Spine J. 2022, 16, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Liu, H.; Wu, J.; Qian, Z.; Qu, R.; Liu, T. A retrospective comparative study of postoperative sagittal balance in isthmic L5–S1 spondylolisthesis using single segment or two-segment pedicle screw fixation. BMC Musculoskelet. Disord. 2022, 23, 145. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jun, C.; Nam, J. Comparison of Fusion Rate and Functional Outcome Between Local Cancellous Bone Plus Demineralized Bone Matrix and Local Bone in 1-Level Posterior Lumbar Interbody Fusion. Clin. Spine Surg. A Spine Publ. 2022, 35, E621–E626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shen, H.; Cui, Y.; Fogel, G.R.; Liao, Z.; Liu, W. Biomechanical Evaluation of Transforaminal Lumbar Interbody Fusion with Coflex-F and Pedicle Screw Fixation: Finite Element Analysis of Static and Vibration Conditions. Orthop. Surg. 2022, 14, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Poetscher, A.W.; Gentil, A.F.; Ferretti, M.; Lenza, M. Interspinous process devices for treatment of degenerative lumbar spine stenosis: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0199623. [Google Scholar] [CrossRef] [PubMed]
- Puzzilli, F.; Gazzeri, R.; Galarza, M.; Neroni, M.; Panagiotopoulos, K.; Bolognini, A.; Callovini, G.; Agrillo, U.; Alfieri, A. Interspinous spacer decompression (X-STOP) for lumbar spinal stenosis and degenerative disk disease: A multicenter study with a minimum 3-year follow-up. Clin. Neurol. Neurosurg. 2014, 124, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Li, J.; Tang, H. Biomechanical comparison of different interspinous process devices in the treatment of lumbar spinal stenosis: A finite element analysis. BMC Musculoskelet. Disord. 2022, 23, 585. [Google Scholar] [CrossRef]
- Erbulut, D.U.; Zafarparandeh, I.; Hassan, C.R.; Lazoglu, I.; Ozer, A.F. Determination of the biomechanical effect of an interspinous process device on implanted and adjacent lumbar spinal segments using a hybrid testing protocol: A finite-element study. J. Neurosurg. Spine 2015, 23, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Chen, Y.-H.; Huang, K.-C.; Ko, P.-C.; Sun, J.-M. Preliminary report on value of Wallis interspinous device for back pain following microdiscectomy. Formos. J. Surg. 2022, 55, 163–170. [Google Scholar] [CrossRef]
- Arriba, M.P.; Maestre, C.; Martín-Gorroño, F.; Plasencia, P. Analysis of Long-Term Results of Lumbar Discectomy With and Without an Interspinous Device. Int. J. Spine Surg. 2022, 16, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ke, W.; Hua, W.; Lu, S.; Zeng, X.; Yang, C. Biomechanical evaluation of anterior and posterior lumbar surgical approaches on the adjacent segment: A finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jin, X.; Wang, C.; Wang, L. Wallis interspinous device versus discectomy for lumbar disc herniation. Orthopade 2018, 48, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Zander, T.; Shirazi-Adl, A.; Puttlitz, C.; Adam, C.; Chen, C.; Goel, V.; Kiapour, A.; Kim, Y.; Labus, K.; et al. Comparison of eight published static finite element models of the intact lumbar spine: Predictive power of models improves when combined together. J. Biomech. 2014, 47, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Galbusera, F.; Rohlmann, A.; Shirazi-Adl, A. What have we learned from finite element model studies of lumbar intervertebral discs in the past four decades? J. Biomech. 2013, 46, 2342–2355. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Lauterborn, S.; Dreischarf, M.; Schmidt, H.; Putzier, M.; Strube, P.; Zander, T. Parameters influencing the outcome after total disc replacement at the lumbosacral junction. Part 1: Misalignment of the vertebrae adjacent to a total disc replacement affects the facet joint and facet capsule forces in a probabilistic finite element analysis. Eur. Spine J. 2013, 22, 2271–2278. [Google Scholar] [CrossRef][Green Version]
- Borrelli, S.; Putame, G.; Pascoletti, G.; Terzini, M.; Zanetti, E.M. In Silico Meta-Analysis of Boundary Conditions for Experimental Tests on the Lumbar Spine. Ann. Biomed. Eng. 2022, 50, 1243–1254. [Google Scholar] [CrossRef]
- Guldeniz, O.; Yesil, O.B.; Okyar, F. Yeditepe spine mesh: Finite element modeling and validation of a parametric CAD model of lumbar spine. Med. Eng. Phys. 2022, 110, 103911. [Google Scholar] [CrossRef]
- Sanjay, D.; Bhardwaj, J.S.; Kumar, N.; Chanda, S. Expandable pedicle screw may have better fixation than normal pedicle screw: Preclinical investigation on instrumented L4-L5 vertebrae based on various physiological movements. Med. Biol. Eng. Comput. 2022, 60, 2501–2519. [Google Scholar] [CrossRef]
- Little, J.; Adam, C. Geometric sensitivity of patient-specific finite element models of the spine to variability in user-selected anatomical landmarks. Comput. Methods Biomech. Biomed. Eng. 2013, 18, 676–688. [Google Scholar] [CrossRef]
- Gazzeri, R.; Galarza, M.; Neroni, M.; Fiore, C.; Faiola, A.; Puzzilli, F.; Callovini, G.; Alfieri, A. Failure rates and complications of interspinous process decompression devices: A European multicenter study. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef]
- Chen, H.-C.; Wu, J.-L.; Huang, S.-C.; Zhong, Z.-C.; Chiu, S.-L.; Lai, Y.-S.; Cheng, C.-K. Biomechanical evaluation of a novel pedicle screw-based interspinous spacer: A finite element analysis. Med. Eng. Phys. 2017, 46, 27–32. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Wang, Y.-F.; Han, J.; Liu, S.-M.; Wang, H. Percutaneously placed lumbar interspinous stabilization devices—A review of current clinical research. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4244–4251. [Google Scholar] [PubMed]
- Nandakumar, A.; Clark, N.A.; Smith, F.W.; Wardlaw, D. Two-Year Results of X-Stop Interspinous Implant for the Treatment of Lumbar Spinal Stenosis. J. Spinal Disord. Tech. 2013, 26, 1–7. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, J.-B.; Chang, D.-G.; Kim, H.J. 15-year survivorship analysis of an interspinous device in surgery for single-level lumbar disc herniation. BMC Musculoskelet. Disord. 2021, 22, 1030. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.-U.; Abraham, I.; León, J.F.R.; Cantú-Leal, R.; Longoria, R.C.; Sánchez, J.A.S.; Yeung, A. A Differential Clinical Benefit Examination of Full Lumbar Endoscopy vs. Interspinous Process Spacers in the Treatment of Spinal Stenosis: An Effect Size Meta-Analysis of Clinical Outcomes. Int. J. Spine Surg. 2022, 16, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Pavan, L.J.; Dalili, D.; De Vivo, A.E.; Hamel-Senecal, A.; Torre, F.; Rudel, A.; Manfré, L.; Amoretti, N. Clinical and radiological outcomes following insertion of a novel removable percutaneous interspinous process spacer: An initial experience. Neuroradiology 2022, 64, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.D.H.; Hudgins, G. In-Situ Curable Interspinous Process Spacer. US 2009/0118833 A1, 12 May 2009. [Google Scholar]
- Khoo, L. Percutaneous Interspinous Process Device And Method. US 8070779 B2, 6 December 2011. [Google Scholar]
- Hess, H. Interspinous Process Implants Having Deployable Engagement Arms. US 8142479 B2, 27 March 2012. [Google Scholar]
- Altarac, M.; Hayes, S.K.; Reglos, J.C.; Gutierrez, R.; Flaherty, J. System and Methods for Posterior Dynamic Stabilization of the Spine. US 2007/0219556A1, 20 September 2007. [Google Scholar]
- James, J.A.C.G.G.; Yue, J. Conical Interspinousapparatus and a Method of Performing Interspnous Distraction. US 8192466 B2, 5 June 2012. [Google Scholar]
- Felix Aschmann, B.B.D.C. Spinous Process Fixator. US 9168073 B2, 27 October 2015. [Google Scholar]
- Aflatoon, K. Dynamic Inter-Spinous Process Spacer. US 9204907 B2, 8 December 2015. [Google Scholar]
- Gabelberger, J. Expandable Interspinous Process Spacer Implant. US 9402732 B2, 2 August 2016. [Google Scholar]
- Hernández, A.F.; Cañizo, R.G.R.; Huerta, O.S.; Cruz, E.A.M.; Pineda, J.M.S.; Hernández, E.P. Aplicación de Metodología de Modelado 3D Utilizando Tomografías Computarizadas Para su Análisis Numérico; Revista Facultad de Ingeniería Universidad de Antioquia: Medellín, Colombia, 2014; pp. 116–126. [Google Scholar]
- Wang, W.; Zhou, C.; Guo, R.; Cha, T.; Li, G. Prediction of biomechanical responses of human lumbar discs - a stochastic finite element model analysis. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1730–1741. [Google Scholar] [CrossRef]
- Guo, L.-X.; Zhang, C. Development and Validation of a Whole Human Body Finite Element Model with Detailed Lumbar Spine. World Neurosurg. 2022, 163, e579–e592. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Cao, J.; Zhang, J.; Rong, Q.; Zhou, D. Response Analysis of Lumbar Vertebra Model under Axial Impact Loading. In IUTAM Symposium on Impact Biomechanics: From Fundamental Insights to Applications; Springer: Berlin/Heidelberg, Germany, 2005; pp. 111–118. [Google Scholar] [CrossRef]
- Heo, M.; Yun, J.; Kim, H.; Lee, S.-S.; Park, S. Optimization of a lumbar interspinous fixation device for the lumbar spine with degenerative disc disease. PLoS ONE 2022, 17, e0265926. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, X.-F.; Liu, Z.-D.; Zhong, G.-B. Biomechanical assessment of a novel L4/5 level interspinous im-plant using three dimensional finite element analysis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 86–94. [Google Scholar] [PubMed]
- Yin, J.-Y.; Guo, L.-X. Biomechanical analysis of lumbar spine with interbody fusion surgery and U-shaped lumbar interspinous spacers. Comput. Methods Biomech. Biomed. Eng. 2020, 24, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, X.; Liu, T.; Dong, L. Biomechanical analysis of a customized lumbar interspinous spacer based on transfacetopedicular screw fixation: A finite element study. Med Eng. Phys. 2022, 107, 103850. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-E.; Hu, H.-T.; Kao, L.-H.; Liu, C.-J.; Chen, K.-C.; Huang, K.-Y. Biomechanical feasibility of semi-rigid stabilization and semi-rigid lumbar interbody fusion: A finite element study. BMC Musculoskelet. Disord. 2022, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Deng, Z.; Chen, X.; Shao, J.; Qiu, L.; Jiang, C.; Niu, W. The Role of Multifidus in the Biomechanics of Lumbar Spine: A Musculoskeletal Modeling Study. Bioengineering 2023, 10, 67. [Google Scholar] [CrossRef] [PubMed]


| ISP | Patients | Complications Related to Device | Reduction of Symptoms | |
|---|---|---|---|---|
| Wallis | 130 | Migration | 1 | NA |
| Incorrect placement of the device | NA | |||
| Spinous process fracture | 0 | |||
| Increased pain | 1 | |||
| U | 209 | Migration | 1 | 157/209 (75%) |
| Implant failure | 2 | |||
| Spinous process fracture | 0 | |||
| Increased pain | NA | |||
| DIAM | 1756 | Infection | 10 | 1505/1756 (85.7%) |
| Spinous process fracture | 10 | |||
| Increased pain | 70 | |||
| X-STOP | 201 | Migration | 2 | 139/201 (69%) |
| Spinous process fracture | 13 | |||
| Increased pain | 13 | |||
| Incorrect placement of the device | NA | |||
| Superion | 190 | Migration | NA | 123/190 (64.7%) |
| Spinous process fracture | 23 | |||
| Increased pain | 8 | |||
| Incorrect placement of the device | NA | |||
| Name | Approach | Characteristics |
|---|---|---|
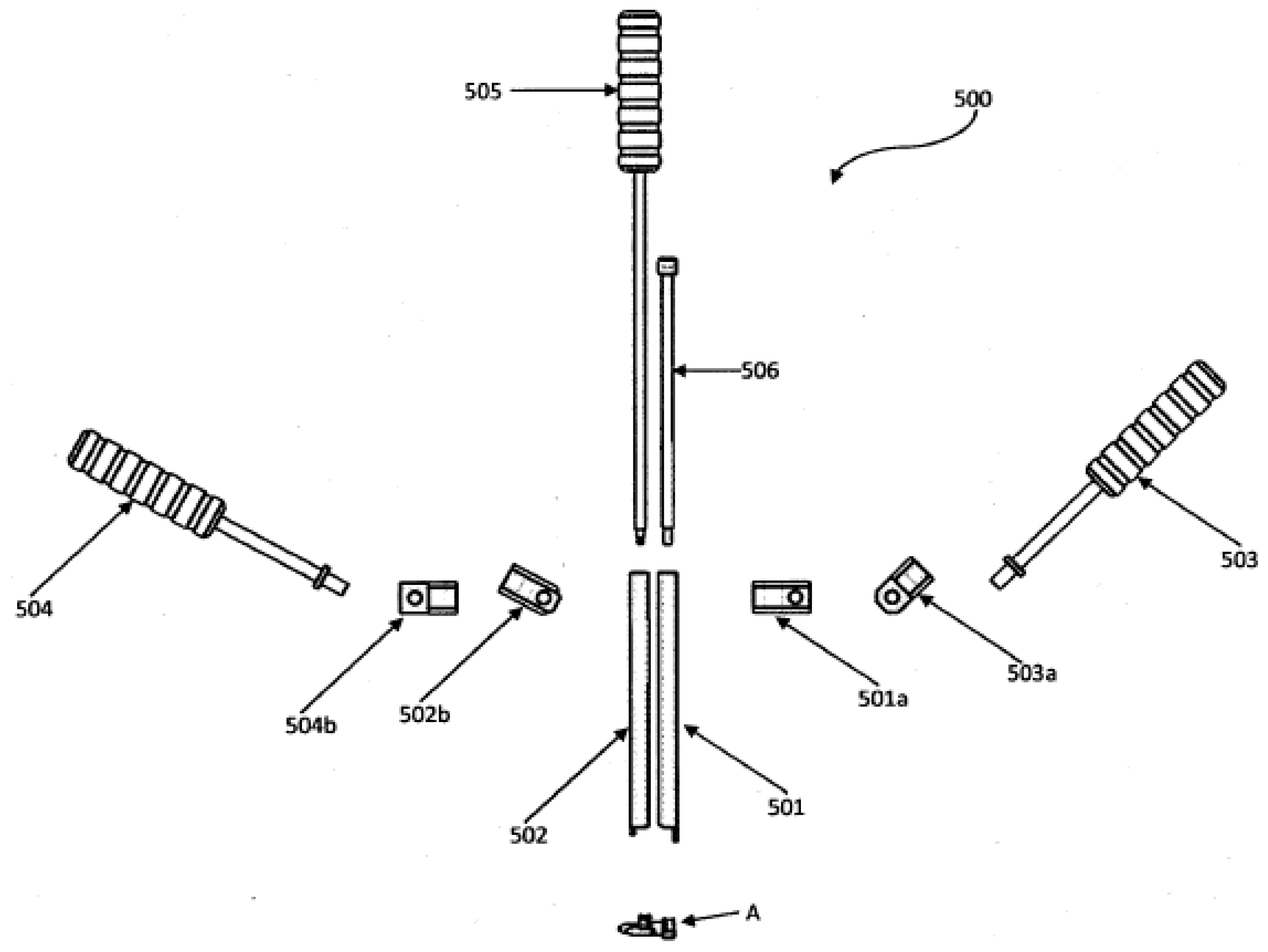
| In Situ Curable ISP [38] | Posterolateral percutaneous approach | The device is expandable, so its placement is compact. Once placed, it expands to the desired size by injecting fluid through a catheter. |
| Percutaneous Interspinous Process Device and Method [39] | Posterolateral percutaneous approach | It has two lateral sections and a central unit. The lateral sections have a dentate contact area to obtain greater adherence to the spiny apophysis. The main section is joined to one of the lateral sections, with an anchor section at the end to join the other lateral area at the time of implantation. |
| Interspinous Process Implants with Deployable Engagement Arms [40] | Posterolateral percutaneous approach | The body part can be partially threaded and have a smooth surface. The body part includes an inner cavity. It comprises deployable coupling members adapted and configured to move simultaneously through an actuating mechanism between a folded and retracted position within the internal cavity. In addition, each coupling arm may include a distal hook portion with teeth for coupling to the spinal process. |
| System and Methods for Posterior Dynamic Stabilization of the Spine [41] | Posterolateral percutaneous approach | It has a main body with a cross-sectional size and shape that allows implantation between adjacent spiny apophyses. It has two pairs of movable arms between a folded and unfolded state. The individual extension arms can have any angle of curvature to facilitate the coupling of multiple spacers. In addition, it may include an element that allows them to be interconnected or joined in a fixed or dynamic manner. |
| Conical Interspinous Apparatus and a Method of Performing Interspinous Distraction [42] | Posterolateral approach | A conical screw ISP apparatus must have an insertion controller, a pair of deployable proximal wings, and a pair of deployable distal wings. |
| Spinous Process Fixator [43] | Subsequent approach | The device has two auxiliary toothed side plates in the clamping mechanism. In addition, the spacer body can include a channel sized and configured to receive the expansion locking mechanism. |
| Dynamic Inter-Spinous Process Spacer [44] | Posterolateral percutaneous approach | The device has two anchors and two retractable adjustment members (upper and lower). In addition, it has an internal mechanism for adjusting the retractable members. |
| Expandable Interspinous Process Spacer Implant [45] | Posterolateral approach | The implant includes an upper casing, a lower casing, and a folding mechanism. The outer surfaces are configured to hook into the apophysis. |
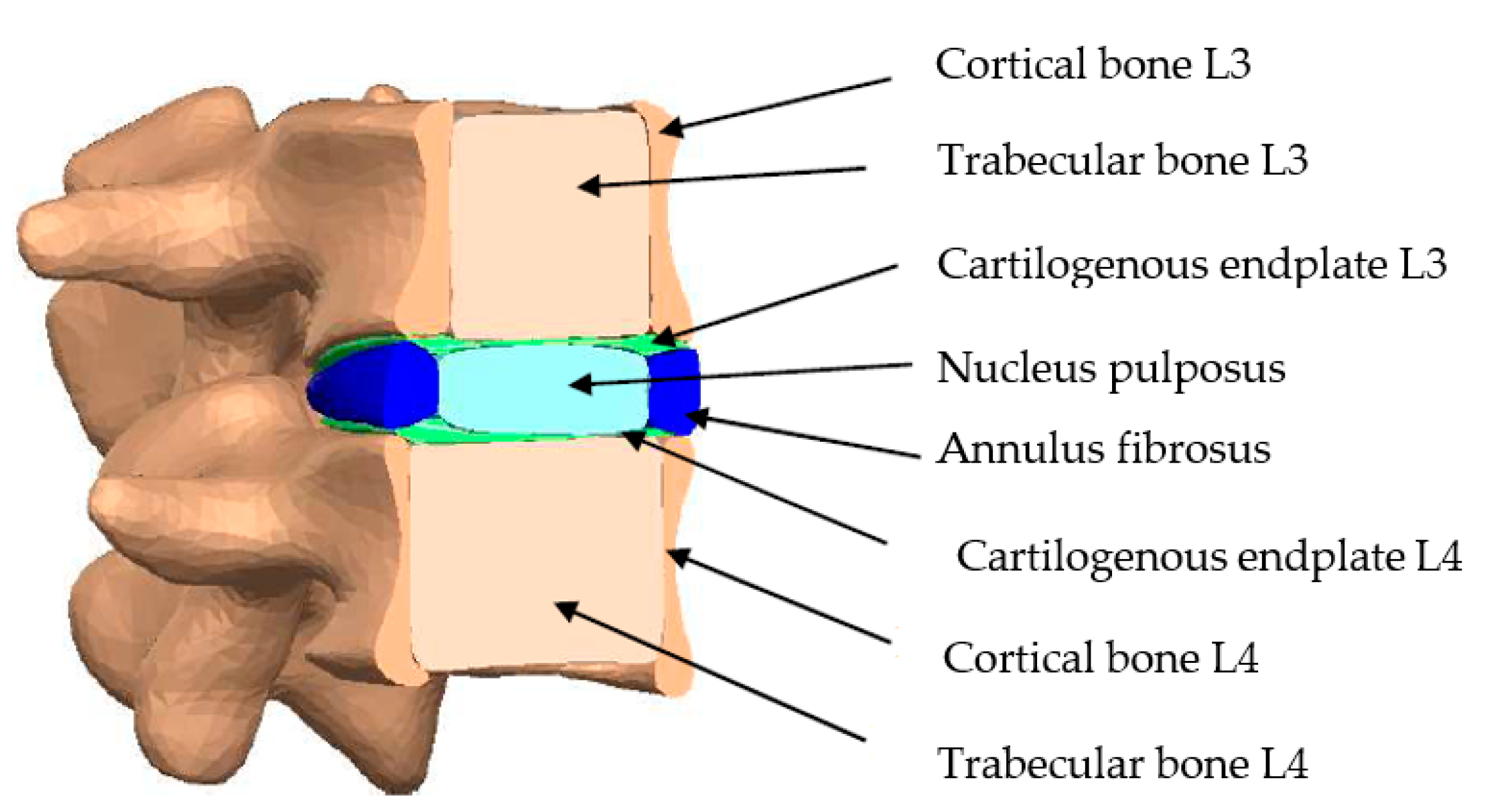
| Material | Young’s Modulus (MPa) | Density (kg/m3) | Poisson’s Ratio |
|---|---|---|---|
| Cortical bone | 12,000 | 1900 | 0.3 |
| Trabecular bone | 100 | 700 | 0.2 |
| Annulus fibrosus | 4.2 | 1065 | 0.45 |
| Nucleus pulposus | 1 | 1000 | 0.4999 |
| Cartilogenous endplates | 500 | --- | 0.4 |
| Ti6Al4V | 113,800 | 4420 | 0.342 |
| Ultra high-density polyethylene | 1100 | 950 | 0.42 |
| Stainless steel AISI 316 L | 200,000 | 7750 | 0.3 |
| Study | Axial Force (N) | Moment or Force | Intervertebral Rotation (Degrees) | Reference |
|---|---|---|---|---|
| Bending movement | 1175 | 8 N-m | 8 | Jia-Yu Yin et al. [52] |
| Lateral bending | 700 | 6 N-m | 7 | |
| Axial rotations | 720 | 4 N-m | 2 | |
| Bending movement | 706.63 | 147.15 N | 11.5 | Current study |
| Lateral bending | 706.63 | 83.3 N | 8 | |
| Axial rotations | 706.63 | 49.05 N | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ortega, M.F.; Torres-SanMiguel, C.R.; Alcántara-Arreola, E.A.; Paredes-Rojas, J.C.; Cabrera-Rodríguez, O.; Urriolagoitia-Calderón, G.M. Numerical Assessment of Interspinous Spacers for Lumbar Spine. Prosthesis 2023, 5, 939-951. https://doi.org/10.3390/prosthesis5030065
Hernández-Ortega MF, Torres-SanMiguel CR, Alcántara-Arreola EA, Paredes-Rojas JC, Cabrera-Rodríguez O, Urriolagoitia-Calderón GM. Numerical Assessment of Interspinous Spacers for Lumbar Spine. Prosthesis. 2023; 5(3):939-951. https://doi.org/10.3390/prosthesis5030065
Chicago/Turabian StyleHernández-Ortega, Marcial Francisco, Christopher René Torres-SanMiguel, Elliot Alonso Alcántara-Arreola, Juan Carlos Paredes-Rojas, Ohtokani Cabrera-Rodríguez, and Guillermo Manuel Urriolagoitia-Calderón. 2023. "Numerical Assessment of Interspinous Spacers for Lumbar Spine" Prosthesis 5, no. 3: 939-951. https://doi.org/10.3390/prosthesis5030065
APA StyleHernández-Ortega, M. F., Torres-SanMiguel, C. R., Alcántara-Arreola, E. A., Paredes-Rojas, J. C., Cabrera-Rodríguez, O., & Urriolagoitia-Calderón, G. M. (2023). Numerical Assessment of Interspinous Spacers for Lumbar Spine. Prosthesis, 5(3), 939-951. https://doi.org/10.3390/prosthesis5030065







