Concomitant Parenchymal, Subarachnoid, and Ventricular Neurocysticercosis in Rome, Italy: A Case Report with a 4-Year Follow-Up
Abstract
1. Introduction
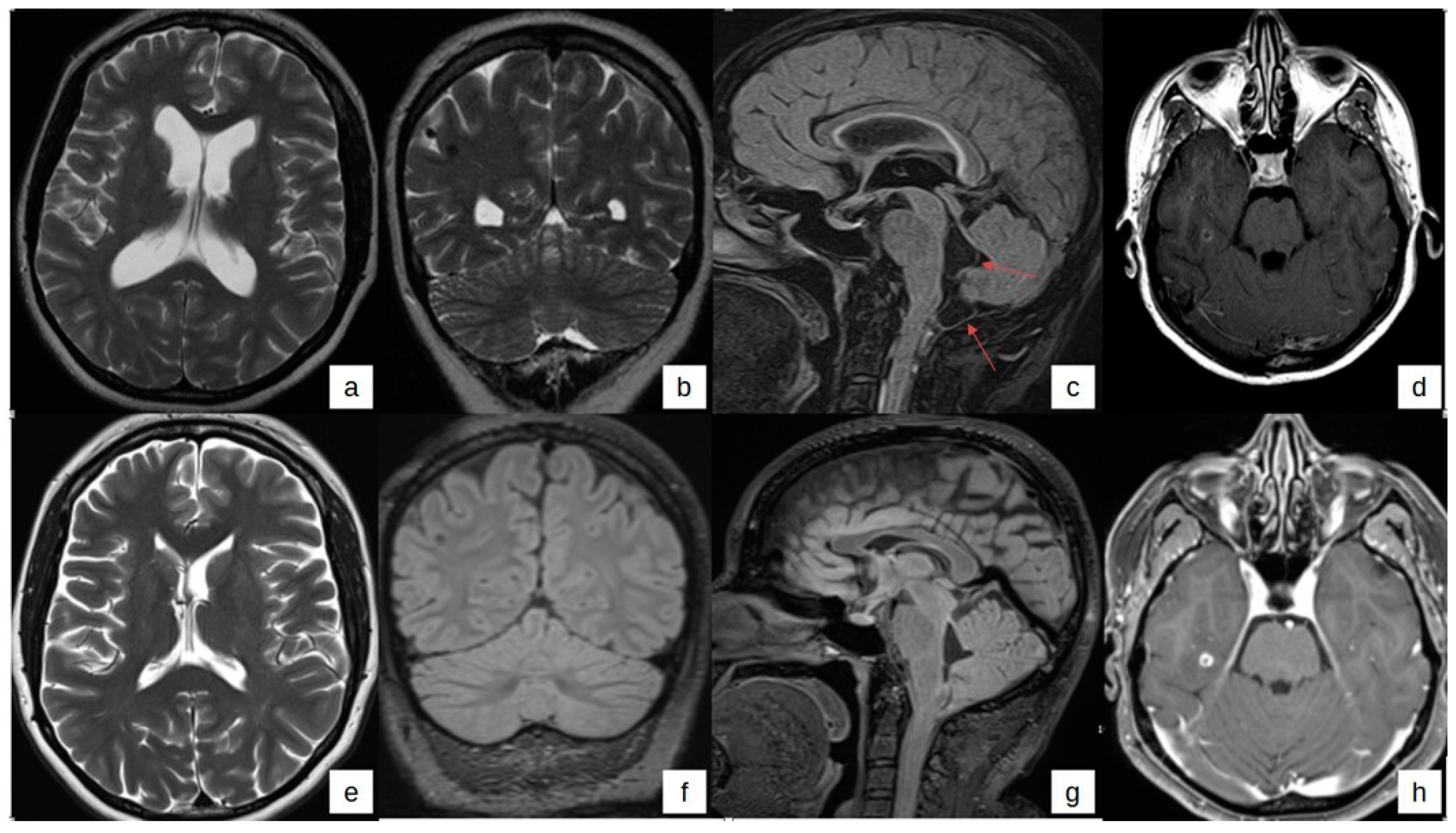
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.paho.org/en/topics/taenia-solium-taeniasiscysticercosis (accessed on 15 April 2024).
- Ndimubanzi, P.C.; Carabin, H.; Budke, C.M.; Nguyen, H.; Qian, Y.J.; Rainwater, E.; Dickey, M.; Reynolds, S.; Stoner, J.A. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl. Trop. Dis. 2010, 4, e870. [Google Scholar] [CrossRef]
- Debacq, G.; Moyano, L.M.; Garcia, H.H.; Boumediene, F.; Marin, B.; Ngoungou, E.B.; Preux, P.-M. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLoS Negl. Trop. Dis. 2017, 11, e0005153. [Google Scholar] [CrossRef]
- O’Neal, S.E.; Flecker, R.H. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg. Infect. Dis. 2015, 21, 969. [Google Scholar] [CrossRef] [PubMed]
- Laranjo-González, M.; Devleesschauwer, B.; Trevisan, C.; Allepuz, A.; Sotiraki, S.; Abraham, A.; Afonso, M.B.; Blocher, J.; Cardoso, L.; da Costa, J.M.C.; et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Western Europe. Parasit. Vectors 2017, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Stelzle, D.; Abraham, A.; Kaminski, M.; Schmidt, V.; De Meijere, R.; Bustos, J.A.; Garcia, H.H.; Sahu, P.S.; Bobic, B.; Cretu, C.; et al. Clinical characteristics and characteristics and management of neurocysticercosis patients: A retrospective assessment of case reports from Europe. J. Travel Med. 2023, 30, taac102. [Google Scholar] [CrossRef] [PubMed]
- von Nickisch-Rosenegk, M.; Lucius, R.; Loos-Frank, B. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J. Mol. Evol. 1999, 48, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Djikeng, A.; Halpin, R.; Kuzmickas, R.; Depasse, J.; Feldblyum, J.; Sengamalay, N.; Afonso, C.; Zhang, X.; Anderson, N.G.; Ghedin, E.; et al. Viral genome sequencing by random priming methods. BMC Genom. 2008, 9, 5. [Google Scholar] [CrossRef]
- O’Connell, E.M.; Harrison, S.; Dahlstrom, E.; Nash, T.; Nutman, T.B. A Novel, Highly Sensitive Quantitative Polymerase Chain Reaction Assay for the Diagnosis of Subarachnoid and Ventricular Neurocysticercosis and for Assessing Responses to Treatment. Clin. Infect. Dis. 2020, 70, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; Ware, J.M.; Mahanty, S. Intraventricular Neurocysticercosis: Experience and Long-Term Outcome from a Tertiary Referral Center in the United States. Am. J. Trop. Med. Hyg. 2018, 98, 1755. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; O’Connell, E.M.; Hammoud, D.A.; Wetzler, L.; Ware, J.M.; Mahanty, S. Natural History of Treated Subarachnoid Neurocysticercosis. Am. J. Trop. Med. Hyg. 2020, 102, 78. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto Filho, P.T.; Norcia, L.F.; Fleury, A.; Zanini, M.A. Current Role of Surgery in the Treatment of Neurocysticercosis. Pathogens 2024, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Arellano, C.A.; Kuschick-Fehér, J.; Romero-Gonzalez, F.G.; Fleury, A. Neurocysticercosis: The Duration of Its Preclinical Phase Relies on the Parasite Location. Trop. Med. Int. Health 2024, 29, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Pérez-Molina, J.A.; Henríquez Camacho, C.A.; Rodriguez-Guardado, A.; Bosch-Nicolau, P.; Calabuig, E.; Domínguez-Castellano, A.; Pérez-Jacoiste, M.A.; de Guevara, M.C.L.; Mena, A.; et al. REDIVI Study Group. Imported cysticercosis in Spain: A retrospective case series from the +REDIVI Collaborative Network. Travel Med. Infect. Dis. 2020, 37, 101683. [Google Scholar] [CrossRef] [PubMed]
- Yera, H.; Dupont, D.; Houze, S.; Ben M’rad, M.; Pilleux, F.; Sulahian, A.; Gatey, C.; Gay Andrieu, F.; Dupouy-Camet, J. Confirmation and follow-up of neurocysticercosis by real-time PCR in cerebrospinal fluid samples of patients living in France. J. Clin. Microbiol. 2011, 49, 4338–4340. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Li, C.; Zhang, Y.; Zhang, H.; Liu, X.; Ji, X.; Shi, Y.; Liu, N.; Wu, M.; Du, F.; et al. Clin Next-generation sequencing of cerebrospinal fluid for the diagnosis of neurocysticercosis. Neurol. Neurosurg. 2020, 9, 471. [Google Scholar] [CrossRef]
- Fleury, A.; Garcia, E.; Hernández, M.; Carrillo, R.; Govezensky, T.; Fragoso, G.; Sciutto, E.; Harrison, L.J.; Parkhouse, R.M. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS Negl. Trop. Dis. 2013, 7, e2096. [Google Scholar] [CrossRef]
- Carrillo Mezo, R.; Lara García, J.; Arroyo, M.; Fleury, A. Relevance of 3D Magnetic Resonance Imaging Sequences in Diagnosing Basal Subarachnoid Neurocysticercosis. Acta. Trop. 2015, 152, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Gothi, R. Consider tuberculoma and cysticercosis in the differential diagnosis of brain tumour in tropical countries. BMJ 2013, 347, f6604. [Google Scholar] [CrossRef] [PubMed]
- White, A.C., Jr.; Coyle, C.M.; Rajshekhar, V.; Singh, G.; Hauser, W.A.; Mohanty, A.; Garcia, H.H.; Nash, T.E. Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2018, 98, 945–966. [Google Scholar] [CrossRef]
| 19 May 2020 | 1 June 2020 | 18 June 2020 | 26 April 2021 | 28 June 2021 | 15 October 2021 | 17 June 2022 | 22 August 2023 | |
|---|---|---|---|---|---|---|---|---|
| WBC (n/mm3) | 105 (N 40%, L 10%, histiocytes) | 80 (N 40%, L 40%) | 20 (L 70%) | 20 (L 40%, histiocytes 40%) | 11 | 14 (L 70%, N 10%) | 8 | 7 (L 70%) |
| Glucose (mg/dL) | 12 | 5 | 23 | 54 | 59 | 48 | 56 | 54 |
| Proteins (mg/dL) | 110 | 120 | 88 | 1112 | 736 | 837 | 808 | 658 |
| Home made PCR for T. solium (INMI) | negative | negative | negative | negative | ||||
| NGS (INMI) | positive for T. solium | |||||||
| T. solium Ag concentration (ng/mL) | * 99800 (NIH) | highly positive (ITG) | highly positive (ITG) | 14.5 (NIH) | negative (NIH) | |||
| qPCR for T. solium (NIH) | * positive (value 24.6 Cq) | negative | negative | |||||
| Therapy | - | - | ||||||
| yes | yes | no | no | no | no | ||
| no | yes | yes | no | no | yes | ||
| no | no | yes | yes | yes | yes | ||
| no | no | no | no | yes | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giancola, M.L.; Haggiag, S.; Corpolongo, A.; Stasolla, A.; Mariano, A.; Menniti, A.; Campioni, P.; Bartolini, B.; Galizia, P.; Vulcano, A.; et al. Concomitant Parenchymal, Subarachnoid, and Ventricular Neurocysticercosis in Rome, Italy: A Case Report with a 4-Year Follow-Up. Trop. Med. Infect. Dis. 2024, 9, 187. https://doi.org/10.3390/tropicalmed9080187
Giancola ML, Haggiag S, Corpolongo A, Stasolla A, Mariano A, Menniti A, Campioni P, Bartolini B, Galizia P, Vulcano A, et al. Concomitant Parenchymal, Subarachnoid, and Ventricular Neurocysticercosis in Rome, Italy: A Case Report with a 4-Year Follow-Up. Tropical Medicine and Infectious Disease. 2024; 9(8):187. https://doi.org/10.3390/tropicalmed9080187
Chicago/Turabian StyleGiancola, Maria Letizia, Shalom Haggiag, Angela Corpolongo, Alessandro Stasolla, Andrea Mariano, Agazio Menniti, Paolo Campioni, Barbara Bartolini, Pierluigi Galizia, Antonella Vulcano, and et al. 2024. "Concomitant Parenchymal, Subarachnoid, and Ventricular Neurocysticercosis in Rome, Italy: A Case Report with a 4-Year Follow-Up" Tropical Medicine and Infectious Disease 9, no. 8: 187. https://doi.org/10.3390/tropicalmed9080187
APA StyleGiancola, M. L., Haggiag, S., Corpolongo, A., Stasolla, A., Mariano, A., Menniti, A., Campioni, P., Bartolini, B., Galizia, P., Vulcano, A., Fontana, C., Gasperini, C., O’Connell, E., Garcia, H. H., Nash, T. E., & Nicastri, E. (2024). Concomitant Parenchymal, Subarachnoid, and Ventricular Neurocysticercosis in Rome, Italy: A Case Report with a 4-Year Follow-Up. Tropical Medicine and Infectious Disease, 9(8), 187. https://doi.org/10.3390/tropicalmed9080187







