One Health Approach to Toxoplasmosis: Owner and Dog Seropositivity as Spatial Indicators of Risk Areas for Acquired, Gestational and Congenital Transmission
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
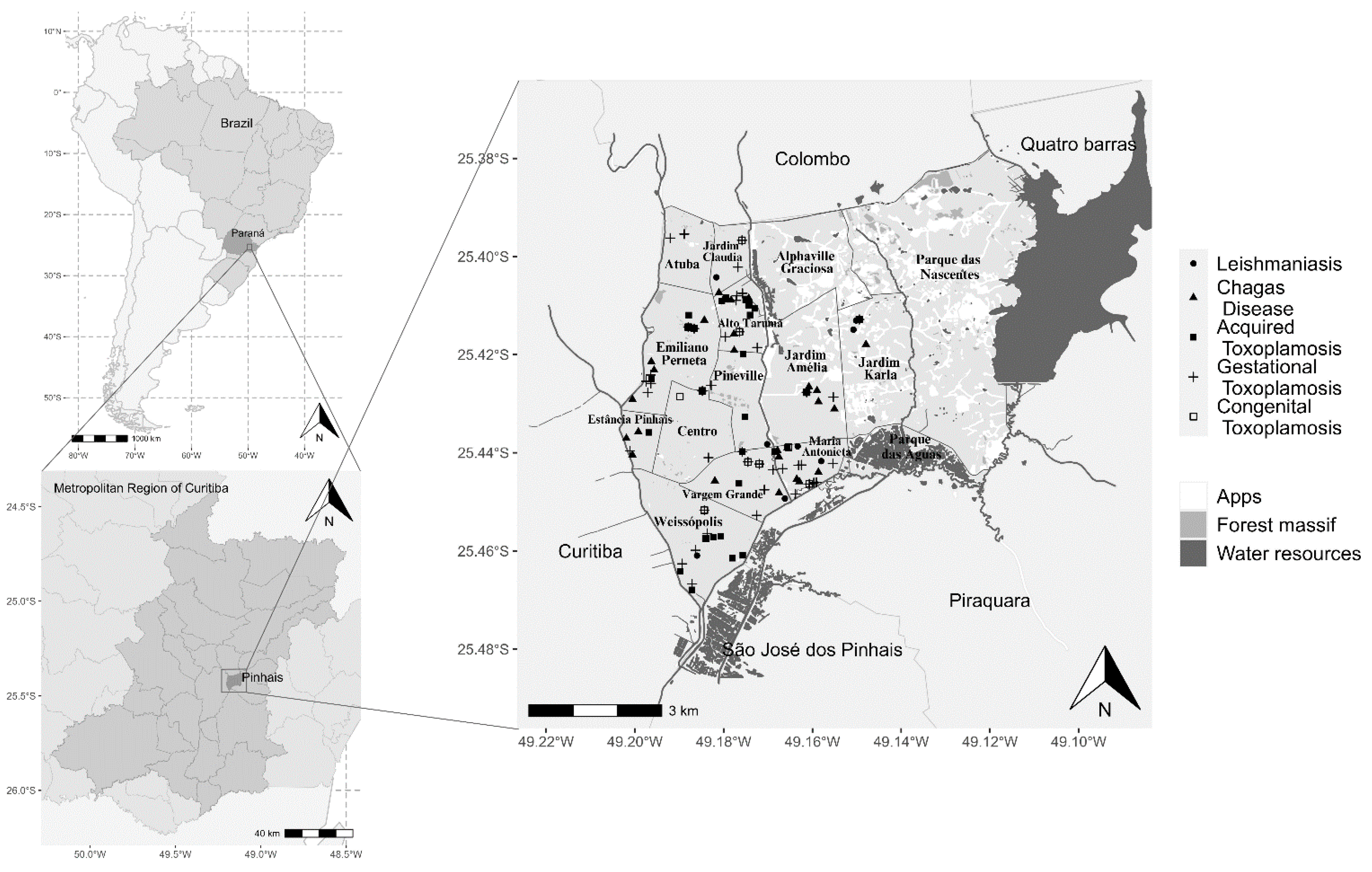
2.2. Study Area
2.3. Samplings and Testing
2.4. Epidemiological Data Collection
2.5. Statistical Analysis
3. Results
3.1. Serological Analysis
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Peng, J.; Mohsin, M.; Huang, X.; Lin, X.; Aguilar-Marcelino, L.; Huang, Z.; Yin, G. Construction and evaluation of the Toxoplasma gondii DNA vaccine targeting DEC-205. Pak. Vet. J. 2022, 42, 256–260. [Google Scholar] [CrossRef]
- Mohsin, M.; Li, Y.; Zhang, X.; Wang, Y.; Huang, Z.; Yin, G.; Zhang, Z. Development of CRISPR-CAS9 based RNA drugs against Eimeria tenella infection. Genomics 2021, 113, 4126–4135. [Google Scholar] [CrossRef]
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef]
- CDC Parasites. 2024. Available online: https://www.cdc.gov/parasites/index.html (accessed on 15 May 2024).
- Almeria, S.; Dubey, J.P. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii as a Parasite in Food: Analysis and Control. Microbiol. Spectr. 2016, 4, 227–247. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P.; Butler, J.M.; Blagburn, B.L. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet. Parasitol. 1997, 73, 27–33. [Google Scholar] [CrossRef]
- FRENKEL, J.K.; PARKER, B.B. An Apparent Role of Dogs in the Transmission of Toxoplasma gondii: The Probable Importance of Xenosmophilia. Ann. N. Y. Acad. Sci. 1996, 791, 402–407. [Google Scholar] [CrossRef]
- Bresciani, K.D.S.; da Costa, A.J.; Navarro, I.T.; Toniollo, G.H.; Sakamoto, C.A.M.; Arantes, T.P.; Gennari, S.M. Toxoplasmose canina: Aspectos clínicos e patológicos. Semin. Ciências Agrárias 2008, 29, 189–201. [Google Scholar] [CrossRef][Green Version]
- Capobiango, J.D.; Mitsuka Breganó, R.; Navarro, I.T.; Rezende Neto, C.P.; Barbante Casella, A.M.; Ruiz Lopes Mori, F.M.; Pagliari, S.; Inoue, I.T.; Reiche, E.M.V. Congenital toxoplasmosis in a reference center of Paraná, Southern Brazil. Braz. J. Infect. Dis. 2014, 18, 364–371. [Google Scholar] [CrossRef]
- Zoonosis y Enfermedades Transmisibles Comunes al Hombre y a los Animales: Parasitosis, v.3, 3 ed. 2024. Available online: https://iris.paho.org/handle/10665.2/3323?show=full&locale-attribute=pt (accessed on 15 May 2024).
- Woodhall, D.; Jones, J.L.; Cantey, P.T.; Wilkins, P.P.; Montgomery, S.P. Neglected parasitic infections: What every family physician needs to know. Am. Fam. Physician 2014, 89, 803–811. [Google Scholar]
- Dubey, J.P.; Lago, E.G.; Gennari, S.M.; Su, C.; Jones, J.L. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef]
- de Moura, L.; Bahia-Oliveira, L.M.G.; Wada, M.Y.; Jones, J.L.; Tuboi, S.H.; Carmo, E.H.; Ramalho, W.M.; Camargo, N.J.; Trevisan, R.; Graça, R.M.T.; et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 2006, 12, 326–329. [Google Scholar] [CrossRef]
- de Almeida, M.J.; de Oliveira, L.H.H.; Freire, R.L.; Navarro, I.T. Aspectos sociopolíticos da epidemia de toxoplasmose em Santa Isabel do ivaí (PR). Cienc. Saude Coletiva 2011, 16, 1363–1373. [Google Scholar] [CrossRef]
- Dal Ponte, S.; Burguez, D.; Andrioli, G. Outbreak of Toxoplasmosis in the City of Santa Maria, Brazil. Prehosp. Disaster Med. 2019, 34, s74. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Ben-Harari, R.R. Tick transmission of toxoplasmosis. Expert Rev. Anti-Infect. Ther. 2019, 17, 911–917. [Google Scholar] [CrossRef]
- Sroka, J.; Wójcik-Fatla, A.; Zwoliński, J.; Zając, V.; Sawczuk, M.; Dutkiewicz, J. Preliminary study on the occurrence of Toxoplasma gondii in Ixodes ricinus ticks from north-western Poland with the use of PCR. Ann. Agric. Environ. Med. 2008, 15, 333–338. [Google Scholar]
- Skotarczak, B.I. The role of ticks in transmission cycle of Toxoplasma gondii. Ann. Parasitol. 2016, 62, 185–191. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Cao, J.; Gong, H.; Zhou, J. Epidemiology of toxoplasmosis: Role of the tick Haemaphysalis longicornis. Infect. Dis. Poverty 2016, 5, 14. [Google Scholar] [CrossRef][Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Pinhais, P.-P.M. de Plano de Habitação e Regularização Fundiária: Diagnóstico Final, 2010. 2024. Available online: https://silo.tips/queue/prefeito-municipal-luis-goularte-alves?&queue_id=-1&v=1713631289&u=MjgwNDozODk6YzAzMjoxZWRiOmFjMDI6NjgwYzphMzk0OjdiZg== (accessed on 15 May 2024).
- Camargo, M.E. Introdução às técnicas de imunofluorescência. Rev. Bras. Patol. Clínica 1974, 10, 112. [Google Scholar]
- Camargo, M.E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev. Inst. Med. Trop. Sao Paulo 1966, 8, 227–235. [Google Scholar]
- Ministério da Saúde. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2014/prt1271_06_06_2014.html (accessed on 18 June 2024).
- Vaz, R.S.; Thomaz-Soccol, V.; Sumikawa, E.; Guimarães, A.T.B. Serological prevalence of Toxoplasma gondii antibodies in pregnant women from Southern Brazil. Parasitol. Res. 2010, 106, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Constantino, C.; Pellizzaro, M.; de Paula, E.F.E.; Vieira, T.S.W.J.; Brandão, A.P.D.; Ferreira, F.; Vieira, R.F.D.C.; Langoni, H.; Biondo, A.W. Serosurvey for Leishmania spp., Toxoplasma gondii, Trypanosoma cruzi and Neospora caninum in neighborhood dogs in Curitiba-Paraná, Brazil. Rev. Bras. Parasitol. Vet. = Braz. J. Vet. Parasitol. Orgao Col. Bras. Parasitol. Vet. 2016, 25, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Frehse, M.; Greca, H.; Ullmann, L.; Camossi, L.; Machado, J.; Langoni, H.; Biondo, A.; Molento, M. Surveillance of canine visceral leishmaniasis in a disease-free area. Rev. Bras. Parasitol. Vet. 2010, 19, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.H.F.D.B.; Lopes-Mori, F.M.R.; Mitsuka-Breganó, R.; Valentim-Zabott, M.; Freire, R.L.; Pinto, S.B.; Navarro, I.T. Soroepidemiologia da toxoplasmose em gestantes a partir da implantação do Programa de Vigilância da Toxoplasmose Adquirida e Congênita em municípios da região oeste do Paraná. Rev. Bras. Ginecol. Obs. 2012, 34, 63–68. [Google Scholar] [CrossRef]
- Benitez, A.; Gonçalves, D.; Nino, B.; Caldart, E.; Freire, R.; Navarro, I. Seroepidemiology of toxoplasmosis in humans and dogs from a small municipality in parana, Brazil. Ciência Anim. Bras. 2017, 18, e42102. [Google Scholar] [CrossRef][Green Version]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef]
- Picone, O.; Fuchs, F.; Benoist, G.; Binquet, C.; Kieffer, F.; Wallon, M.; Wehbe, K.; Mandelbrot, L.; Villena, I. Toxoplasmosis screening during pregnancy in France: Opinion of an expert panel for the CNGOF. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101814. [Google Scholar] [CrossRef]
- Nogareda, F.; Le Strat, Y.; Villena, I.; De Valk, H.; Goulet, V. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: Model-based estimation. Epidemiol. Infect. 2014, 142, 1661–1670. [Google Scholar] [CrossRef]
- Ministério da Saúde (BR). Protocolo de Notificação e Investigação: Toxoplasmose Gestacional e Congênita. 2024. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_notificacao_investigacao_toxoplasmose_gestacional_congenita.pdf (accessed on 15 May 2024).
- Pinto-Ferreira, F.; Pasquali, A.K.S.; Thomaz-Soccol, V.; Mitsuka-Breganó, R.; Caldart, E.T.; Leandro, A.D.S.; Chiyo, L.; Pozzolo, E.M.; Cubas, P.; Giordano, L.G.P.; et al. Epidemiological relevance of dogs for the prevention of Toxoplasma gondii, Neospora caninum and Leptospira spp. Rev. Bras. Parasitol. Veterinária 2019, 28, 383–394. [Google Scholar] [CrossRef]
- Freitas, A.R.; Delai, R.R.; Kmetiuk, L.B.; da Silva, E.C.; Martini, R.; Brandão, A.P.D.; Giuffrida, R.; de Barros-Filho, I.R.; Costa da Silva, R.; Langoni, H.; et al. Seropositivity of Anti-Toxoplasma gondii Antibodies in Owners and Their Dogs Living on Island and Mainland Seashore Areas of Southern Brazil. Trop. Med. Infect. Dis. 2022, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.; Filho, M.; Oliveira, S.; Oliveira, K.; Nascente, F.; Rezende, H.; Castro, A.; Avelar, J. Soroprevalência e fatores de risco para toxoplasmose em gestantes na região metropolitana de Goiânia, Goiás, Brasil. Braz. J. Health Rev. 2020, 3, 729–746. [Google Scholar] [CrossRef][Green Version]
- de Paula Dreer, M.K.; Goncalves, D.D.; da Silva Caetano, I.C.; Geronimo, E.; Menegas, P.H.; Bergo, D.; Ruiz Lopes-Mori, F.M.; Benitez, A.; de Freitas, J.C.; Evers, F.; et al. Toxoplasmosis, leptospirosis and brucellosis in stray dogs housed at the shelter in Umuarama municipality, Parana, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Caldart, E.T.; Constantino, C.; Sbruzzi Pasquali, A.K.; Benitez, A.D.N.; Hamada, F.N.; Ferreira Dias, R.C.; Rorato-Nascimento, A.M.; Marangoni Marana, E.R.; Navarro, I.T.; Freres Mascarenhas, N.M.; et al. Zoonosis in dogs and cats attended by the Birth Control Project: Toxoplasma gondii, Leishmania spp. and Leptospira spp., serodiagnosis and epidemiology. Semin. Agrar. 2015, 36, 253–265. [Google Scholar] [CrossRef][Green Version]
- da Cunha, G.R.; Pellizzaro, M.; Martins, C.M.; Rocha, S.M.; Yamakawa, A.C.; da Silva, E.C.; dos Santos, A.P.; Morikawa, V.M.; Langoni, H.; Biondo, A.W. Spatial serosurvey of anti-Toxoplasma gondii antibodies in individuals with animal hoarding disorder and their dogs in Southern Brazil. PLoS ONE 2020, 15, e0233305. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Carlos, R.; Albuquerque, G.; Bezerra, R.; Sicupira, P.; Munhoz, A.; Lopes, C. Ocurrence of anti-Toxoplasma gondii antibodies and the risk factors associated with canine infection at ilhéus-itabuna region in the state of Bahia. Rev. Bras. Med. Vet. 2010, 32, 115–121. [Google Scholar]
- Dantas, S.B.A.; da Fonseca Fernandes, A.R.; Neto, O.L.D.S.; Mota, R.A.; Alves, C.J.; de Azevedo, S.S. Ocorrência e fatores de risco associados às infecções por Toxoplasma gondii e Neospora caninum em c ães no município de Natal, Estado do Rio Grande do Norte, Nordeste do Brasil. Cienc. Rural 2013, 43, 2042–2049. [Google Scholar] [CrossRef]
- De Souza, S.L.P.; Gennari, S.M.; Yai, L.E.O.; D’Auria, S.R.N.; Cardoso, S.M.S.; Junior, J.S.G.; Dubey, J.P. Occurrence of Toxoplasma gondii antibodies in sera from dogs of the urban and rural areas from Brazil. Rev. Bras. Parasitol. Vet. 2003, 12, 1–3. [Google Scholar]
- Machado, F.P.; Kmetiuk, L.B.; Teider-Junior, P.I.; Pellizzaro, M.; Yamakawa, A.C.; Martins, C.M.; Bach, R.V.W.; Morikawa, V.M.; de Barros-Filho, I.R.; Langoni, H.; et al. Seroprevalence of anti-Toxoplasma gondii antibodies in wild boars (Sus scrofa), hunting dogs, and hunters of Brazil. PLoS ONE 2019, 14, e0223474. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Yang, Y.; Su, C. Toxoplasma gondii infections in dogs: 2009–2020. Vet. Parasitol. 2020, 287, 109223. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.G.; Cordeiro, M.D.; Batista, Y.; Alonso, J.C.; Márquez, M.; Roque, E.; Fonseca, A. Serological detection of Toxoplasma gondii in domestic dogs in the western region of Cuba. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, E.Z.; Sarba, E.J.; Tola, G.K.; Endalew, S.S.; Marami, L.M.; Melkamsew, A.T.; Lo Presti, V.D.M.; Vitale, M. Prevalence and risk factors of Toxoplasma gondii and Leishmania spp. infections in apparently healthy dogs in west Shewa zone, Oromia, Ethiopia. BMC Vet. Res. 2021, 17, 284. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Tian, Y.-M.; Li, B.-F.; Yang, J.-F.; Liu, Z.-L.; Yuan, F.-Z.; Zhu, X.-Q.; Zou, F.-C. Seroprevalence of Toxoplasma gondii infection in pet dogs in Kunming, Southwest China. Parasit. Vectors 2012, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Langoni, H.; Modolo, J.; Pezerico, S.; Silva, R.; Castro, A.; da Silva, A.; Padovani, C. Serological profile of anti-Toxoplasma gondii antibodies in apparently healthy dogs of the city of Botucatu, São Paulo State, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 142–148. [Google Scholar] [CrossRef][Green Version]
- Jabur Lot Rodrigues, N.; Manzini, S.; Koeler Fonseca Pereira, J.; Siqueira Cruz, T.; Valente Bertozzo, T.; Nunes de Moraes, G.; Francisco Abbade, J.; Langoni, H. Atualizações e padrões da toxoplasmose humana e animal: Revisão de literatura. Vet. Zootec. 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Havlik, O. Experimentálnĭ prenos toxoplasmosy klĭśtĕtem Ornithodorus moubata. Casopís Lékarů Českých 1951, 90. Available online: https://pubmed.ncbi.nlm.nih.gov/14905455/ (accessed on 15 May 2024).
- Woke, P.A.; Jacobs, L.; Jones, F.E.; Melton, M.L. Experimental results on possible arthropod transmission of toxoplasmosis. J. Parasitol. 1953, 39, 523–532. [Google Scholar] [CrossRef]
- Tanaka, T.; Maeda, H.; Galay, R.L.; Boldbattar, D.; Umemiya-Shirafuji, R.; Suzuki, H.; Xuan, X.; Tsuji, N.; Fujisaki, K. Tick longicin implicated in the arthropod transmission of Toxoplasma gondii. J. Vet. Sci. Technol. 2012, 3. [Google Scholar] [CrossRef]
- Sroka, J.; Chmielewska-Badora, J.; Dutkiewicz, J. Ixodes ricinus as a potential vector of Toxoplasma gondii. Ann. Agric. Environ. Med. 2003, 10, 121–123. [Google Scholar] [PubMed]
- Gidel, R.; Provost, A. Isolement de Toxoplasma gondii chez des ixodidés du genre Amblyomma naturellement infectés. Ann. l”Institut. Pasteur. 1965, 109, 613–616. [Google Scholar]
- Kim, J.Y.; Kwak, Y.S.; Lee, I.Y.; Yong, T.S. Molecular Detection of Toxoplasma gondii in Haemaphysalis Ticks in Korea. Korean J. Parasitol. 2020, 58, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.C.; Pasquali, A.K.; Thomaz-Soccol, V.; Pozzolo, E.M.; Chiyo, L.; Alban, S.M.; Fendrich, R.C.; Almeida, R.A.; Ferreira, F.P.; Caldart, E.T.; et al. Autochthonous canine visceral leishmaniasis cases occur in Paraná state since 2012: Isolation and identification of Leishmania infantum. Rev. Bras. Parasitol. Veterinária 2020, 29, e009819. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.G.; do Carmo Moura, L.; Cambraia, R.P. Distribuição espacial dos casos de leishmaniose visceral humana e canina na área urbana do município de Virgem da Lapa, Minas Gerais, Brasil. Rev. Cerrados 2022, 20, 347–367. [Google Scholar] [CrossRef]
- de Souza Abreu, M.; Macedo Torquato de Siqueira, J.M.; Cleves da Silva Maia, J.; Barguil Nepomuceno, D.; Barros Araújo Lopes Luz, E.; Ferreira Mendes-Sousa, A. Aspectos epidemiológicos e distribuição espacial da leishmaniose visceral em Picos, Piauí-, Brasil. Saúde Coletiva (Barueri) 2021, 11, 5846–5857. [Google Scholar] [CrossRef]
- Almeida, C.P.; Cavalcante, F.R.A.; de Oliveira Moreno, J.; Florêncio, C.M.G.D.; de Sousa Cavalcante, K.K.; Alencar, C.H. Visceral Leishmaniasis: Temporal and spatial distribution in Fortaleza, Ceará State, Brazil, 2007–2017. Epidemiol. Serv. Saude 2020, 29, e2019422. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.D.B.P.F.D.; Mendonça, A.J.; Sousa, V.R.F. Prevalence and epidemiology of visceral leishmaniasis in dogs and humans in the city Cuiaba, Mato Grosso, Brazil. Cienc. Rural 2010, 40, 1610–1615. [Google Scholar] [CrossRef]
- Maia, C.S.; Pimentel, D.S.; Santana, M.A.; Oliveira, G.M.; Pedrosa, N.A.; Nascimento, L.A.; Faustino, M.A.G.; Alves, L.C. Análise espacial da Leishmaniose Visceral Americana no município de Petrolina, Pernambuco, Brasil. Hygeia-Rev. Bras. Geogr. Médica Saúde 2014, 10, 167–176. [Google Scholar] [CrossRef]
- de Marchi, M.N.A.; Caldart, E.T.; Martins, F.D.C.; Freire, R.L. Spatial analysis of leishmaniasis in Brazil: A systematized review. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e68. [Google Scholar] [CrossRef]
- Melo, H.A.; Rossoni, D.F.; Teodoro, U. Spatial distribution of cutaneous leishmaniasis in the state of Paraná, Brazil. PLoS ONE 2017, 12, e0185401. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Garcia, F.A.; Feitosa, M.M. Diagnostic methods of canine visceral leishmaniasis. Clínica Veterinária 2006, 11, 32–38. [Google Scholar]
- Valadas, S.; Minervino, A.H.H.; Lima, V.M.F.; Soares, R.M.; Ortolani, E.L.; Gennari, S.M. Occurrence of antibodies anti-Neospora caninum, anti-Toxoplasma gondii, and anti-Leishmania chagasi in serum of dogs from Pará State, Amazon, Brazil. Parasitol. Res. 2010, 107, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.; Sousa, M.; Meireles, L.; Kesper, N.; Umezawa, E. Canine visceral leishmaniasis and Chagas disease among dogs in Araguaína, Tocantins. Rev. Bras. Parasitol. Vet. 2013, 22, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Troncarelli, M.Z.; Camargo, J.B.; Machado, J.G.; Lucheis, S.B.; Langoni, H. Leishmania spp. and/or Trypanosoma cruzi diagnosis in dogs from endemic and nonendemic areas for canine visceral leishmaniasis. Vet. Parasitol. 2009, 164, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Nogueira, F.D.S.; Menz, I.; Tabanez, P.; da Silva, S.M.; Ribeiro, V.M.; Miró, G.; Cardoso, L.; Petersen, C.; Baneth, G.; et al. Vaccination against canine leishmaniasis in Brazil. Int. J. Parasitol. 2020, 50, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.P.; Paiva, T.R.; Nogueira, L.M.V.; de Paula Souza E Guimarães, R.J.; Rodrigues, I.L.A.; André, S.R. Spatial distribution of Chagas disease and its correlation with health services. Rev. Esc. Enferm. USP 2020, 54, e03565. [Google Scholar] [CrossRef] [PubMed]
- Sousa Júnior, A.D.S.; Palácios, V.R.D.C.M.; Miranda, C.D.S.; Da Costa, R.J.F.; Catete, C.P.; Chagasteles, E.J.; Pereira, A.L.R.R.; Gonçalves, N.V. Análise espaço-temporal da doença de chagas e seus fatores de risco ambientais e demográficos no município de Barcarena, Pará, Brasil. Rev. Bras. Epidemiol. 2017, 20, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.; Silva, A.M.; Sobral-Souza, T.; Vancine, M.H.; Muylaert, R.L.; de Abreu, A.P.; Pelloso, S.M.; de Barros Carvalho, M.D.; de Andrade, L.; Ribeiro, M.C.; et al. Spatial prediction of risk areas for vector transmission of Trypanosoma cruzi in the State of Paraná, southern Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006907. [Google Scholar] [CrossRef]
- Mareze, M.; Benitez, A.D.N.; Brandão, A.P.D.; Pinto-Ferreira, F.; Miura, A.C.; Martins, F.D.C.; Caldart, E.T.; Biondo, A.W.; Freire, R.L.; Mitsuka-Breganó, R.; et al. Socioeconomic vulnerability associated to Toxoplasma gondii exposure in southern Brazil. PLoS ONE 2019, 14, e0212375. [Google Scholar] [CrossRef]
- Garcia Bahia-Oliveira, L.M.; Jones, J.L.; Azevedo-Silva, J.; Alves, C.C.F.; Oréfice, F.; Addiss, D.G. Highly endemic, waterborne toxoplasmosis in North Rio de Janeiro State, Brazil. Emerg. Infect. Dis. 2003, 9, 55. [Google Scholar] [CrossRef]
- Rosso, F.; Les, J.T.; Agudelo, A.; Villalobos, C.; Chaves, J.A.; Tunubala, G.A.; Messa, A.; Remington, J.S.; Montoya, J.G. Prevalence of infection with Toxoplasma gondii among pregnant women in Cali, Colombia, South America. Am. J. Trop. Med. Hyg. 2008, 78, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Torres-Castorena, A.; Liesenfeld, O.; García-López, C.R.; Estrada-Martínez, S.; Sifuentes-Alvarez, A.; Marsal-Hernández, J.F.; Esquivel-Cruz, R.; Sandoval-Herrera, F.; Castañeda, J.A.; et al. Seroepidemiology of Toxoplasma gondii infection in pregnant women in rural Durango, Mexico. J. Parasitol. 2009, 95, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Sroka, S.; Bartelheimer, N.; Winter, A.; Heukelbach, J.; Ariza, L.; Ribeiro, H.; Oliveira, F.A.; Queiroz, A.J.; Alencar, C., Jr.; Liesenfeld, O. Prevalence and risk factors of toxoplasmosis among pregnant women in Fortaleza, Northeastern Brazil. Am. J. Trop. Med. Hyg. 2010, 83, 528–533. [Google Scholar] [CrossRef]
- Lopes-Mori, F.M.; Mitsuka-Breganó, R.; Bittencourt, L.H.; Dias, R.C.; Gonçalves, D.D.; Capobiango, J.D.; Reiche, E.M.; Morimoto, H.K.; Freire, R.L.; Navarro, I.T. Gestational toxoplasmosis in Paraná State, Brazil: Prevalence of IgG antibodies and associated risk factors. Braz. J. Infect. Dis. 2013, 17, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Olbera, A.V.G.; Fornazari, F.; Babboni, S.D.; Rossi, R.S.; Sevá, A.P.; Latosinski, G.S.; Silva, M.; Modolo, J.R.; Langoni, H. Cumulative incidence and spatial distribution of dogs exposed to Toxoplasma gondii. Rev. Bras. Parasitol. Vet. 2020, 29, e000820. [Google Scholar] [CrossRef]
- Arruda, I.F.; Millar, P.R.; Barbosa, A.D.S.; Abboud, L.C.D.S.; dos Reis, I.C.; Moreira, A.S.D.C.; Guimarães, M.P.D.P.; Amendoeira, M.R.R. Toxoplasma gondii in domiciled dogs and cats in urban areas of Brazil: Risk factors and spatial distribution. Parasite 2021, 28, 56. [Google Scholar] [CrossRef]
- Shapiro, K. Climate and coastal habitat change: A recipe for a dirtier ocean. Mar. Pollut. Bull. 2012, 64, 1079–1080. [Google Scholar] [CrossRef]
- Liberg, O.; Sandell, M.; Pontier, D.; Natoli, E. Density spatial organisation and reproductive tactics in the domestic cat and other felids. Incollection 2000, 119–148. Available online: https://hal.science/hal-00427051/document (accessed on 15 May 2024).
- Heim, R.R. An overview of weather and climate extremes—Products and trends. Weather Clim. Extrem. 2015, 10. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Vector Control Response 2017–2030; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- de Barros, R.A.M.; Torrecilhas, A.C.; Marciano, M.A.M.; Mazuz, M.L.; Pereira-Chioccola, V.L.; Fux, B. Toxoplasmosis in Human and Animals Around the World. Diagnosis and Perspectives in the One Health Approach. Acta Trop. 2022, 231, 106432. [Google Scholar] [CrossRef] [PubMed]
- Miguel, D.C.; Brioschi, M.B.C.; Rosa, L.B.; Minori, K.; Grazzia, N. The impact of COVID-19 on neglected parasitic diseases: What to expect? Trends Parasitol. 2021, 37, 694–697. [Google Scholar] [CrossRef] [PubMed]

| Variable | Result/Titer | N | % | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Seropositivity | Seropositive | 20 | 14.8 | 9.8 | 21.8 |
| Seronegative | 115 | 85.2 | 78.2 | 90.2 | |
| RIFI | 16 | 16 | 80 | 58.4 | 91.9 |
| 64 | 3 | 15 | 5.2 | 36.0 | |
| 256 | 1 | 5 | 0.9 | 23.6 | |
| Variable | Result/Titer | N | % | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Seropositivity | Seropositive | 13 | 9.77 | 5.8 | 16.0 |
| Seronegative | 120 | 90.23 | 84.0 | 94.2 | |
| RIFI | 16 | 10 | 76.92 | 49.7 | 91.8 |
| 64 | 3 | 23.08 | 8.2 | 50.3 | |
| Variables | Mod1 | Mod2 | Mod3 | Mod4 | Mod5 | Mod6 |
|---|---|---|---|---|---|---|
| (Intercept) | 0.995 | 0.995 | 0.995 | 0.378 | 0.195 | 0.372 |
| When it rains, water accumulates inside the house: Yes | 0.115 | 0.115 | 0.118 | 0.103 | ||
| Occurred at home: Not bitten by ticks | 0.995 | 0.995 | ||||
| Occurred at home: Yes | 0.995 | 0.995 | ||||
| Occurred after visiting the forest: Not bitten by ticks | 0.999 | |||||
| Occurred after visiting the forest: Yes | 0.999 | |||||
| Time of year: Not bitten by ticks | 0.999 | 0.999 | 0.995 | |||
| Time of year: Do not know | 0.992 | 0.992 | 0.992 | |||
| Consumes raw or pasteurized milk: Does not drink milk | 0.156 | 0.156 | 0.123 | 0.052 | 0.058 | 0.058 |
| Consume raw or pasteurized milk: Pasteurized and/or UHT | 0.263 | 0.263 | 0.195 | 0.061 | 0.070 | 0.069 |
| Frequent contact with sand or earth: Yes | 0.041 | 0.041 | 0.057 | 0.071 | 0.110 |
| Variables | Mod1 | Mod2 | Mod3 | Mod4 | Mod5 | Mod6 | Mod7 | Mod8 | Mod9 | Mod10 |
|---|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | 0.305 | 0.355 | 0.728 | 0.234 | 0.136 | 0.016 | 0.005 | <0.001 | <0.001 | <0.001 |
| Breed: mixed | 0.018 | 0.025 | 0.029 | 0.034 | 0.045 | 0.044 | 0.064 | 0.041 | 0.071 | 0.097 |
| Body score | 0.724 | 0.752 | 0.917 | 0.920 | 0.960 | |||||
| Number of ticks: 1 to 5 | 0.998 | |||||||||
| Number of ticks: 6 to 10 | 0.999 | |||||||||
| Number of ticks: >10 | 0.998 | |||||||||
| Tick collection locations: both | 0.999 | 0.727 | 0.857 | 0.822 | 0.889 | 0.881 | ||||
| Tick collection locations: dog | 0.998 | 0.507 | 0.405 | 0.384 | 0.308 | 0.281 | ||||
| Tick collection locations: no tick collection | 0.415 | 0.461 | 0.462 | 0.427 | 0.594 | 0.574 | ||||
| Dog household location: backyard | 0.214 | 0.158 | 0.130 | |||||||
| Dog household location: street | 0.997 | 0.997 | 0.997 | |||||||
| Raw meat: yes | 0.072 | 0.068 | 0.101 | 0.141 | 0.119 | 0.115 | 0.120 | 0.093 | 0.093 | |
| Control ticks: yes | 0.360 | 0.389 | 0.202 | 0.233 | 0.208 | 0.206 | 0.234 | |||
| Vaccination: both | 0.142 | 0.201 | 0.880 | 0.950 | ||||||
| Vaccination: antirabies | 0.193 | 0.246 | 0.945 | 0.874 | ||||||
| Vaccination: do not know | 0.890 | 0.883 | 0.323 | 0.209 | ||||||
| Vaccination: multipurpose | 0.995 | 0.995 | 0.995 | 0.993 | ||||||
| Deworming: do not know | 0.998 | 0.998 | ||||||||
| Deworming: yes | 0.099 | 0.117 | ||||||||
| Animal hygiene: dirty | 0.144 | 0.129 | 0.225 | 0.299 | 0.144 | 0.140 | 0.106 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn-Hausner, N.; Correa, R.G.; Kmetiuk, L.B.; da Silva, E.C.; de Moraes, G.N.; Rocha, G.d.S.; Langoni, H.; Biondo, A.W. One Health Approach to Toxoplasmosis: Owner and Dog Seropositivity as Spatial Indicators of Risk Areas for Acquired, Gestational and Congenital Transmission. Trop. Med. Infect. Dis. 2024, 9, 143. https://doi.org/10.3390/tropicalmed9070143
Sohn-Hausner N, Correa RG, Kmetiuk LB, da Silva EC, de Moraes GN, Rocha GdS, Langoni H, Biondo AW. One Health Approach to Toxoplasmosis: Owner and Dog Seropositivity as Spatial Indicators of Risk Areas for Acquired, Gestational and Congenital Transmission. Tropical Medicine and Infectious Disease. 2024; 9(7):143. https://doi.org/10.3390/tropicalmed9070143
Chicago/Turabian StyleSohn-Hausner, Natacha, Ricardo Guedes Correa, Louise Bach Kmetiuk, Evelyn Cristine da Silva, Gustavo Nunes de Moraes, Gabrielle dos Santos Rocha, Helio Langoni, and Alexander Welker Biondo. 2024. "One Health Approach to Toxoplasmosis: Owner and Dog Seropositivity as Spatial Indicators of Risk Areas for Acquired, Gestational and Congenital Transmission" Tropical Medicine and Infectious Disease 9, no. 7: 143. https://doi.org/10.3390/tropicalmed9070143
APA StyleSohn-Hausner, N., Correa, R. G., Kmetiuk, L. B., da Silva, E. C., de Moraes, G. N., Rocha, G. d. S., Langoni, H., & Biondo, A. W. (2024). One Health Approach to Toxoplasmosis: Owner and Dog Seropositivity as Spatial Indicators of Risk Areas for Acquired, Gestational and Congenital Transmission. Tropical Medicine and Infectious Disease, 9(7), 143. https://doi.org/10.3390/tropicalmed9070143








