Therapeutic Modulation of Arginase with nor-NOHA Alters Immune Responses in Experimental Mouse Models of Pulmonary Tuberculosis including in the Setting of Human Immunodeficiency Virus (HIV) Co-Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Cell Lines, Human Tissues
2.2. Arginase Inhibitor
2.3. Bacteria and Virus Strains
2.4. In Vitro Macrophage Assays
2.5. Animal Infections and Treatments
2.6. Nitrite (NO) Assay
2.7. Arginase Activity Assay
2.8. Cytokine and Chemokine Quantification
2.9. Assessment of Mϕ Polarization
2.10. Histopathology and Fluorescent Immunocytochemistry
2.11. Metabolite Assessment Using LC-MS-MS
2.12. Statistical Analysis
3. Results
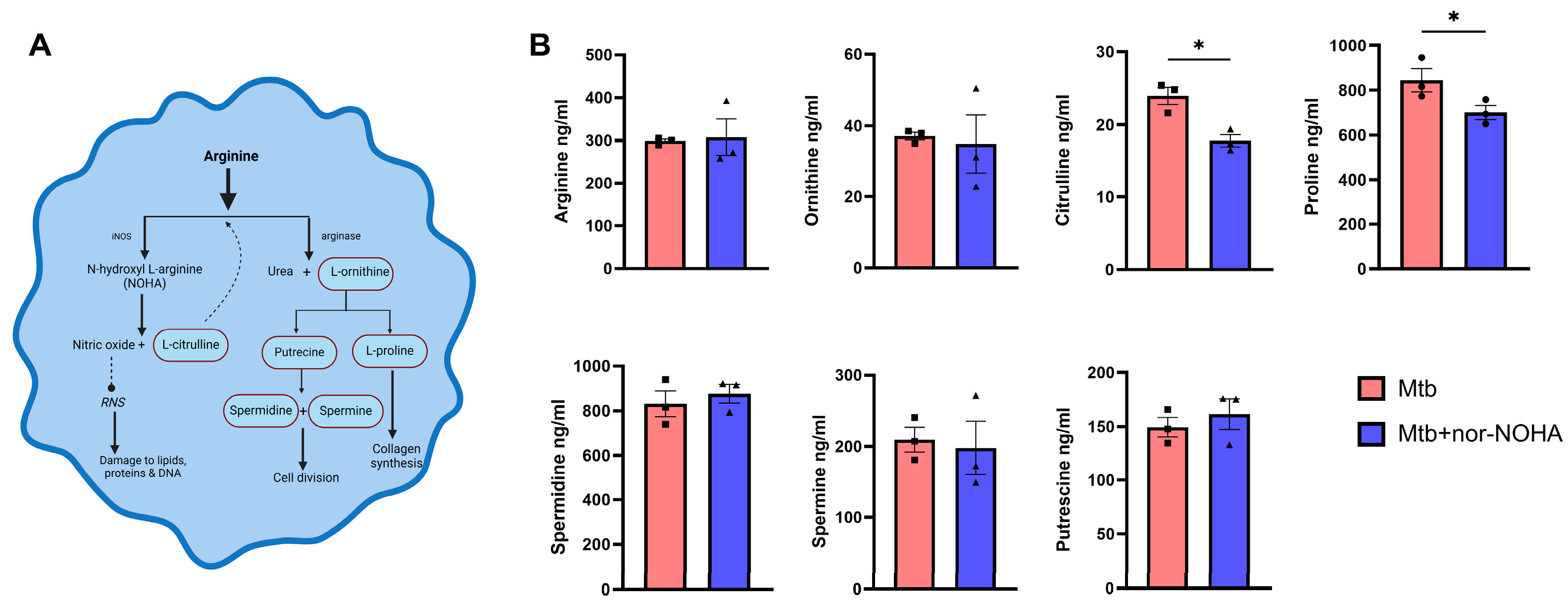
3.1. Treatment with nor-NOHA Augments M1 Mϕ Phenotype and Antibacterial Activity of RAW Cells Infected with Mtb
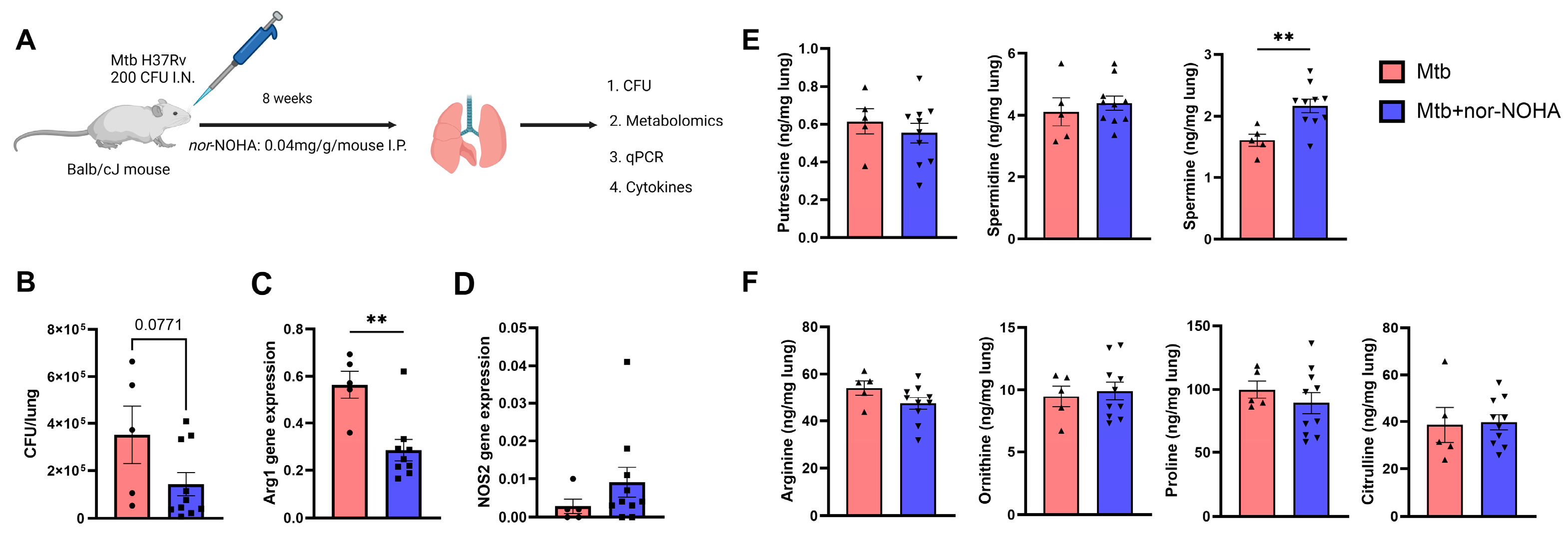
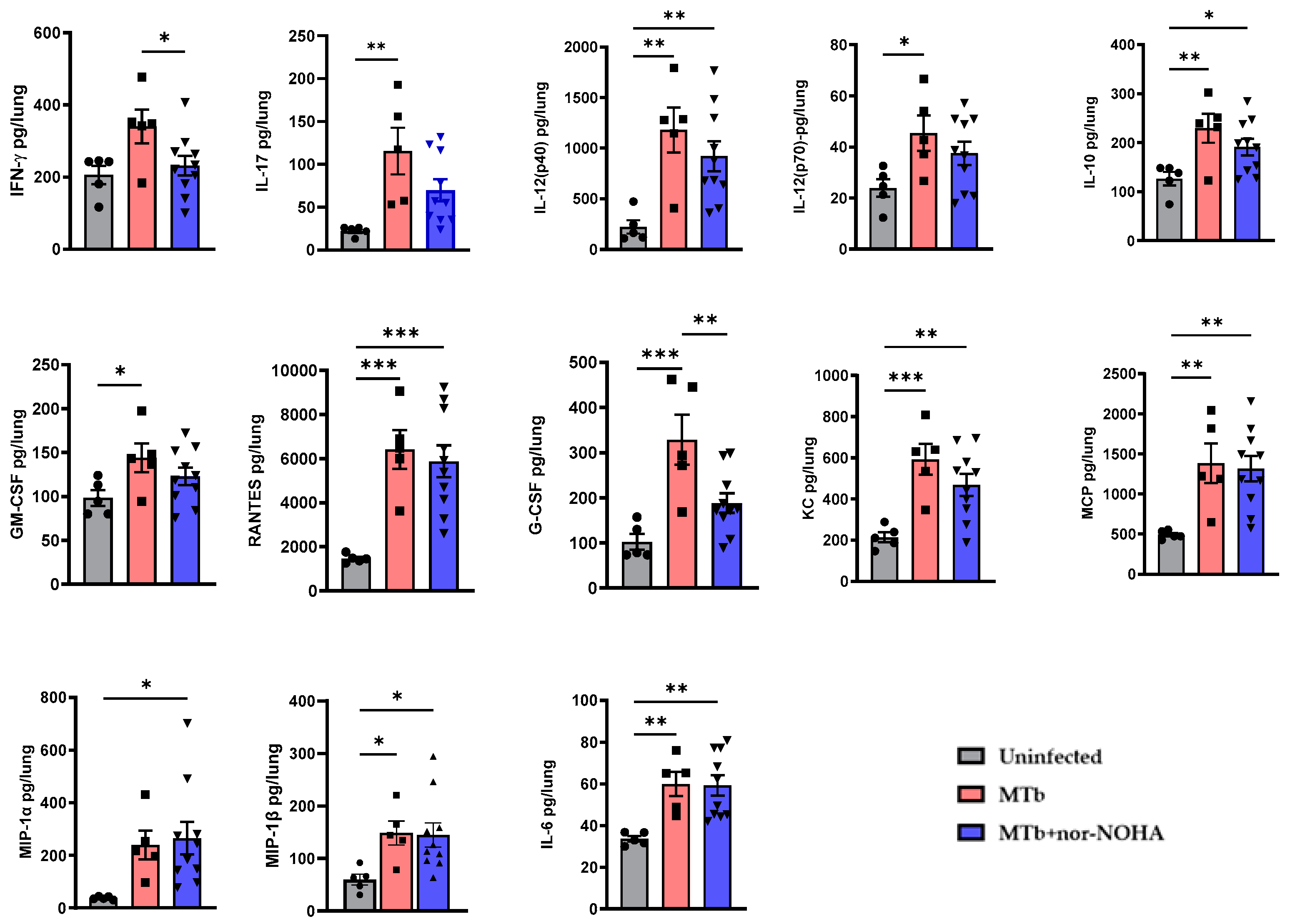
3.2. Moderate Effects of nor-NOHA in Balb/cJ Mice Infected with Mtb
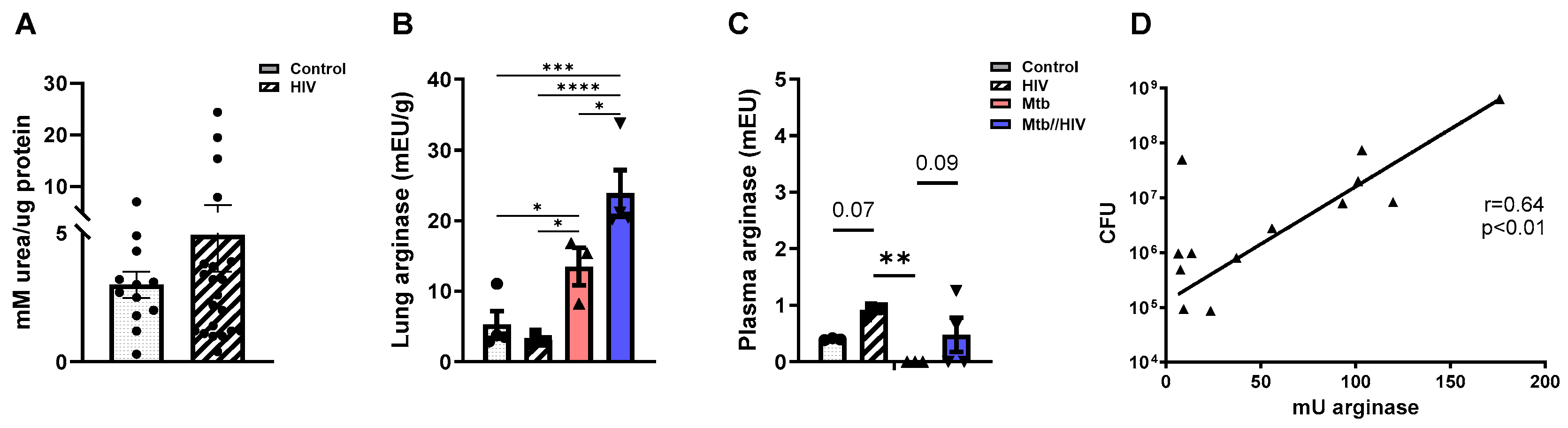
3.3. HIV Co-Infection Promotes Increased Arginase in the Mtb-Infected Lung
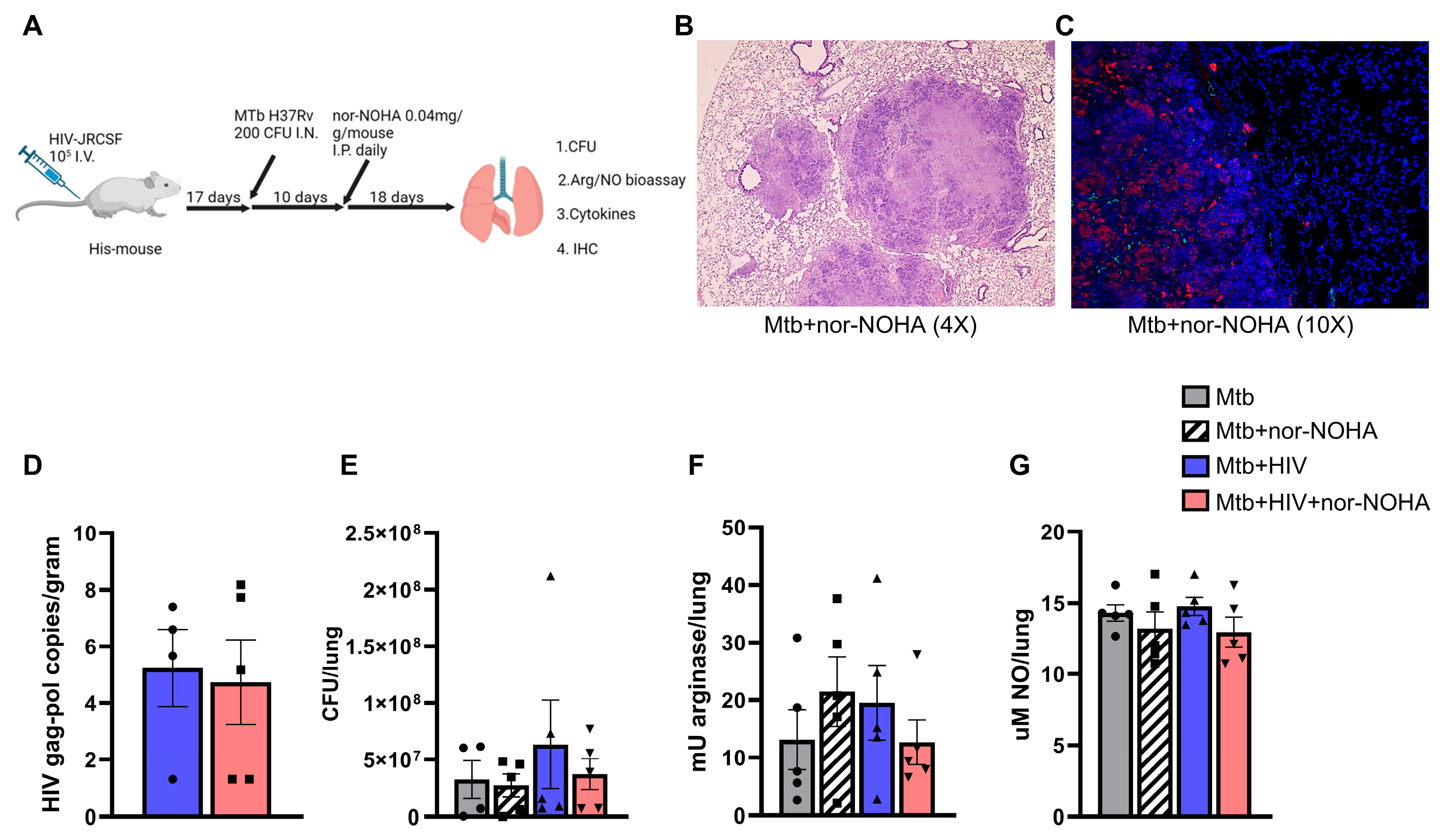
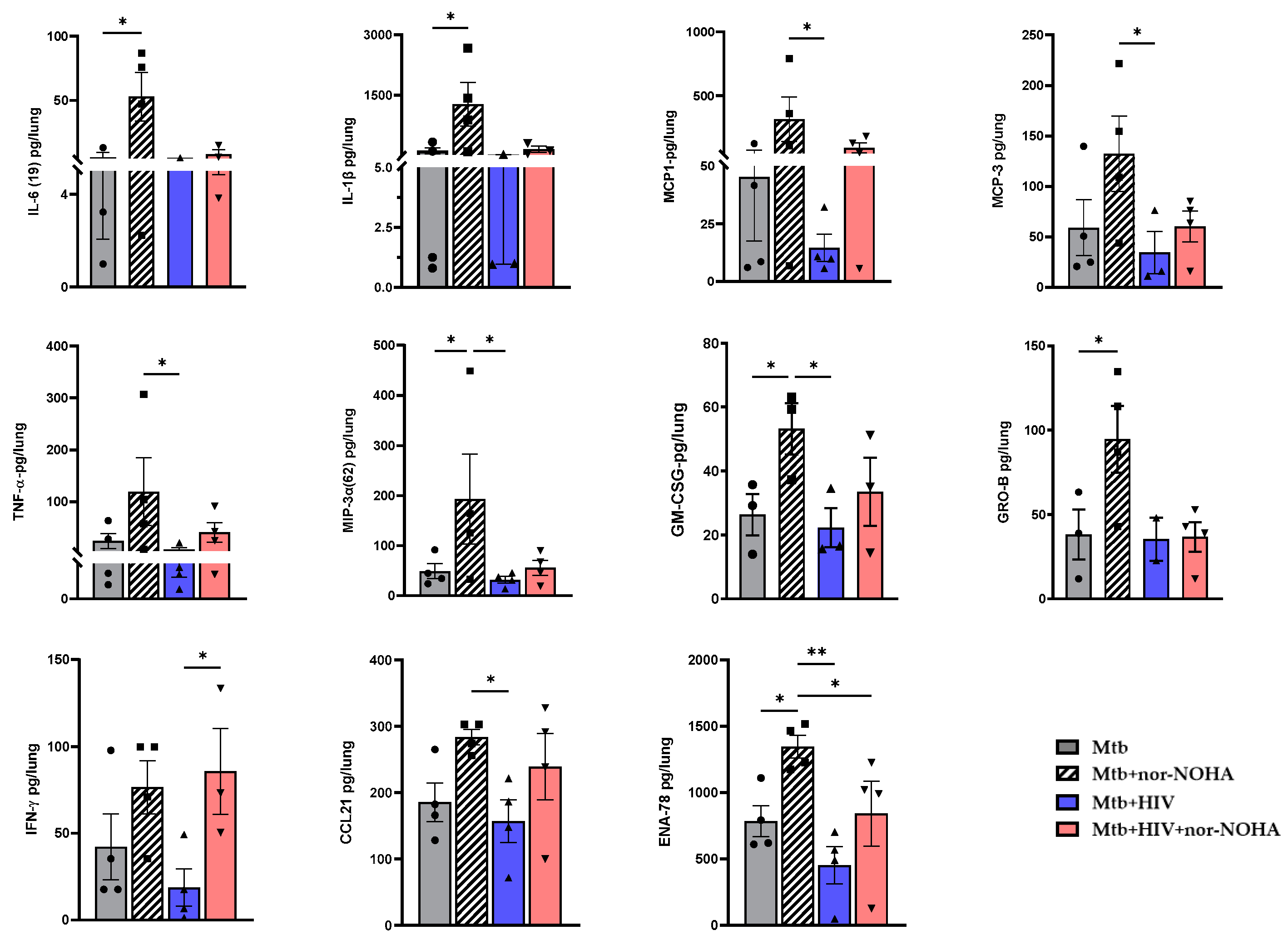
3.4. nor-NOHA Treatment Differentially Effects the Lung Cytokine Response of HIS Mice with Mtb and Mtb+ HIV in the Absence of Effects on Pathogen Burden
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022; p. 16.
- Whalen, C.; Horsburgh, C.R.; Hom, D.; Lahart, C.; Simberkoff, M.; Ellner, J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am. J. Respir. Crit. Care Med. 1995, 151, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Goletti, D.; Weissman, D.; Jackson, R.W.; Graham, N.M.; Vlahov, D.; Klein, R.S.; Munsiff, S.S.; Ortona, L.; Cauda, R.; Fauci, A.S. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 1996, 157, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Solá, E.; Rivera, C.; Mangual, M.; Martinez, J.; Rivera, K.; Fernandez, R. Diabetes mellitus: An important risk factor for reactivation of tuberculosis. Endocrinol. Diabetes Metab Case Rep. 2016, 2016, 16-0035. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Lin, H.C.; Huang, A.S.; Wei, S.H.; Lai, M.S.; Lin, H.H. Diabetes and risk of tuberculosis relapse: Nationwide nested case-control study. PLoS ONE 2014, 9, e92623. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Consolidated Guidelines on Tuberculosis: Tuberculosis Preventive Treatment: Module 1: Prevention; World Health Organization: Geneva, Switzerland, 2020.
- Sinha, P.; Ponnuraja, C.; Gupte, N.; Prakash Babu, S.; Cox, S.R.; Sarkar, S.; Mave, V.; Paradkar, M.; Cintron, C.; Govindarajan, S.; et al. Impact of Undernutrition on Tuberculosis Treatment Outcomes in India: A Multicenter, Prospective, Cohort Analysis. Clin. Infect. Dis. 2023, 76, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Xun, J.; Qi, T.; Zou, L.; Tang, Q.; Shen, Y.; Yang, J.; Xie, L.; Ji, Y.; Zhang, R.; Liu, L.; et al. Mycobacterium tuberculosis co-infection is associated with increased surrogate marker of the HIV reservoir. AIDS Res. Ther. 2020, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Luies, L.; Loots, D.T.; Williams, A.A. The metabolic consequences of HIV/TB co-infection. BMC Infect. Dis. 2023, 23, 536. [Google Scholar] [CrossRef] [PubMed]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef]
- Cronan, M.R. In the Thick of It: Formation of the Tuberculous Granuloma and Its Effects on Host and Therapeutic Responses. Front. Immunol. 2022, 13, 820134. [Google Scholar] [CrossRef]
- Bo, H.; Moure, U.A.E.; Yang, Y.; Pan, J.; Li, L.; Wang, M.; Ke, X.; Cui, H. Mycobacterium tuberculosis-macrophage interaction: Molecular updates. Front. Cell Infect. Microbiol. 2023, 13, 1062963. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994, 269, 13725–13728. [Google Scholar] [CrossRef]
- Wong, J.M.; Billiar, T.R. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv. Pharmacol. 1995, 34, 155–170. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Chakravortty, D.; Hensel, M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003, 5, 621–627. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, J.; Schluger, N.W. What is the role of nitric oxide in murine and human host defense against tuberculosis?Current knowledge. Am. J. Respir. Cell Mol. Biol. 2001, 25, 606–612. [Google Scholar] [CrossRef]
- Adams, L.B.; Dinauer, M.C.; Morgenstern, D.E.; Krahenbuhl, J.L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung Dis. 1997, 78, 237–246. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef]
- Beisiegel, M.; Kursar, M.; Koch, M.; Loddenkemper, C.; Kuhlmann, S.; Zedler, U.; Stäber, M.; Hurwitz, R.; Kaufmann, S.H. Combination of host susceptibility and virulence of Mycobacterium tuberculosis determines dual role of nitric oxide in the protection and control of inflammation. J. Infect. Dis. 2009, 199, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Scanga, C.A.; Tanaka, K.E.; Chan, J. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 1998, 160, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Tanaka, K.; Carroll, D.; Flynn, J.; Bloom, B.R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 1995, 63, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Thiriot, J.D.; Martinez-Martinez, Y.B.; Endsley, J.J.; Torres, A.G. Hacking the host: Exploitation of macrophage polarization by intracellular bacterial pathogens. Pathog. Dis. 2020, 78, ftaa009. [Google Scholar] [CrossRef] [PubMed]
- Cloke, T.E.; Garvey, L.; Choi, B.S.; Abebe, T.; Hailu, A.; Hancock, M.; Kadolsky, U.; Bangham, C.R.; Munder, M.; Müller, I.; et al. Increased level of arginase activity correlates with disease severity in HIV-seropositive patients. J. Infect. Dis. 2010, 202, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Ribeiro, S.P.; Chan, C.N.; Aid, M.; Deleage, C.; Micci, L.; Pino, M.; Cervasi, B.; Raghunathan, G.; Rimmer, E.; et al. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J. Clin. Investig. 2022, 132, e155251. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Chikatsu, D.; Inomata, E.; Oshima, T.; Kawai, G. Docking simulation of polyamines on a kissing-loop RNA dimer. Nucleic Acids Symp. Ser. 2009, 53, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Arginase Inhibitors: A Rational Approach Over One Century. Med. Res. Rev. 2017, 37, 475–513. [Google Scholar] [CrossRef]
- Kavalukas, S.L.; Uzgare, A.R.; Bivalacqua, T.J.; Barbul, A. Arginase inhibition promotes wound healing in mice. Surgery 2012, 151, 287–295. [Google Scholar] [CrossRef]
- van den Berg, M.P.M.; Kurhade, S.H.; Maarsingh, H.; Erceg, S.; Hulsbeek, I.R.; Boekema, P.H.; Kistemaker, L.E.M.; van Faassen, M.; Kema, I.P.; Elsinga, P.H.; et al. Pharmacological Screening Identifies SHK242 and SHK277 as Novel Arginase Inhibitors with Efficacy against Allergen-Induced Airway Narrowing In Vitro and In Vivo. J. Pharmacol. Exp. Ther. 2020, 374, 62–73. [Google Scholar] [CrossRef]
- Nahidi, S.; Gholami, E.; Taslimi, Y.; Habibzadeh, S.; Seyed, N.; Davarpanah, E.; Ghanadan, A.; Rafati, S.; Taheri, T. The outcome of arginase activity inhibition in BALB/c mice hosting Leishmania tropica. Parasite Immunol. 2020, 42, e12691. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Das, P.; Chakravortty, D. Arginase modulates Salmonella induced nitric oxide production in RAW264.7 macrophages and is required for Salmonella pathogenesis in mice model of infection. Microbes Infect. 2008, 10, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Lahiri, A.; Chakravortty, D. Modulation of the arginase pathway in the context of microbial pathogenesis: A metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010, 6, e1000899. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, R.J.; Calderon, V.E.; Huante, M.B.; Sutjita, P.; Vijayakumar, S.; Lancaster, K.L.; Hunter, R.L.; Actor, J.K.; Cirillo, J.D.; Aronson, J.; et al. Pulmonary Tuberculosis in Humanized Mice Infected with HIV-1. Sci. Rep. 2016, 6, 21522. [Google Scholar] [CrossRef] [PubMed]
- Huante, M.B.; Saito, T.B.; Nusbaum, R.J.; Naqvi, K.F.; Chauhan, S.; Hunter, R.L.; Actor, J.K.; Rudra, J.S.; Endsley, M.A.; Lisinicchia, J.G.; et al. Small Animal Model of Post-chemotherapy Tuberculosis Relapse in the Setting of HIV Co-infection. Front. Cell Infect. Microbiol. 2020, 10, 150. [Google Scholar] [CrossRef]
- Calderon, V.E.; Valbuena, G.; Goez, Y.; Judy, B.M.; Huante, M.B.; Sutjita, P.; Johnston, R.K.; Estes, D.M.; Hunter, R.L.; Actor, J.K.; et al. A humanized mouse model of tuberculosis. PLoS ONE 2013, 8, e63331. [Google Scholar] [CrossRef]
- Martinez-Martinez, Y.B.; Huante, M.B.; Chauhan, S.; Naqvi, K.F.; Bharaj, P.; Endsley, J.J. Helper T cell bias following tuberculosis chemotherapy identifies opportunities for therapeutic vaccination to prevent relapse. NPJ Vaccines 2023, 8, 165. [Google Scholar] [CrossRef]
- Morgello, S.; Gelman, B.B.; Kozlowski, P.B.; Vinters, H.V.; Masliah, E.; Cornford, M.; Cavert, W.; Marra, C.; Grant, I.; Singer, E.J. The National NeuroAIDS Tissue Consortium: A new paradigm in brain banking with an emphasis on infectious disease. Neuropathol. Appl. Neurobiol. 2001, 27, 326–335. [Google Scholar] [CrossRef]
- Larsen, M.H.; Biermann, K.; William, R.; Jacobs, J. Laboratory Maintenance of Mycobacterium tuberculosis. Curr. Protoc. Microbiol. 2007, 6, 10. [Google Scholar] [CrossRef]
- Corraliza, I.M.; Campo, M.L.; Soler, G.; Modolell, M. Determination of arginase activity in macrophages: A micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Cloke, T.E.; Abebe, T.; Hailu, A.; Munder, M.; Taylor, G.P.; Müller, I.; Kropf, P. Antiretroviral therapy abrogates association between arginase activity and HIV disease severity. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 746–748. [Google Scholar] [CrossRef]
- Krug, S.; Parveen, S.; Bishai, W.R. Host-Directed Therapies: Modulating Inflammation to Treat Tuberculosis. Front. Immunol. 2021, 12, 660916. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal. Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, M.U.; Nathan, C.F. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 2000, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Gotoh, T. Arginine metabolic enzymes, nitric oxide and infection. J. Nutr. 2004, 134, 2820S–2825S, discussion 2853S. [Google Scholar] [CrossRef] [PubMed]
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Bordy, R.; Quirié, A.; Marie, C.; Wendling, D.; Totoson, P.; Demougeot, C. Vascular Arginase Is a Relevant Target to Improve Cerebrovascular Endothelial Dysfunction in Rheumatoid Arthritis: Evidence from the Model of Adjuvant-Induced Arthritis. Transl. Stroke Res. 2020, 11, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Onyilagha, C.; Kuriakose, S.; Ikeogu, N.; Jia, P.; Uzonna, J. Myeloid-Derived Suppressor Cells Contribute to Susceptibility to. J. Immunol. 2018, 201, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Namouchi, A.; Gómez-Muñoz, M.; Frye, S.A.; Moen, L.V.; Rognes, T.; Tønjum, T.; Balasingham, S.V. The Mycobacterium tuberculosis transcriptional landscape under genotoxic stress. BMC Genom. 2016, 17, 791. [Google Scholar] [CrossRef]
- Botha, T.; Ryffel, B. Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology 2002, 107, 350–357. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.H.; Hsu, M.C.; Chang, R.W.; Wang, C.H.; Lee, T.S. Advances in the molecular mechanisms of statins in regulating endothelial nitric oxide bioavailability: Interlocking biology between eNOS activity and L-arginine metabolism. Biomed. Pharmacother. 2024, 171, 116192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.N.; Uversky, V.N. Biological importance of arginine: A comprehensive review of the roles in structure, disorder, and functionality of peptides and proteins. Int. J. Biol. Macromol. 2024, 257, 128646. [Google Scholar] [CrossRef]
- Chen, B.; Jin, Y.; Pool, C.M.; Liu, Y.; Nelin, L.D. Hypoxic pulmonary endothelial cells release epidermal growth factor leading to vascular smooth muscle cell arginase-2 expression and proliferation. Physiol. Rep. 2022, 10, e15342. [Google Scholar] [CrossRef] [PubMed]
- Havlínová, Z.; Hroch, M.; Nagy, A.; Sišpera, L.; Holeček, M.; Chládek, J. Single- and multiple-dose pharmacokinetics of arginase inhibitor Nω-hydroxy-nor-L-arginine, and its effect on plasma amino acids concentrations in Wistar rats. Gen. Physiol. Biophys. 2014, 33, 189–198. [Google Scholar] [CrossRef]
- Zanatta, J.M.; Acuña, S.M.; de Souza Angelo, Y.; de Almeida Bento, C.; Peron, J.P.S.; Stolf, B.S.; Muxel, S.M. Putrescine supplementation shifts macrophage L-arginine metabolism related-genes reducing Leishmania amazonensis infection. PLoS ONE 2023, 18, e0283696. [Google Scholar] [CrossRef]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide-derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef]
- Momma, T.Y.; Ottaviani, J.I. Arginase inhibitor, N-omega-hydroxy-L-norarginine, spontaneously releases biologically active NO-like molecule: Limitations for research applicatiuons. Free Radic. Biol. Med. 2020, 152, 74–82. [Google Scholar] [CrossRef]
- Sao Emani, C.; Reiling, N. Spermine enhances the activity of anti-tuberculosis drugs. Microbiol. Spectr. 2024, 12, e0356823. [Google Scholar] [CrossRef] [PubMed]
- Hey, C.; Boucher, J.L.; Vadon-Le Goff, S.; Ketterer, G.; Wessler, I.; Racké, K. Inhibition of arginase in rat and rabbit alveolar macrophages by N omega-hydroxy-D,L-indospicine, effects on L-arginine utilization by nitric oxide synthase. Br. J. Pharmacol. 1997, 121, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Myers, A.; Flynn, J.L.; Kirschner, D.E. TNF and IL-10 are major factors in modulation of the phagocytic cell environment in lung and lymph node in tuberculosis: A next-generation two-compartmental model. J. Theor. Biol. 2010, 265, 586–598. [Google Scholar] [CrossRef]
- Dorhoi, A.; Kaufmann, S.H. Perspectives on host adaptation in response to Mycobacterium tuberculosis: Modulation of inflammation. Semin. Immunol. 2014, 26, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.; Ehlers, S.; Heitmann, L.; Rausch, A.; Mages, J.; Murray, P.J.; Lang, R.; Hölscher, C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J. Immunol. 2009, 183, 1301–1312. [Google Scholar] [CrossRef]
- Dowling, J.K.; Afzal, R.; Gearing, L.J.; Cervantes-Silva, M.P.; Annett, S.; Davis, G.M.; De Santi, C.; Assmann, N.; Dettmer, K.; Gough, D.J.; et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat. Commun. 2021, 12, 1460. [Google Scholar] [CrossRef]
- Jang, S.; Uematsu, S.; Akira, S.; Salgame, P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 2004, 173, 3392–3397. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.M.; Jobe, O.; Lazarevic, V.; Vasquez, K.; Bronson, R.; Glimcher, L.H.; Kramnik, I. Increased susceptibility of mice lacking T-bet to infection with Mycobacterium tuberculosis correlates with increased IL-10 and decreased IFN-gamma production. J. Immunol. 2005, 175, 4593–4602. [Google Scholar] [CrossRef] [PubMed]
- Othieno, C.; Hirsch, C.S.; Hamilton, B.D.; Wilkinson, K.; Ellner, J.J.; Toossi, Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect. Immun. 1999, 67, 5730–5735. [Google Scholar] [CrossRef]
- Sin, Y.Y.; Ballantyne, L.L.; Mukherjee, K.; St Amand, T.; Kyriakopoulou, L.; Schulze, A.; Funk, C.D. Inducible arginase 1 deficiency in mice leads to hyperargininemia and altered amino acid metabolism. PLoS ONE 2013, 8, e80001. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Ilies, M.; Thorn, K.J.; Christianson, D.W. Inhibition of human arginase I by substrate and product analogues. Arch. Biochem. Biophys. 2010, 496, 101–108. [Google Scholar] [CrossRef]
- Havlinova, Z.; Babicova, A.; Hroch, M.; Chladek, J. Comparative pharmacokinetics of N(ω)-hydroxy-nor-L-arginine, an arginase inhibitor, after single-dose intravenous, intraperitoneal and intratracheal administration to brown Norway rats. Xenobiotica 2013, 43, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Gómez-Nieto, L.C.; Corraliza, I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 2001, 193, 777–784. [Google Scholar] [CrossRef]
- Zhu, X.; Pribis, J.P.; Rodriguez, P.C.; Morris, S.M.; Vodovotz, Y.; Billiar, T.R.; Ochoa, J.B. The central role of arginine catabolism in T-cell dysfunction and increased susceptibility to infection after physical injury. Ann. Surg. 2014, 259, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.; Schneider, H.; Luckner, C.; Giese, T.; Langhans, C.D.; Fuentes, J.M.; Kropf, P.; Mueller, I.; Kolb, A.; Modolell, M.; et al. Suppression of T-cell functions by human granulocyte arginase. Blood 2006, 108, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E.; Klein, E.; Kirschner, D.E.; et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, E.F.; Donato, M.; Keren, L.; Chen, Z.; Delmastro, A.; Fitzpatrick, M.B.; Gupta, S.; Greenwald, N.F.; Baranski, A.; Graf, W.; et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 2022, 23, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Takele, Y.; Abebe, T.; Weldegebreal, T.; Hailu, A.; Hailu, W.; Hurissa, Z.; Ali, J.; Diro, E.; Sisay, Y.; Cloke, T.; et al. Arginase activity in the blood of patients with visceral leishmaniasis and HIV infection. PLoS Negl. Trop. Dis. 2013, 7, e1977. [Google Scholar] [CrossRef][Green Version]
- Kupani, M.; Sharma, S.; Pandey, R.K.; Kumar, R.; Sundar, S.; Mehrotra, S. IL-10 and TGF-β Induced Arginase Expression Contributes to Deficient Nitric Oxide Response in Human Visceral Leishmaniasis. Front. Cell Infect. Microbiol. 2020, 10, 614165. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, S.; Nusbaum, R.J.; Huante, M.B.; Holloway, A.J.; Endsley, M.A.; Gelman, B.B.; Lisinicchia, J.G.; Endsley, J.J. Therapeutic Modulation of Arginase with nor-NOHA Alters Immune Responses in Experimental Mouse Models of Pulmonary Tuberculosis including in the Setting of Human Immunodeficiency Virus (HIV) Co-Infection. Trop. Med. Infect. Dis. 2024, 9, 129. https://doi.org/10.3390/tropicalmed9060129
Chauhan S, Nusbaum RJ, Huante MB, Holloway AJ, Endsley MA, Gelman BB, Lisinicchia JG, Endsley JJ. Therapeutic Modulation of Arginase with nor-NOHA Alters Immune Responses in Experimental Mouse Models of Pulmonary Tuberculosis including in the Setting of Human Immunodeficiency Virus (HIV) Co-Infection. Tropical Medicine and Infectious Disease. 2024; 9(6):129. https://doi.org/10.3390/tropicalmed9060129
Chicago/Turabian StyleChauhan, Sadhana, Rebecca J. Nusbaum, Matthew B. Huante, Alex J. Holloway, Mark A. Endsley, Benjamin B. Gelman, Joshua G. Lisinicchia, and Janice J. Endsley. 2024. "Therapeutic Modulation of Arginase with nor-NOHA Alters Immune Responses in Experimental Mouse Models of Pulmonary Tuberculosis including in the Setting of Human Immunodeficiency Virus (HIV) Co-Infection" Tropical Medicine and Infectious Disease 9, no. 6: 129. https://doi.org/10.3390/tropicalmed9060129
APA StyleChauhan, S., Nusbaum, R. J., Huante, M. B., Holloway, A. J., Endsley, M. A., Gelman, B. B., Lisinicchia, J. G., & Endsley, J. J. (2024). Therapeutic Modulation of Arginase with nor-NOHA Alters Immune Responses in Experimental Mouse Models of Pulmonary Tuberculosis including in the Setting of Human Immunodeficiency Virus (HIV) Co-Infection. Tropical Medicine and Infectious Disease, 9(6), 129. https://doi.org/10.3390/tropicalmed9060129




