Toxoplasma gondii in a Remote Subsistence Hunting-Based Indigenous Community of the Peruvian Amazon
Abstract
1. Introduction
2. Materials and Methods
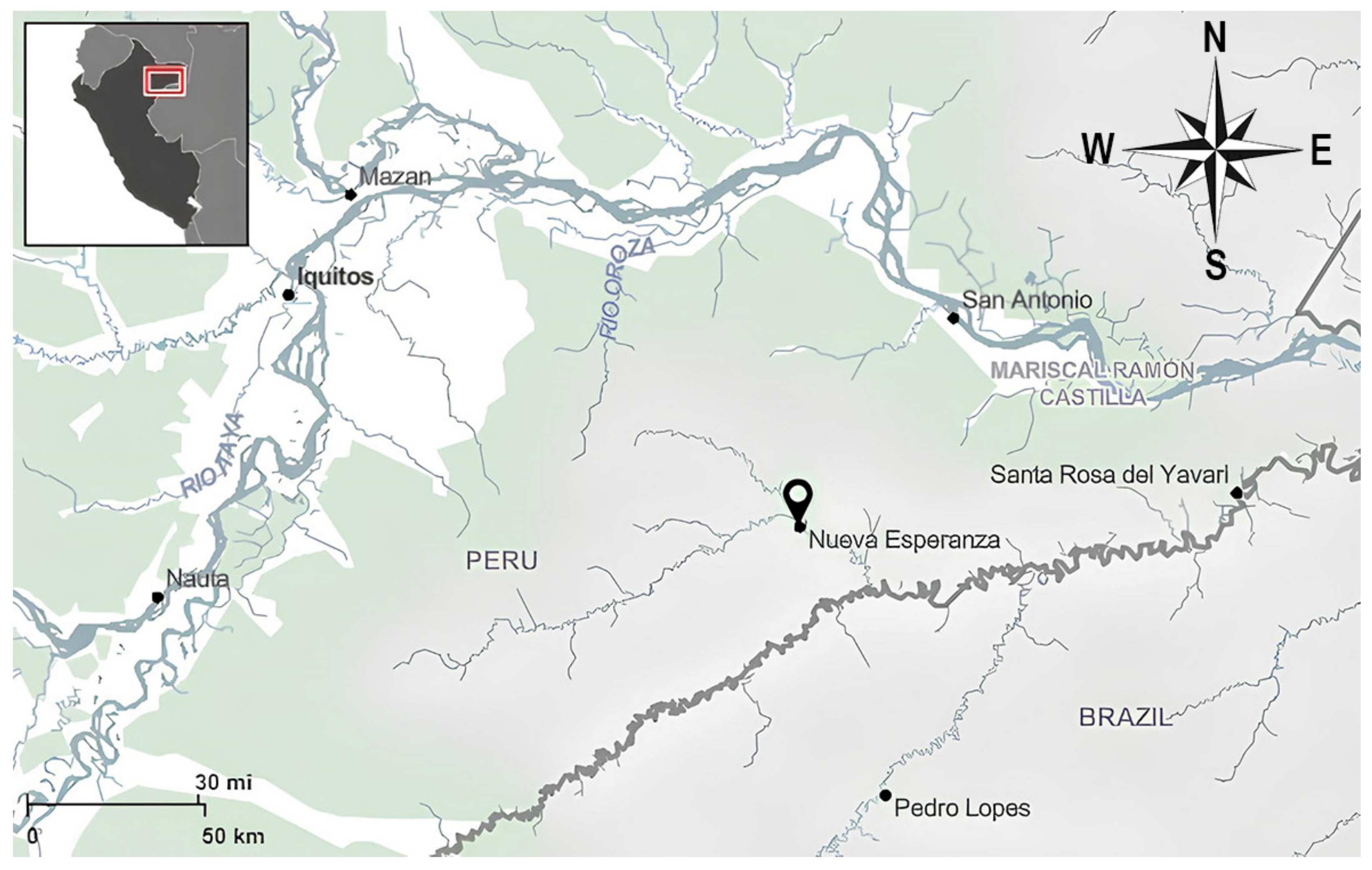
2.1. Study Area
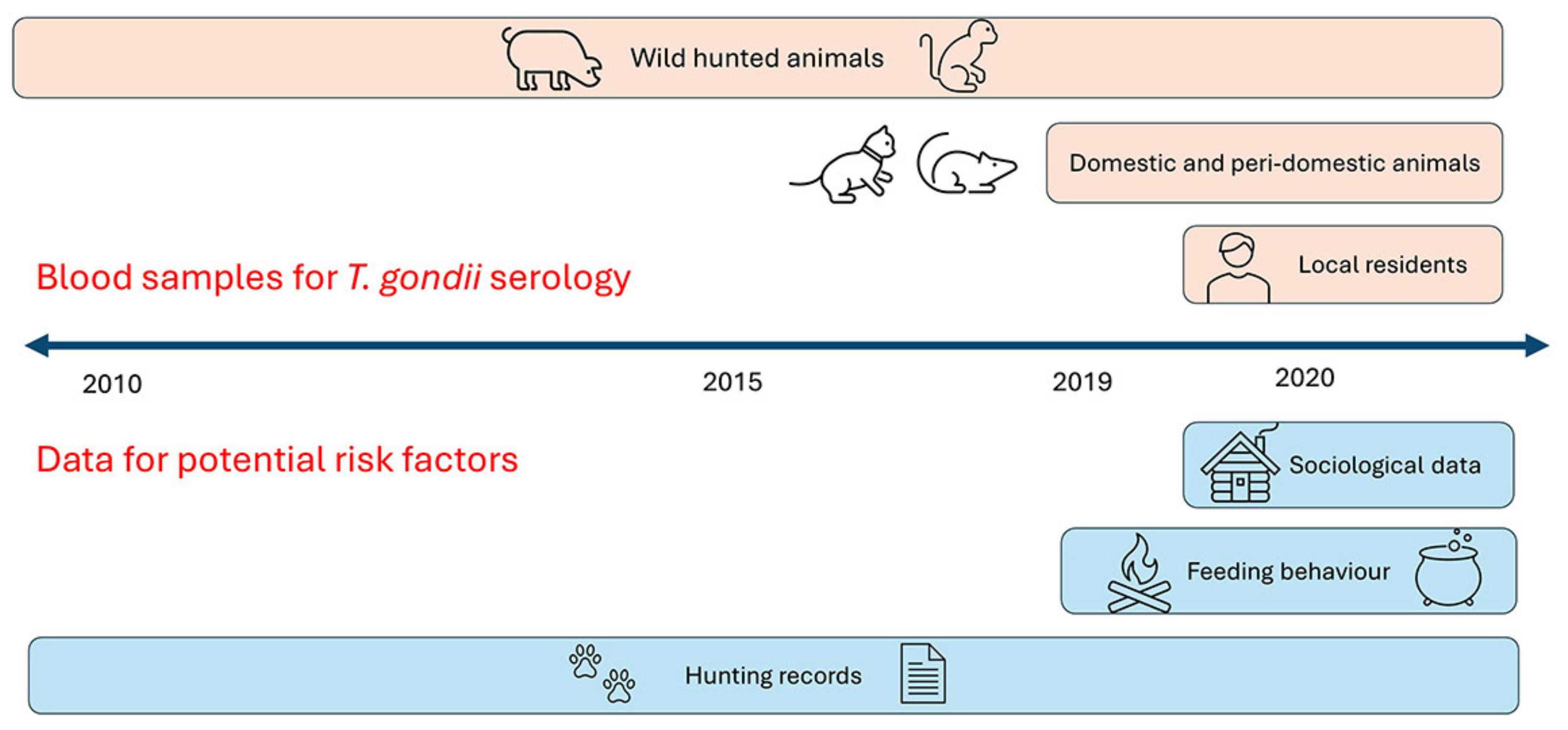
2.2. Blood Samples from Animals and Humans
2.3. Laboratory Procedures
2.4. Social Structure, Hunting and Feeding Characteristics as Potential Risk Factors
2.4.1. Sociological Data
2.4.2. Hunting Registers and Preference of Wild Meat Consumption
2.4.3. Feeding Behavior
2.5. Statistical Analyses
2.5.1. Wildlife
2.5.2. Humans
3. Results
3.1. Wildlife and Domestic/Peri-Domestic Animals
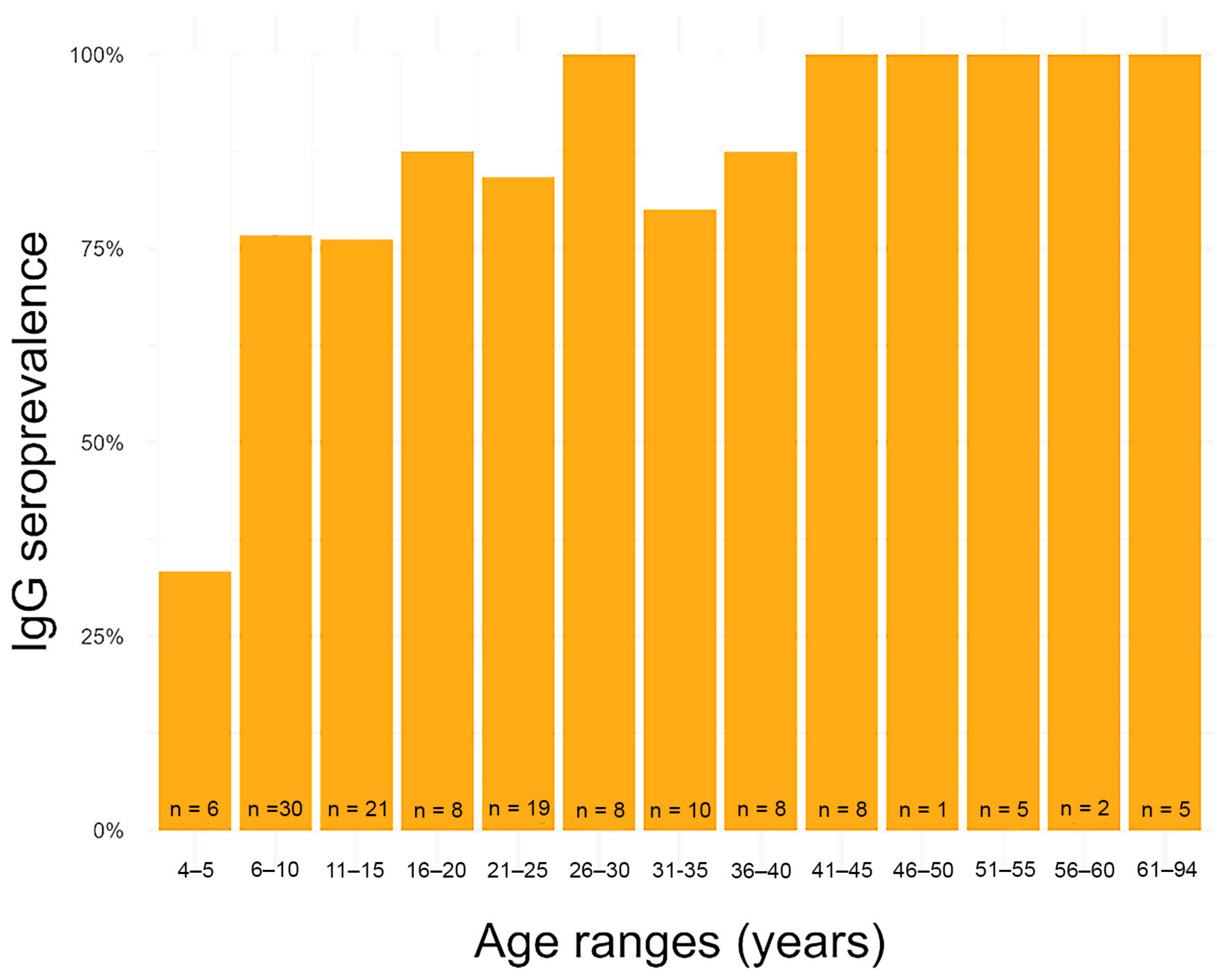
3.2. Humans
3.3. Risk Factors
3.3.1. Sociological Data
3.3.2. Hunting Records
3.3.3. Feeding Behaviors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Ratón, FL, USA, 2021. [Google Scholar]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Dubey, J.P. Biology of toxoplasmosis. In Toxoplasmosis: A Comprehensive Clinical Guide; Cambridge University Press: Cambridge, UK, 2001; pp. 1–42. [Google Scholar]
- Montoya, J.G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 2002, 185 (Suppl. S1), S73–S82. [Google Scholar] [CrossRef]
- Cook, A.J.C.; Holliman, R.; Gilbert, R.E.; Buffolano, W.; Zufferey, J.; Petersen, E.; Jenum, P.A.; Foulon, W.; Semprini, A.E.; Dunn, D.T.; et al. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study Commentary: Congenital toxoplasmosis—Further thought for food. BMJ 2000, 321, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasmosis—A Waterborne Zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.; Heckeroth, A.; Weiss, L. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Parasite zoonoses and wildlife: One Health, spillover and human activity. Int. J. Parasitol. 2013, 43, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Crozier, G.K.D.; Schulte-Hostedde, A.I. The ethical dimensions of wildlife disease management in an evolutionary context. Evol. Appl. 2014, 7, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.J.; Simon, A.; Bachand, N.; Stephen, C. Wildlife parasites in a One Health world. Trends Parasitol. 2015, 31, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Mayor, P.; El Bizri, H.R.; Morcatty, T.Q.; Moya, K.; Bendayán, N.; Solis, S.; Neto, C.F.A.V.; Kirkland, M.; Arevalo, O.; Fang, T.G.; et al. Wild meat trade over the last 45 years in the Peruvian Amazon. Conserv. Biol. 2022, 36, e13801. [Google Scholar] [CrossRef]
- Carme, B.; Demar, M.; Ajzenberg, D.; Dardé, M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 2009, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Vitaliano, S.N.; Mendonça, G.M.D.; Sandres, F.A.M.D.; Camargo, J.D.S.A.A.; Tarso, P.D.; Basano, S.D.A.; Silva, J.C.D.E.; de Souza, V.K.G.; Cartonilho, G.; da Silva de Almeida, A.T.; et al. Epidemiological aspects of Toxoplasma gondii infection in riverside communities in the Southern Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2015, 48, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaizot, R.; Nabet, C.; Laghoe, L.; Faivre, B.; Escotte-Binet, S.; Djossou, F.; Mosnier, E.; Henaff, F.; Blanchet, D.; Mercier, A.; et al. Outbreak of Amazonian toxoplasmosis: A One Health investigation in a remote Amerindian community. Front. Cell. Infect. 2020, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Aston, E.J.; Mayor, P.; Bowman, D.D.; Mohammed, H.O.; Liotta, J.L.; Kwok, O.; Dubey, J.P. Use of filter papers to determine seroprevalence of Toxoplasma gondii among hunted ungulates in remote Peruvian Amazon. Int. J. Parasitol. Parasites Wildl. 2014, 3, 15–19. [Google Scholar] [CrossRef][Green Version]
- Bodmer, R.; Puertas, P.; Fang, T. Comanaging wildlife in the Amazon and the salvation of the Pacaya-Samiria National Reserve in Peru. In Wildlife and Society: The Science of Human Dimensions; Island Press: Washington, DC, USA, 2009; pp. 104–142. [Google Scholar]
- Pitman, N.; Vriesendorp, C.; Moskovits, D. Rapid Biological Inventory 11: Peru: Yavari; The Field Museum: Chicago, IL, USA, 2003. [Google Scholar]
- Bernárdez-Rodríguez, G.B.; Bowler, M.; Braga-Pereira, F.; McNaughton, M.; Mayor, P. Conservation education promotes positive short- and medium-term changes in perceptions and attitudes towards a threatened primate species. Ethnobiol. Conserv. 2021, 10, 31. [Google Scholar] [CrossRef]
- Aysanoa, E.; Mayor, P.; Mendoza, A.P.; Zariquiey, C.M.; Morales, E.A.; Pérez, J.G.; Bowler, M.; González, C.; Ventocilla, J.A.; Baldeviano, G.C.; et al. Molecular epidemiology of Trypanosomatids and Trypanosoma cruzi in Primates from Perú. Ecohealth 2017, 14, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Nobuto, K. Toxoplasmosis in animal and laboratory diagnosis. Proc. Crop Sci. Soc. Jpn. 1963, 14, 45–46. [Google Scholar]
- Steinberg, H.E.; Bowman, N.M.; Diestra, A.; Ferradas, C.; Russo, P.; Clark, D.E.; Zhu, D.; Magni, R.; Malaga, E.; Diaz, M.; et al. Toxoplasmosis working group in Peru and Bolivia. Detection of toxoplasmic encephalitis in HIV positive patients in urine with hydrogel nanoparticles. PLoS Negl. Trop. Dis. 2021, 15, e0009199. [Google Scholar] [CrossRef]
- Joynson, D.H.M.; Guy, E.C. Laboratory diagnosis of Toxoplasma infection. In Toxoplasmosis: A Comprehensive Clinical Guide; Joynson, D.H.M., Wreghitt, T.G., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 296–318. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 15 November 2023).
- Van Vliet, N.; Muhindo, J.; Nyumu, J.; Enns, C.; Massé, F.; Bersaglio, B.; Cerutti, P.; Nasi, R. Understanding Factors that Shape Exposure to Zoonotic and Food-Borne Diseases Across Wild Meat Trade Chains. Hum. Ecol. 2022, 50, 983–995. [Google Scholar] [CrossRef]
- Furtado, M.M.; Gennari, S.M.; Ikuta, C.Y.; Jácomo, A.T.D.A.; de Morais, Z.M.; Pena, H.F.D.J.; Porfírio, G.E.d.O.; Silveira, L.; Sollmann, R.; de Souza, G.O.; et al. Serosurvey of smooth Brucella, Leptospira spp. and Toxoplasma gondii in free-ranging jaguars (Panthera onca) and domestic animals from Brazil. PLoS ONE 2015, 10, e0143816. [Google Scholar] [CrossRef]
- Carme, B.; Aznar, C.; Motard, A.; Demar, M.; De Thoisy, B. Serologic survey of Toxoplasma gondii in noncarnivorous free-ranging neotropical mammals in French Guiana. Vector Borne Zoonotic Dis. 2002, 2, 11–17. [Google Scholar] [CrossRef]
- Minervino, A.H.H.; Soares, H.S.; Barrêto-Júnior, R.A.; Neves, K.A.L.; de Jesus Pena, H.F.; Ortolani, E.L.; Dubey, J.P.; Gennari, S.M. Seroprevalence of Toxoplasma gondii antibodies in captive wild mammals and birds in Brazil. J. Zoo Wildl. Med. 2010, 41, 572–574. [Google Scholar] [CrossRef]
- de Souza Jesus, A.; Castilla Torres, R.I.; de Quadros, J.C.; Cruz, A.N.; Valsecchi, J.; El Bizri, H.R.; Mayor, P. Are larger primates less faunivorous? Consumption of arthropods by Amazonian primates does not fulfil the Jarman-Bell and Kay models. Acta Amaz. 2022, 52, 208–217. [Google Scholar] [CrossRef]
- de Souza Jesus, A.; El Bizri, H.R.; Fa, J.E.; Valsecchi, J.; Rabelo, R.M.; Mayor, P. Comparative gastrointestinal organ lengths among Amazonian primates (Primates: Platyrrhini). Am. J. Biol. Anthropol. 2023, 181, 440–453. [Google Scholar] [CrossRef]
- Blake, J.G.; Guerra, J.; Mosquera, D.; Torres, R.; Loiselle, B.A.; Romo, D. Use of mineral licks by white-bellied spider monkeys (Ateles belzebuth) and red howler monkeys (Alouatta seniculus) in eastern Ecuador. Int. J. Primatol. 2010, 31, 471–483. [Google Scholar] [CrossRef]
- Link, A.; De Luna, A.G.; Arango, R.; Diaz, M.C. Geophagy in brown spider monkeys (Ateles hybridus) in a lowland tropical rainforest in Colombia. Folia Primatol. 2011, 82, 25–32. [Google Scholar] [CrossRef]
- Johnson, C.J.; McKenzie, D.; Pedersen, J.A.; Aiken, J.M. Meat and bone meal and mineral feed additives may increase the risk of oral prion disease transmission. J. Toxicol. Environ. Health Part A 2011, 74, 161–166. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wen, Z.; Wang, Y.; Feijó, A.; Fu, Q.; Ran, J. Ecological significance and risks of mineral licks to mammals in a nature reserve on the Eastern Qinghai-Tibet Plateau. Ecosyst. Health Sustain. 2022, 8, 2052764. [Google Scholar] [CrossRef]
- Minervino, A.H.; Cassinelli, A.B.M.; de Lima, J.T.; Soares, H.S.; Malheiros, A.F.; Marcili, A.; Gennari, S.M. Prevalence of anti-Neospora caninum and anti-Toxoplasma gondii antibodies in dogs from two different indigenous communities in the Brazilian Amazon Region. J. Parasitol. 2012, 98, 1276–1278. [Google Scholar] [CrossRef]
- Cavalcante, G.T.; Aguiar, D.M.D.; Chiebao, D.; Dubey, J.P.; Ruiz, V.L.A.; Dias, R.A.; Camargo, L.M.A.; Labruna, M.B.; Gennari, S.M. Seroprevalence of Toxoplasma gondii antibodies in cats and pigs from rural Western Amazon, Brazil. J. Parasitol. 2006, 92, 863–864. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Hiramoto, R.M.; Aureliano, D.P.; da Silva-Nunes, M.; da Silva, N.S.; Malafronte, R.S.; Muniz, P.T. A community-based survey of human toxoplasmosis in rural Amazonia: Seroprevalence, seroconversion rate, and associated risk factors. Am. J. Trop. Med. Hyg. 2009, 81, 171–176. [Google Scholar] [CrossRef]
- Morais, R.d.A.P.B.; Carmo, E.L.d.; Costa, W.S.; Marinho, R.R.; Póvoa, M.M. T. gondii infection in urban and rural areas in the Amazon: Where is the risk for Toxoplasmosis? Int. J. Environ. Res. Public Health 2021, 18, 8664. [Google Scholar] [CrossRef]
- Ferraroni, J.J.; Reed, S.G.; Speer, C.A. Prevalence of Toxoplasma antibodies in humans and various animals in the Amazon. Proc. Helminthol. Soc. Wash. 1980, 47, 148–150. [Google Scholar]
- Mercier, A.; Ajzenberg, D.; Devillard, S.; Demar, M.P.; De Thoisy, B.; Bonnabau, H.; Collinet, F.; Boukhari, R.; Blanchet, D.; Simon, S.; et al. Human impact on genetic diversity of Toxoplasma gondii: Example of the anthropized environment from French Guiana. Infect. Genet. Evol. 2011, 11, 1378–1387. [Google Scholar] [CrossRef]
- Tumelty, L.; Fa, J.E.; Coad, L.; Friant, S.; Mbane, J.; Kamogne, C.T.; Tata, C.Y.; Ickowitz, A. A systematic mapping review of links between handling wild meat and zoonotic diseases. One Health 2023, 17, 100637. [Google Scholar] [CrossRef]
- Carme, B. Les parasitoses humaines en Guyane Française [Human parasitoses in French Guiana]. Presse Med. 2001, 30, 1601–1608. [Google Scholar]
- Cavalcante, G.T.; Aguiar, D.M.D.; Camargo, L.M.A.; Labruna, M.B.; De Andrade, H.F.; Meireles, L.R.; Dubey, J.P.; Thulliez, P.; Dias, R.A.; Gennari, S.M. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. J. Parasitol. 2006, 92, 647–649. [Google Scholar] [CrossRef]
| Order, Family | Species | Tested | Positive (%) | 95% CI |
|---|---|---|---|---|
| O. Carnivora | 22 | 2 (9.1%) | 2.5–27.8% | |
| Felidae | Leopardus pardalis | 1 | 0 (0.0%) | 0.0–79.4% |
| Panthera onca | 2 | 0 (0.0%) | 0.0–65.8% | |
| Procyonidae | Nasua nasua | 19 | 2 (10.5%) | 2.9–30.4% |
| O. Cingulata | 38 | 17 (44.7%) | 30.2–60.3% | |
| Dasypodidae | Dasypus novemcinctus | 38 | 17 (44.7%) | 30.2–60.3% |
| O. Primates | 155 | 39 (25.2%) | 19.0–32.5% | |
| Atelidae | Alouatta seniculus | 3 | 1 (33.3%) | 6.2–79.2% |
| Ateles chamek | 20 | 3 (15.0%) | 5.2–36.0% | |
| Lagothrix l. poeppigii | 66 | 15 (22.7%) | 14.3–34.2% | |
| Pitheciidae | Cacajao clavus | 16 | 1 (6.25%) | 1.1–28.3% |
| Plecturocebus cupreus | 4 | 1 (25.0%) | 4.6–69.9% | |
| Pithecia monachus | 6 | 0 (0.0%) | 0.0–39.0% | |
| Callitrichidae | Leontocebus fuscicolis | 1 | 0 (0.0%) | 0.0–79.4% |
| Cebidae | Cebus albiforns | 7 | 3 (42.9%) | 15.8–75.0% |
| Sapajus macrocephalus | 32 | 15 (46.9%) | 30.9–63.6% | |
| O. Rodentia | 148 | 59 (39.9%) | 32.3–47.9% | |
| Cuniculidae | Cuniculus paca | 139 | 57 (41.0%) | 33.2–49.3% |
| Dasyproctidae | Dasyprocta fuliginosa | 6 | 1 (16.7%) | 3.0–56.4% |
| Caviidae | Galea musteloides | 1 | 0 (0.0%) | 0.0–79.4% |
| Hydrochoerus hydrochaeris | 1 | 1 (100%) | 20.7–100% | |
| Sciuridae | Sciurus igniventris | 1 | 0 (0.0%) | 0.0–79.4% |
| O. Cetartiodactyla | 171 | 48 (28.1%) | 21.9–35.2% | |
| Cervidae | Mazama americana | 51 | 13 (25.5%) | 15.6–38.9% |
| Mazama nemorivaga | 1 | 1 (100%) | 20.7–100% | |
| Tayassuidae | Pecari tajacu | 65 | 18 (27.7%) | 18.3–39.6% |
| Tayassu pecari | 54 | 16 (29.6%) | 19.1–42.8% | |
| O. Perissodactyla | 21 | 4 (19.1%) | 7.7–40.0% | |
| Tapiridae | Tapirus terrestris | 21 | 4 (19.1%) | 7.7–40.0% |
| Total | 555 | 169 (30.45%) | 26.8–34.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menajovsky, M.F.; Espunyes, J.; Ulloa, G.; Calderon, M.; Diestra, A.; Malaga, E.; Muñoz, C.; Montero, S.; Lescano, A.G.; Santolalla, M.L.; et al. Toxoplasma gondii in a Remote Subsistence Hunting-Based Indigenous Community of the Peruvian Amazon. Trop. Med. Infect. Dis. 2024, 9, 98. https://doi.org/10.3390/tropicalmed9050098
Menajovsky MF, Espunyes J, Ulloa G, Calderon M, Diestra A, Malaga E, Muñoz C, Montero S, Lescano AG, Santolalla ML, et al. Toxoplasma gondii in a Remote Subsistence Hunting-Based Indigenous Community of the Peruvian Amazon. Tropical Medicine and Infectious Disease. 2024; 9(5):98. https://doi.org/10.3390/tropicalmed9050098
Chicago/Turabian StyleMenajovsky, María Fernanda, Johan Espunyes, Gabriela Ulloa, Maritza Calderon, Andrea Diestra, Edith Malaga, Carmen Muñoz, Stephanie Montero, Andres G. Lescano, Meddly L. Santolalla, and et al. 2024. "Toxoplasma gondii in a Remote Subsistence Hunting-Based Indigenous Community of the Peruvian Amazon" Tropical Medicine and Infectious Disease 9, no. 5: 98. https://doi.org/10.3390/tropicalmed9050098
APA StyleMenajovsky, M. F., Espunyes, J., Ulloa, G., Calderon, M., Diestra, A., Malaga, E., Muñoz, C., Montero, S., Lescano, A. G., Santolalla, M. L., Cabezón, O., & Mayor, P. (2024). Toxoplasma gondii in a Remote Subsistence Hunting-Based Indigenous Community of the Peruvian Amazon. Tropical Medicine and Infectious Disease, 9(5), 98. https://doi.org/10.3390/tropicalmed9050098






