Finding Priority Areas in the Evaluation of Strategies for the Prevention of Leishmaniasis in an Endemic Municipality of Brazil
Abstract
1. Introduction
2. Materials and Methods
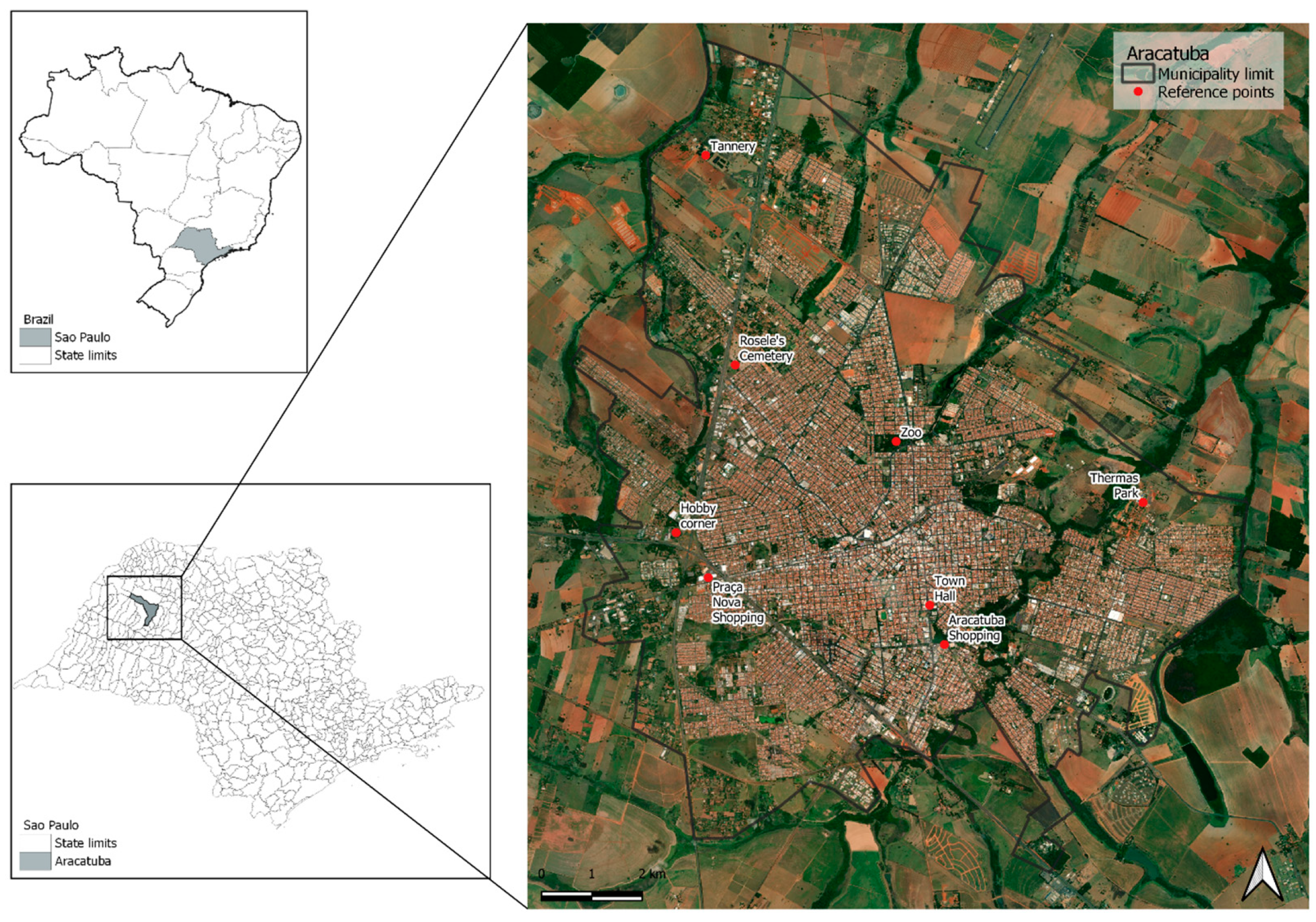
2.1. Study Area
2.2. Data of Tested Dogs
2.3. Data Analysis
Descriptive Analysis
2.4. Analyses of Comparisons
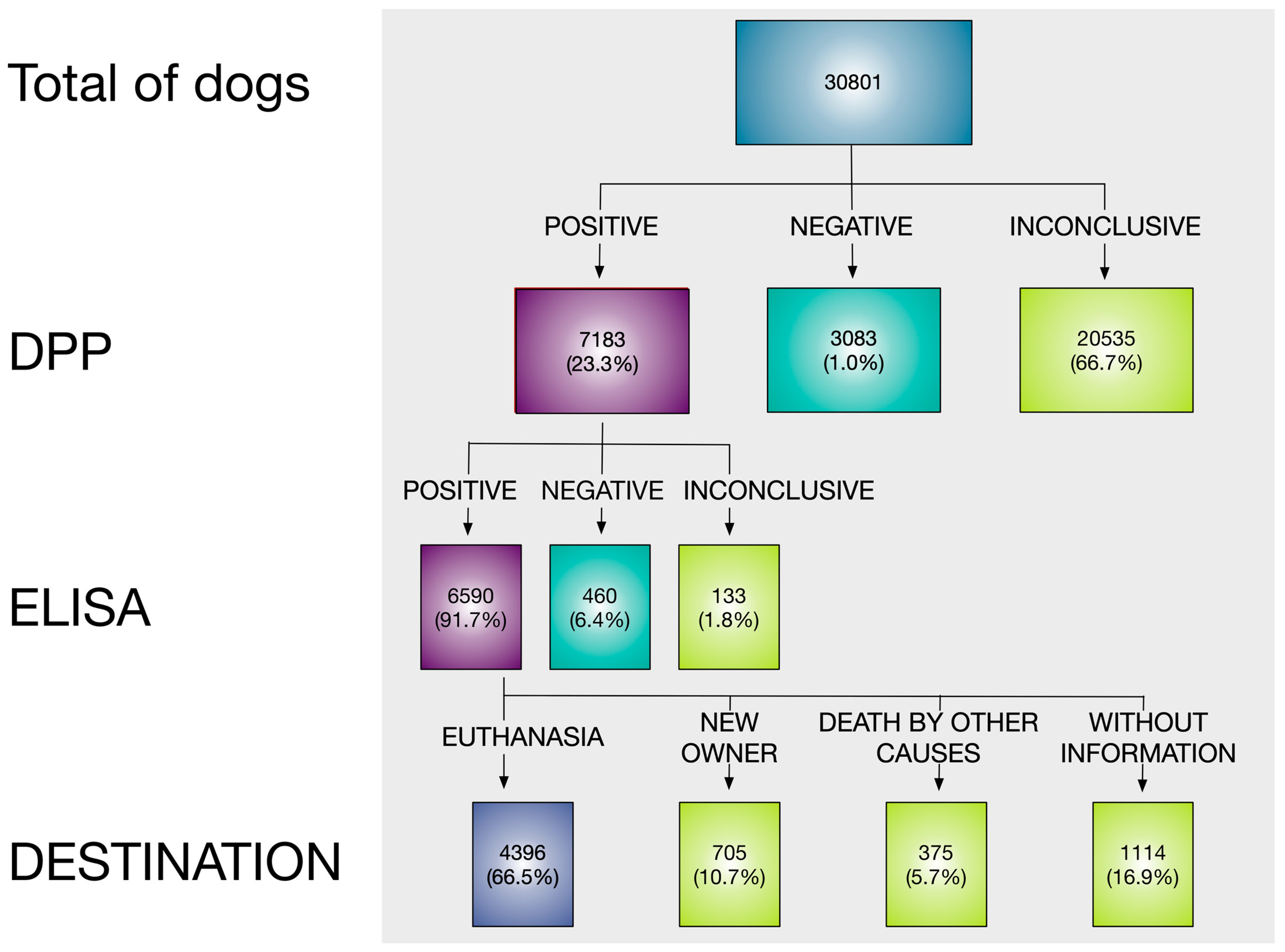
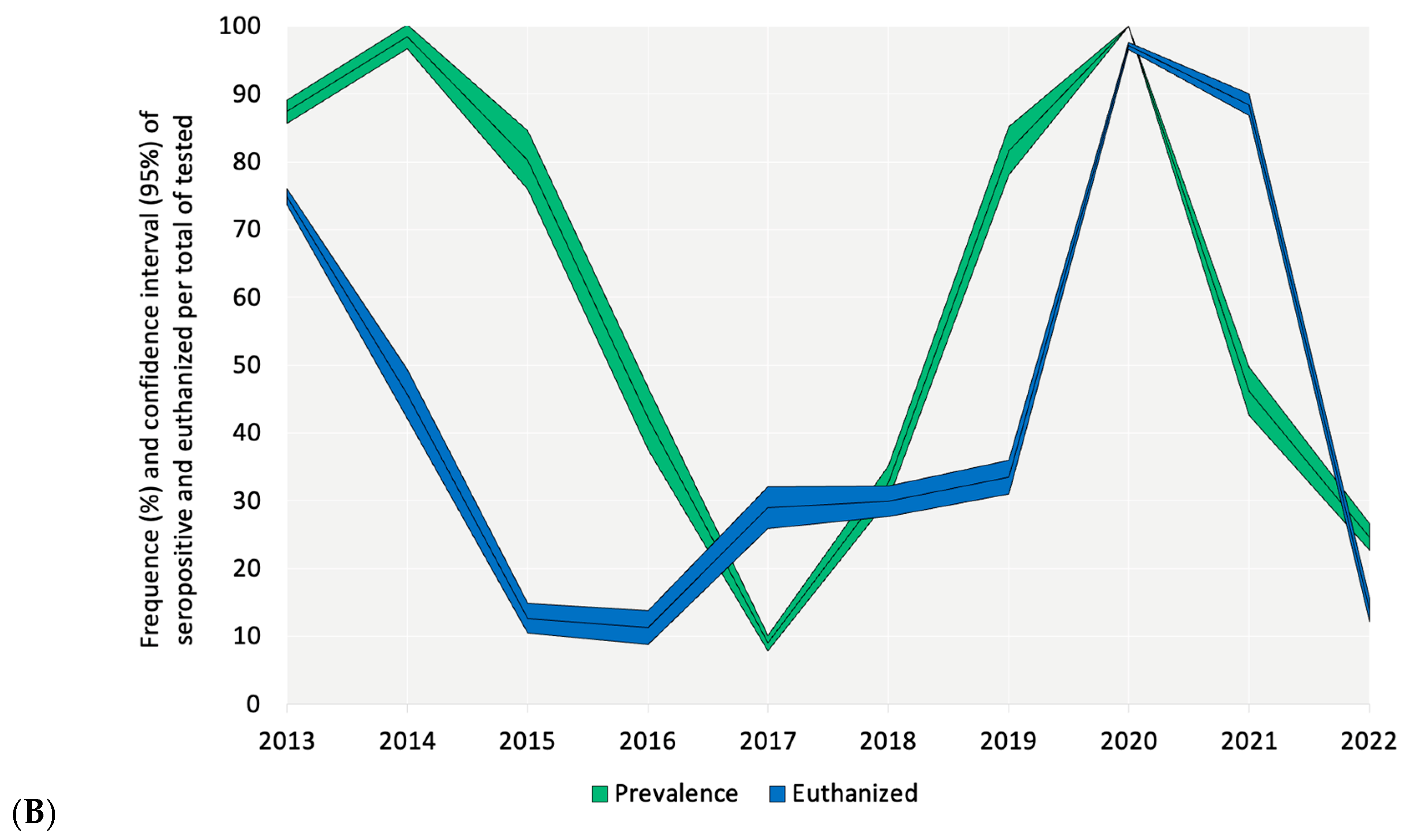
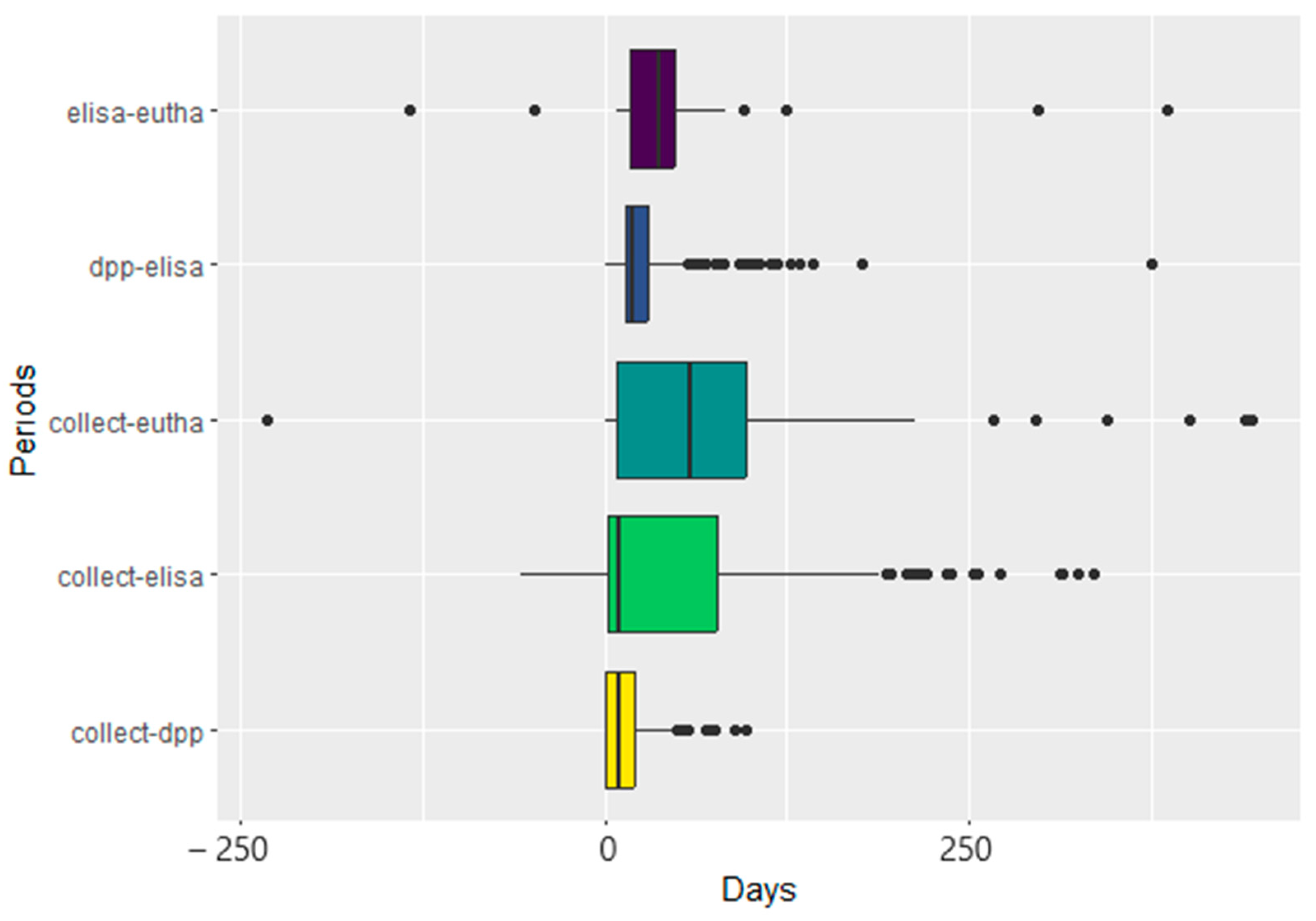
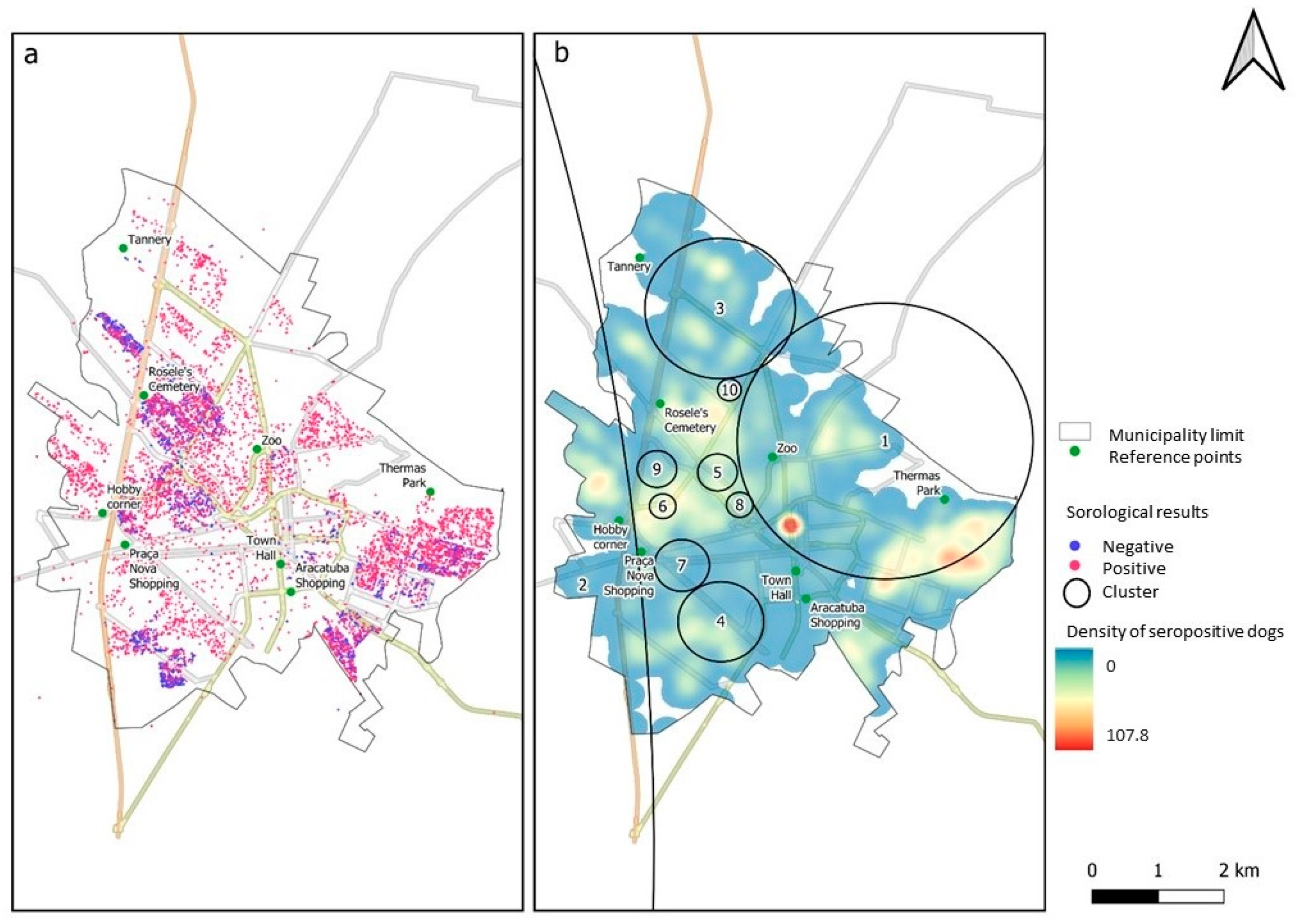
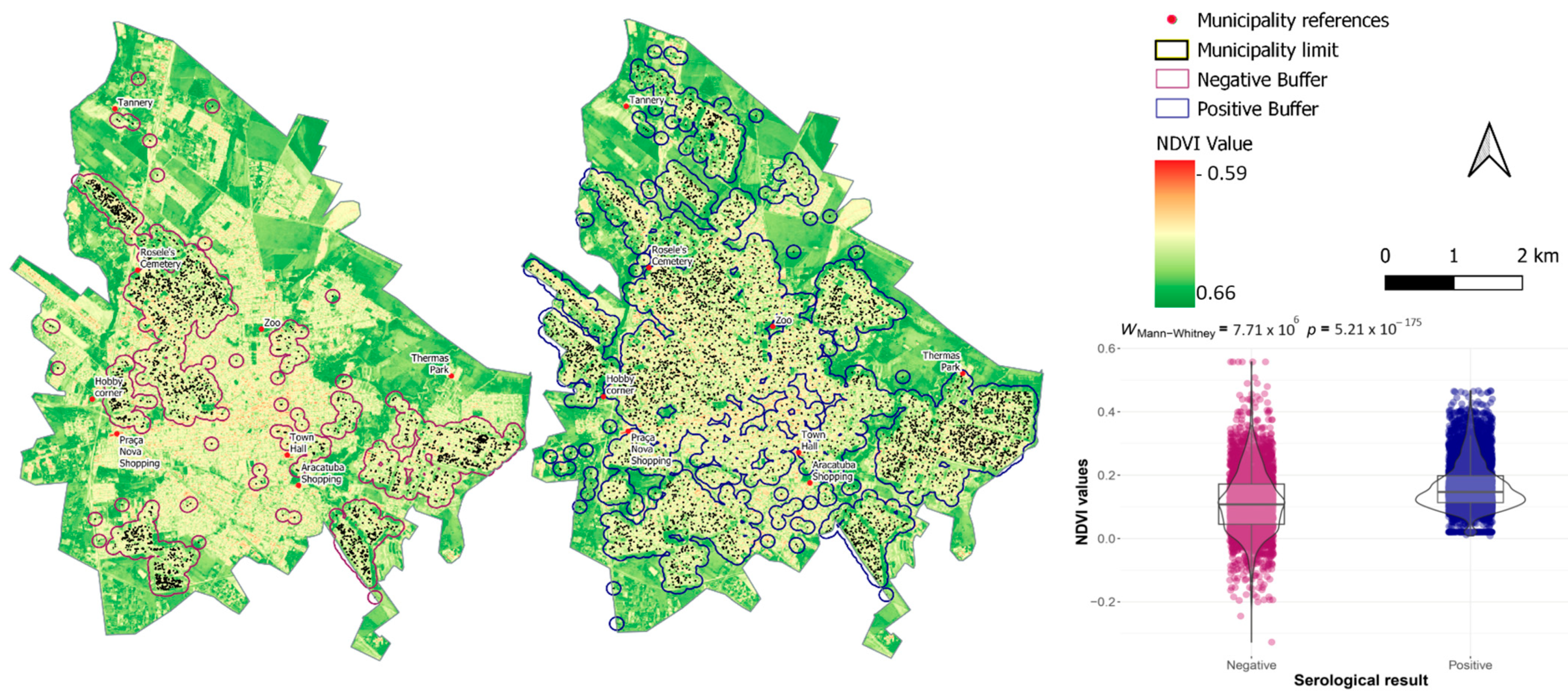
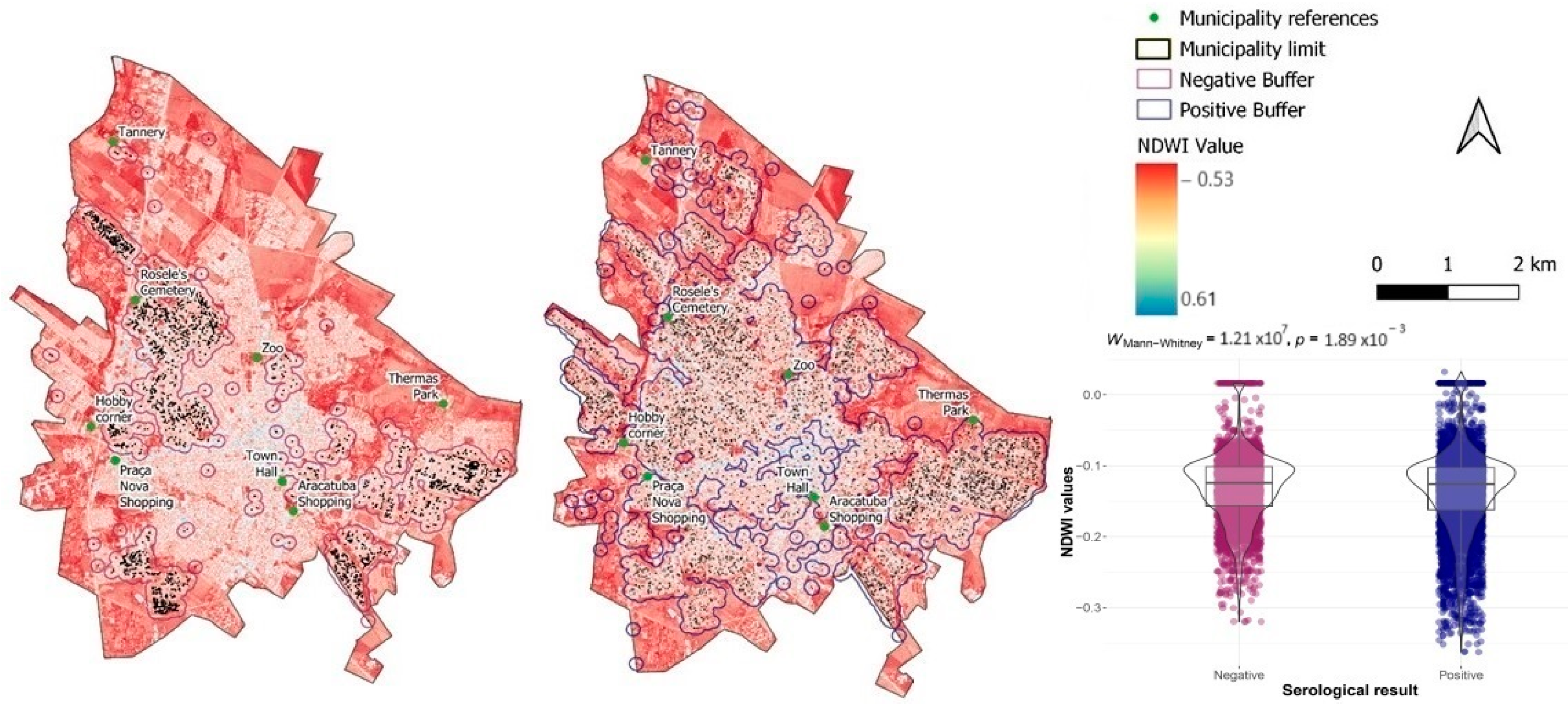
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins-Melo, F.R.; Lima, M.D.S.; Ramos, A.N.; Alencar, C.H.; Heukelbach, J. Mortality and case fatality due to visceral leishmaniasis in Brazil: A nationwide analysis of epidemiology, trends and spatial patterns. PLoS ONE 2014, 9, e93770. [Google Scholar] [CrossRef]
- Brasília. Guia de Vigilância em Saúde; Ministério da Saúde: Brasília, Brasil, 2016; p. 773. [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.T.P.; Marques, L.D.F.V.; Lamounier, K.C.C.; Castro, J.M.; Cabrera, G.P.B. Leishmaniose visceral humana: Reflexões éticas e jurídicas acerca do controle do reservatório canino no Brasil. Rev. Bioética Derecho Perspect. Bioét. 2017, 39, 135–151. [Google Scholar] [CrossRef]
- da Saude, M.; de V em Saude, S. Estratificação de Risco da Leishmaniose Visceral por Município de Infecção. Brasil, 2020 a 2022. 2022. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leishmaniose-visceral/arquivos/estratificacaolv_20a22.pdf (accessed on 3 February 2023).
- Camargo-Neves, V.L.F.D.; Katz, G.; Rodas, L.A.C.; Poletto, D.W.; Lage, L.C.; Spínola, R.M.F.; Cruz, O.G. Utilização de ferramentas de análise espacial na vigilância epidemiológica de leishmaniose visceral americana—Araçatuba, São Paulo, Use of spatial analysis tools in the epidemiological surveillance of American visceral leishmaniasis. Cad. Saúde Pública 2001, 17, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-torres, F. The prevention of canine leishmaniasis and its impact on public health. Trends Parasitol. 2013, 29, 339–345. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.; Assunção, R.M.; Reis, I.A.; Proietti, F.A. Spatial distribution of human and canine visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994–1997. Cad. Saúde Pública 2001, 17, 1231–1239. [Google Scholar] [CrossRef]
- da Paixão Sevá, A.; Mao, L.; Galvis-Ovallos, F.; Lima, J.M.T.; Valle, D. Risk analysis and prediction of visceral leishmaniasis dispersion in Sao Paulo State, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005353. [Google Scholar] [CrossRef]
- Maroli, M.; Rossi, L.; Baldelli, R.; Capelli, G.; Ferroglio, E.; Genchi, C.; Gramiccia, M.; Mortarino, M.; Pietrobelli, M.; Gradoni, L. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop. Med. Int. Health 2008, 13, 256–264. [Google Scholar] [CrossRef]
- Feliciangeli, M.D.; Delgado, O.; Suarez, B.; Bravo, A. Leishmania and sand flies: Proximity to woodland as a risk factor for infection in a rural focus of visceral leishmaniasis in west central Venezuela. Trop. Med. Int. Health 2006, 11, 1785–1791. [Google Scholar] [CrossRef]
- Chaniotis, B.N.; Neely, J.M.; Correa, M.A.; Tesh, R.B.; Johnson, K.M. Natural population dynamics of phlebotomine sandflies in Panama. J. Med. Entomol. 1971, 8, 339–352. [Google Scholar] [CrossRef]
- de Toledo, C.R.S.; de Almeida, A.S.; de Miranda Chaves, S.A.; Sabroza, P.C.; Toledo, L.M.; Caldas, J.P. Vulnerability to the transmission of human visceral leishmaniasis in a Brazilian urban area. Rev. Saude Publica 2017, 51, 49. [Google Scholar] [CrossRef][Green Version]
- OPAS. Plano de Ação Para a Eliminação de Doenças Infecciosas Negligenciadas e Ações Pós-Eliminação 2016–2022. 55° Cons Dir—68a SESSÃO DO Com Reg DA OMS PARA AS Américas—Washington, DC, USA, 26–30 Setembro 2016. pp. 1–6. Available online: https://www.paho.org/pt/documentos/cd55r9-plano-acao-para-eliminacao-doencas-infecciosas-negligenciadas-e-acoes-pos (accessed on 3 February 2023).
- PAHO/WHO. Plan of Action to Strengthen the Surveillance and Control of Leishmaniasis in the Americas 2017–2022; PAHO/WHO: Washington, DC, USA, 2017. [Google Scholar]
- Brasil. MDSSDVESDDVE. In Manual de Vigilância e Controle Da Leishmaniose Visceral; Ministério da Saúde: Brasília, Brasil, 2006. [Google Scholar] [CrossRef]
- Ministério da Saúde. Leishmaniose Visceral. In Gráfico e Mapas dos Casos de Leishmaniose Visceral; Ministério da Saúde: Brasília, Brazil, 2022. [Google Scholar]
- Carneiro, L.A.; Lima, L.V.; Campos, M.B.; Santos, T.V.D.; Ramos, P.K.; Laurenti, M.D.; Silveira, F.T. Prevalence and incidence of canine visceral leishmaniasis and its clinical–immunological features in an endemic area of the Brazilian Amazon. Vet. Med. Sci. 2023, 9, 2463–2474. [Google Scholar] [CrossRef]
- de Souza Pimentel, D.; Ramos, R.A.N.; de Andrade Santana, M.; Maia, C.S.; de Carvalho, G.A.; da Silva, H.P.; Alves, L.C. Prevalence of zoonotic visceral leishmaniasis in dogs in an endemic area of Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 491–493. [Google Scholar] [CrossRef][Green Version]
- das Graças da Silva Bernardino, M.; Angelo, D.F.D.S.; Silva, R.B.S.; da Silva, E.G.; e Silva, L.F.F.; Vaz, A.F.D.M.; de Melo, M.A.; de Sousa Américo Batista Santos, C.; Alves, C.J.; de Azevedo, S.S. High seroprevalence and associated factors for visceral leishmaniasis in dogs in a transmission area of Paraíba state, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e016919. [Google Scholar] [CrossRef]
- Silva, S.S.; de Macedo, L.O.; de Oliveira, J.C.P.; Alves, L.C.; de Carvalho, G.A.; Ramos, R.A.N. Canine visceral leishmaniasis: Risk factors and spatial analysis in an endemic area of Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2023, 32, e003223. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, A.T.; Silva, C.F.D.C.; Rocha, T.B.; Coutinho, D.J.B.; da Costa, A.P.; Costa, F.B.; Souza, F.A.; de Maria Seabra Nogueira, R. Exposure to and infection by Leishmania infantum among domestic dogs in an area of the Cerrado biome, Maranhão, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2023, 39, 100851. [Google Scholar] [CrossRef]
- Evaristo, A.M.d.C.F.; Sevá, A.d.P.; de Oliveira, G.M.B.; da Silva, I.W.G.; Ferreira, M.S.; de Souza, E.A.R.; Silva, J.A.M.; Azevedo, S.S.; Horta, M.C. Canine leishmaniasis in the semi-arid region of pernambuco, northeastern Brazil: Epidemiology, factors associated with seropositivity and spatial analysis. Rev. Bras. Parasitol. Vet. 2020, 29, e001120. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; Benitez, A.D.N.; da Paixão Sevá, A.; Okamura, L.H.; Galvão, A.B.; Gomes, J.F.; Bresciani, K.D.S.; Cardoso, T.C. Spatial and seroepidemiology of canine visceral leishmaniasis in an endemic southeast Brazilian area. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190525. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Meneguetti, D.U.; de Oliveira, J.; Camargo, L.M.A. Leishmanioses No Brasil: Aspectos Epidemiológicos, Desafios e Perspectivas. In Atualidades em Medicina Tropical no Brasil: Protozoários; Stricto Sensu: Rio Branco, Acre, Brasil, 2020. [Google Scholar] [CrossRef]
- da Paixão Sevá, A.; Ferreira, F.; Amaku, M. How much does it cost to prevent and control visceral leishmaniasis in Brazil? Comparing different measures in dogs. PLoS ONE 2020, 15, e0236127. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.B.; Figueiredo, F.B.; Rocha, M.F.; Castro, M.C.; Werneck, G.L. Effectiveness of insecticide-impregnated collars for the control of canine visceral leishmaniasis. Prev. Vet. Med. 2020, 182, 105104. [Google Scholar] [CrossRef] [PubMed]
- BRASIL M da SS de V em S. Nota Técnica No 5/2021-CGZV/DEIDT/SVS/MS. 2021; pp. 1–6. Available online: https://www.gov.br/saude/pt-br/media/pdf/2021/maio/27/sei_ms-nota-tecnica-n-5_leishpdf.pdf (accessed on 3 February 2023).
- DATASUS. Tutorial de Navegação. 2023. Available online: https://datasus.saude.gov.br/wp-content/uploads/2020/05/Tutorial-de-Navega%C3%A7%C3%A3o-e-SUS-VE_atualizado-15-05.pdf (accessed on 3 February 2023).
- de Arruda, M.M.; Figueiredo, F.B.; Cardoso, F.A.; Hiamamoto, R.M.; Brazuna, J.C.M.; de Oliveira, M.R.F.; Noronha, E.F.; Romero, G.A.S. Validity and Reliability of Enzyme Immunoassays Using Leishmania major or L. infantum Antigens for the Diagnosis of Canine Visceral Leishmaniasis in Brazil. PLoS ONE 2013, 8, 8–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulldorff, M.; Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 1995, 14, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. Theory and Methods A spatial scan statistic. Commun. Stat. 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Wheeler, D.C. A comparison of spatial clustering and cluster detection techniques for childhood leukemia incidence in Ohio, 1996–2003. Int. J. Health Geogr. 2007, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Ovallos, F.; Casanova, C.; Pimentel Bergamaschi, D.; Bianchi Galati, E.A. A field study of the survival and dispersal pattern of Lutzomyia longipalpis in an endemic area of visceral leishmaniasis in Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006333. [Google Scholar] [CrossRef] [PubMed]
- INPE B. Instituto Nacional de Pesquisas Espaciais. 2024. Available online: http://www.dgi.inpe.br/catalogo/explore (accessed on 3 February 2023).
- da Saúde, M. Nota Técnica No. 30/2021-CGABR/DEIDT/SVS/MS. 2021. Available online: https://www.saude.ba.gov.br/wp-content/uploads/2022/09/NOTA-TECNICA-N-30-2021-CGARB-DEIDT-SVS-MS.pdf (accessed on 3 February 2023).
- FIOCRUZ. Índice de Estado da Vegetação (NDVI). 2022. Available online: https://climaesaude.icict.fiocruz.br/indicador/indice-de-estado-da-vegetacao-ndvi#:~:text=Interpretação%3A,altosentre0.5e1.0 (accessed on 3 February 2023).
- Grimaldi, G.; Teva, A.; Ferreira, A.L.; dos Santos, C.B.; Pinto, I.D.-S.; De-Azevedo, C.T.; Falqueto, A. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP ® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, L.C.; Brod, C.S.; Radin, J.; Simon, C.F.; Recuero, A.L.C. Leishmaniose Visceral Canina: Comparação de Métodos Sorológicos em Cães de Área Indene do Rio Grande do Sul no Brasil. Rev. Patol. Trop. 2015, 44, 33–44. [Google Scholar] [CrossRef]
- Peixoto, H.M.; de Oliveira, M.R.F.; Romero, G.A.S. Serological diagnosis of canine visceral leishmaniasis in Brazil: Systematic review and meta-analysis. Trop. Med. Int. Health 2015, 20, 334–352. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Ker, H.G.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Leal, G.G.d.A.; Moreira, N.d.D.; Oliveira, L.A.M.; Machado, E.M.d.M.; Morais, M.H.F.; Corrêa-Oliveira, R.; et al. Evaluation of change in canine diagnosis protocol adopted by the visceral leishmaniasis control program in Brazil and a new proposal for diagnosis. PLoS ONE 2014, 9, e91009. [Google Scholar] [CrossRef]
- de Arruda, M.M.; Figueiredo, F.B.; Marcelino, A.P.; Barbosa, J.R.; Werneck, G.L.; Noronha, E.F.; Romero, G.A.S. Sensitivity and Specificity of Parallel or Serial Serological Testing for Detection of Canine Leishmania Infection. Mem. Inst. Oswaldo Cruz 2016, 111, 168–173. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762016000300168 (accessed on 8 February 2023). [CrossRef] [PubMed]
- Ribeiro, V.M.; Miranda, J.B.; Marcelino, A.P.; de Andrade, H.M.; Reis, I.A.; Cardoso, M.S.; Gontijo, C.M.F.; Paz, G.F. Performance of different serological tests in the diagnosis of natural infection by Leishmania infantum in dogs. Vet. Parasitol. 2019, 274, 108920. [Google Scholar] [CrossRef] [PubMed]
- OPAS. Informe epidemiológico das Américas. Leishmaniases Epidemiol. Rep. Am. 2022, 11, 1–12. [Google Scholar]
- Alvar, J.; Yactayo, S.; Bern, C. Leishmaniasis and poverty. Trends Parasitol. 2006, 22, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Bermudi, P.M.M.; Costa, D.N.C.C.; Nunes, C.M.; Tolezano, J.E.; Hiramoto, R.M.; Rodas, L.A.C.; Cipriano, R.S.; Blangiardo, M.; Chiaravalloti-Neto, F.; Bermudi, P.M.M.; et al. Canine serological survey and dog culling ant its relationship with human visceral leishmaniasis in an endemic urban area. BMC Infect. Dis. 2020, 20, 401. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Shaw, J.J.; Dye, C. Infectiousness in a cohort of brazilian dogs: Why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 2002, 186, 1314–1320. [Google Scholar] [CrossRef]
- de Almeida Leal, G.G.; Carneiro, M.; da Costa Pinheiro, A.; Marques, L.A.; Ker, H.G.; Reis, A.B.; Coura-Vital, W. Risk profile for Leishmania infection in dogs coming from an area of visceral leishmaniasis reemergence. Prev. Vet. Med. 2018, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Teva, A.; Santos, C.B.; Ferreira, A.L.; Falqueto, A. The effect of removing potentially infectious dogs on the numbers of canine Leishmania infantum infections in an endemic area with high transmission rates. Am. J. Trop. Med. Hyg. 2012, 86, 966–971. [Google Scholar] [CrossRef][Green Version]
- Werneck, G.L.; Costa, C.H.N.; de Carvalho, F.A.A.; do Socorro Pires e Cruz, M.; Maguire, J.H.; Castro, M.C. Effectiveness of Insecticide Spraying and Culling of Dogs on the Incidence of Leishmania infantum Infection in Humans: A Cluster Randomized Trial in Teresina, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3172. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B.; Santos, W.R.D.; França-Silva, J.C.; da Costa, R.T.; Reis, A.B.; Palatnik, M.; Mayrink, W.; Genaro, O. Impact of Canine Control on the Epidemiology of Canine and Human. Am. J. Trop. Med. Hyg. 2001, 65, 510–517. [Google Scholar] [CrossRef]
- Nunes, C.M.; Pires, M.M.; da Silva, K.M.; Assis, F.D.; Filho, J.G.; Perri, S.H.V. Relationship between dog culling and incidence of human visceral leishmaniasis in an endemic area. Vet. Parasitol. 2010, 170, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.A.; David, J.R.; Freire, M.; David, R.; Sherlock, I.; Eulálio, M.C.; Sampaio, D.P.; Badaro, R. Studies on control of visceral leishmaniasis: Impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am. J. Trop. Med. Hyg. 1998, 59, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Sevá, A.P.; Ovallos, F.G.; Amaku, M.; Carrillo, E.; Moreno, J.; Galati, E.A.B.; Lopes, E.G.; Soares, R.M.; Ferreira, F. Canine-based strategies for prevention and control of visceral leishmaniasis in Brazil. PLoS ONE 2016, 11, e0160058. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.M.; Acosta-Serrano, A.; Bern, C.; Boelaert, M.; Boer, M.D.; Burza, S.; Chapman, L.A.C.; Chaskopoulou, A.; Coleman, M.; Courtenay, O.; et al. Understanding the transmission dynamics of Leishmania donovani to provide robust evidence for interventions to eliminate visceral leishmaniasis in Bihar, India the LCNTDR Collection: Advances in scientific research for NTD control. Parasites Vectors 2016, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.P.; Meneses, E.C.P.; Lopes, E.R.; Brito, F.R.D.S.; Mota, R.R.; Batista, M.C.A.; Albarado, K.V.P.; Freitas, D.D.S. Conhecimento da população sobre leishmaniose: Revisão integrativa. Peer Rev. 2023, 5, 383–397. [Google Scholar] [CrossRef]
- De Castro, J.M.; Rodrigues, S.M.; da Silva, S.T.P.; Costa, F.C.D.L.; Rodrigues, A.C.D.C.P.; Vieira, L.D.F.; Lima, M.R.; Borja-Cabrera, G.P. Conhecimento, Percepções de Individuos em Relação à Leishmaniose Visceral Humana como Novas Ferramentas de Controle. Ens. Ciênc. C Biol. Agrárias Saúde 2016, 20, 93. [Google Scholar] [CrossRef]
- Paranhos-Silva, M.; Freitas, L.A.R.; Santos, W.C.; Grimaldi, G.; Pontes-De-Carvalho, L.C.; Oliveira-Dos-Santos, A.J. A cross-sectional serodiagnostic survey of canine leishmaniasis due to Leishmania chagasi. Am. J. Trop. Med. Hyg. 1996, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Brandão-Filho, S.P. Visceral leishmaniasis in Brazil: Revisiting paradigms of epidemiology and control. Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 151–156. [Google Scholar] [CrossRef]
- Salles, S.A.C.; Schraiber, L.B. Gestores do SUS: Apoio e resistências à Homeopatia. Cad. Saude Publica 2009, 25, 195–202. [Google Scholar] [CrossRef][Green Version]
- Bezerra Da Silva, L. Sistemas De Informações Em Saúde Como Ferramenta Para Gestão Do Sus Health Information System As a Tool for Public Health Service’s Management. Saúde Desenvolv. 2016, 8, 20–30. [Google Scholar]
- Silva, L.S.; Machado, E.L.; Oliveira HN de Ribeiro, A.P. Condições de trabalho e falta de informações sobre o impacto da COVID-19 entre trabalhadores da saúde. Rev. Bras. Saúde Ocup. 2020, 45, e24. [Google Scholar] [CrossRef]
- Saúde, S.d.V.E. Saúde S de V em. Nota Informativa No. 24/2019-CGDT/DEVIT/SVS/MS 1. Do Plano De Ação Como Instrumento Para O Controle Da Leishmaniose Visceral. 2019; pp. 2–3. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/leishmaniose/5-nota-informativa-n-24-2019-cgdt-devit-svs-ms#:~:text=O%20Plano%20de%20A%C3%A7%C3%A3o%20%C3%A9,Minist%C3%A9rio%20da%20Sa%C3%BAde%20(MS) (accessed on 3 February 2023).
- Rollin, B.E. Euthanasia, moral stress, and chronic Illness in veterinary medicine. Vet. Clin. N. Am.—Small Anim. Pract. 2011, 41, 651–659. [Google Scholar] [CrossRef] [PubMed]
- COMAC. RADAR; COMAC: Shanghai, China, 2020. [Google Scholar]
- Tolezano, J.E.; Matsumoto PS, S.; Taniguchi, H.H.; Bertollo, D.M.B.; Pierre, M.K.; Barbosa, J.E.D.R. Evaluation of the effectiveness of using deltamethrin- impregnated collars to control visceral leishmaniasis in the municipality of Votuporanga, State of São Paulo, Brazil, 2014–2016. Rev. Inst. Adolfo Lutz. 2018, 1–10. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Gomes de Almeida Leal, G.; Marques, L.A.; Da Costa Pinheiro, A.; Carneiro, M.; Reis, A.B. Effectiveness of deltamethrin-impregnated dog collars on the incidence of canine infection by Leishmania infantum: A large scale intervention study in an endemic area in Brazil. PLoS ONE 2018, 13, e0208613. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.B.; de Oliveira, T.C.B.; Rodas, L.C.; Rozza, D.B.; Nakamura, A.A.; Ferrari, E.D.; da Silva, D.R.R.; dos Santos, G.M.; Calemes, E.B.; Requena, K.A.M.L.; et al. Efficacy of imidacloprid/flumethrin collar in preventing canine leishmaniosis in Brazil. Transbound. Emerg. Dis. 2022, 69, e2302–e2311. [Google Scholar] [CrossRef] [PubMed]
- Bray, D.P.; Alves, G.B.; Dorval, M.E.; Brazil, R.P.; Hamilton, J.G.C. Synthetic sex pheromone attracts the leishmaniasis vector Lutzomyia longipalpis to experimental chicken sheds treated with insecticide. Parasites Vectors 2010, 3, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, M.J.; Sedda, L.; Gonzalez, M.A.; de Souza, C.F.; Dilger, E.; Brazil, R.P.; Courtenay, O.; Hamilton, J.G.C. Attraction of Lutzomyia longipalpis to synthetic sex-aggregation pheromone: Effect of release rate and proximity of adjacent pheromone sources. PLoS Negl. Trop. Dis. 2018, 12, e0007007. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.G.; Sevá, A.P.; Ferreira, F.; Nunes, C.M.; Keid, L.B.; Hiramoto, R.M.; Ferreira, H.L.; Oliveira, T.M.F.S.; Ovallos, F.G.; Galati, E.A.B.; et al. Vaccine effectiveness and use of collar impregnated with insecticide for reducing incidence of Leishmania infection in dogs in an endemic region for visceral leishmaniasis, in Brazil. Epidemiol. Infect. 2018, 146, 401–406. [Google Scholar] [CrossRef]
- Retkute, R.; Dilger, E.; Hamilton, J.G.C.; Keeling, M.J.; Courtenay, O. Modelling sand fly lutzomyia longipalpis attraction to host odour: Synthetic sex-aggregation pheromone dominates the response. Microorganisms 2021, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- de Camargo-Neves, V.; Calemes, E.; Rodas, L.; Galvis-Ovallos, F.; Silva, L. Control of Canine Visceral Leishmaniasis: A Success Case Based on Deltamethrin 4% Collars. Epidemiologia 2021, 2, 502–518. [Google Scholar] [CrossRef]
- Otranto, D.; de Caprariis, D.; Lia, R.; Tarallo, V.; Lorusso, V.; Testini, G.; Dantas-Torres, F.; Latrofa, S.; Diniz, P.; Mencke, N.; et al. Prevention of endemic canine vector-borne diseases using imidacloprid 10% and permethrin 50% in young dogs: A longitudinal field study. Vet. Parasitol. 2010, 172, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Nota Informativa No. 13/2020-CGZV/DEIDT/SVS/MS. 2020; Volume 21, pp. 1–9. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/estudos-e-notas-informativas/2020/nota-informativa-miltefosina.pdf/view#:~:text=Orienta%C3%A7%C3%B5es%20sobre%20o%20uso%20da,do%20Sistema%20%C3%9Anico%20de%20Sa%C3%BAde (accessed on 3 February 2023).
- Ikeda-Garcia, F.A.; Lopes, R.S.; Marques, F.J.; Ciarlini, P.C.; de Lima, V.M.F.; Morinishi, C.K.; Zanette, M.F.; Perri, S.H.V.; Marcondes, M. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate and allopurinol. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 218–223. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Moura, E.P.; Pimentel, V.M.; Sampaio, W.M.; Silva, S.M.; Schettini, D.A.; Alves, C.F.; Melo, F.A.; Tafuri, W.L.; Demicheli, C.; et al. Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrob. Agents Chemother. 2008, 52, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- CFMV. Medicamento para Tratamento de LVC Deve ser Emitido Somente via SIPEAGRO. 2022; pp. 10–13. Available online: https://www.cfmv.gov.br/medicamento-para-tratamento-de-lvc-deve-ser-emitido-somente-via-sipeagro/comunicacao/noticias/2019/02/12/ (accessed on 3 February 2023).
- Chagas, Ú.M.R.; de Avelar, D.M.; Marcelino, A.P.; Paz, G.F.; Gontijo, C.M.F. Correlations between tissue parasite load and common clinical signs in dogs naturally infected by Leishmania infantum. Vet. Parasitol. 2021, 291, 109368. [Google Scholar] [CrossRef] [PubMed]
- Michalsky, M.; Rocha, M.F.; da Rocha Lima, A.C.V.M.; França-Silva, J.C.; Pires, M.Q.; Oliveira, F.S.; Pacheco, R.S.; dos Santos, S.L.; Barata, R.A.; Romanha, J.; et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet. Parasitol. 2007, 147, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.R.A.; de Mendonça, I.L.; Bonfim, J.M.D.; Rodrigues, J.A.; Werneck, G.L.; Costa, C.H.N. Canine visceral leishmaniasis in Teresina, Brazil: Relationship between clinical features and infectivity for sand flies. Acta Trop. 2011, 117, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.D.; Rossi, C.N.; da Matta, V.L.R.; Tomokane, T.Y.; Corbett, C.E.P.; Secundino, N.F.C.; Pimenta, P.F.P.; Marcondes, M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet. Parasitol. 2013, 196, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Rebelo, J.M.M.; Caldas, A.; Gomes, R.B.B.; Vinhas, V.S.; Barral, A.M.P.; Silva, A.A.M.; Costa, J.M.L. História natural da infecção causada por Leishmania chagasi em cães (Canis familiaris) domiciliados em área endêmica da Ilha de São Luis—Maranhão, Brasil. Gaz. Méd. Bahia 2009, 79, 147–155. [Google Scholar]
- Marzochi, M.C.d.A.; Coutinho, S.G.; Sabroza, P.C.; de Souza, M.A.; de Souza, P.P.M.; Toledo, L.; Filho, F.B.R. Leishmaniose visceral canina no Rio de Janeiro—Brasil. Cad. Saude Publica 1985, 1, 432–446. [Google Scholar] [CrossRef]
- Marcondes, M.; Rossi, C.N. Leishmaniase visceral no Brasil. Braz. J. Vet. Res. Anim. Sci. 2013, 50, 341–352. [Google Scholar] [CrossRef]
- Andrade, A.M.; Queiroz, L.H.; Perri, S.H.V.; Nunes, C.M. Estudo descritivo da estrutura populacional canina da área urbana de Araçatuba, São Paulo, Brasil, no período de 1994 a 2004. Cad. Saude Publica 2008, 24, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.N.C.C.; Bermudi, P.M.M.B.; Rodas, L.A.C.; Nunes, C.M.; Hiramoto, R.M.; Tolezano, J.E.; Cipriano, R.S.; Cardoso, G.C.D.; Codeço, C.T.; Chiaravalloti-Neto, F. Human visceral leishmaniasis and relationship with vector and canine control measures. Rev. Saude Publica 2018, 52, 92. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Gradoni, L.; Bettini, M.; Gramiccia, M. Leishmaniasis in Tuscny (Italy): VI. Canine leishmaniasis in the focus of Monte Argentario (Grosseto). Acta Trop. 1981, 38, 383–393. [Google Scholar]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. Adv. Parasitol. 2004, 57, 88. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.; Silva, J.; Haddad, J.; Moreira, E.; Magalhães, D.; Ribeiro, L.; Fiúza, V. Presença de animais associada ao risco de transmissão da leishmaniose visceral em humanos em Belo Horizonte, Minas Gerais. Arq. Bras. Med. Vet. Zootec. 2009, 61, 1035–1043. [Google Scholar] [CrossRef][Green Version]
- Chaves, L.F.; Cohen, J.M.; Pascual, M.; Wilson, M.L. Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PLoS Negl. Trop. Dis. 2008, 2, e176. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.M.; Gutierrez, J.D.; Xiao, Y.; Branscum, A.J.; Cuadros, D.F. Spatial epidemiology of cutaneous leishmaniasis in Colombia: Socioeconomic and demographic factors associated with a growing epidemic. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 560–568. [Google Scholar] [CrossRef]
- Casanova, C.; Colla-Jacques, F.E.; Hamilton, J.G.C.; Brazil, R.P.; Shaw, J.J. Distribution of Lutzomyia longipalpis Chemotype Populations in São Paulo State, Brazil. PLoS Negl. Trop. Dis. 2015, 9, e0003620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pereira, N.C.L.; Michalsky, M.; Lara-Silva, F.O.; Lana, R.S.; de Paula, A.J.V.; Pereira, D.M.; Lopes, J.V.; Fortes-Dias, C.L.; Dias, E.S. Ecology of phlebotomine sand flies in a Brazilian area with recent leishmaniasis transmission (Itaúna, in Minas Gerais state). Rev. Soc. Bras. Med. Trop. 2020, 53, e20190538. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.; Bavia, M.E.; Rocha, W.; Lobão, J.; Madureira Filho, C.; Oliveira, J.B.D.; da Silva, C.E.; das Graças Barbosa, M.; Rios, R. Identificação de áreas de risco para a leishmaniose visceral americana, através de estudos epidemiológicos e sensoriamento remoto orbital, em feira de santana, bahia, brasil (2000–2002). Rev. Baiana Saúde Pública 2004, 28, 19–32. [Google Scholar] [CrossRef]
- Paulan, S.d.C.; Silva, H.R.; de Freitas Lima, E.A.C.; Flores, E.F.; Tachibana, V.M.; Kanda, C.Z.; Junior, A.C.F.d.N.; Dobre, P.R. Spatial distribution of canine visceral leishmaniasis in ilha solteira, são paulo, Brazil. Eng. Agric. 2012, 32, 765–774. [Google Scholar] [CrossRef][Green Version]
- Desjeux, P. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Forattini, O.P. Entomologia Médica—V4; Editora Universidade de São Paulo: São Paulo, Brasil, 1962. [Google Scholar]
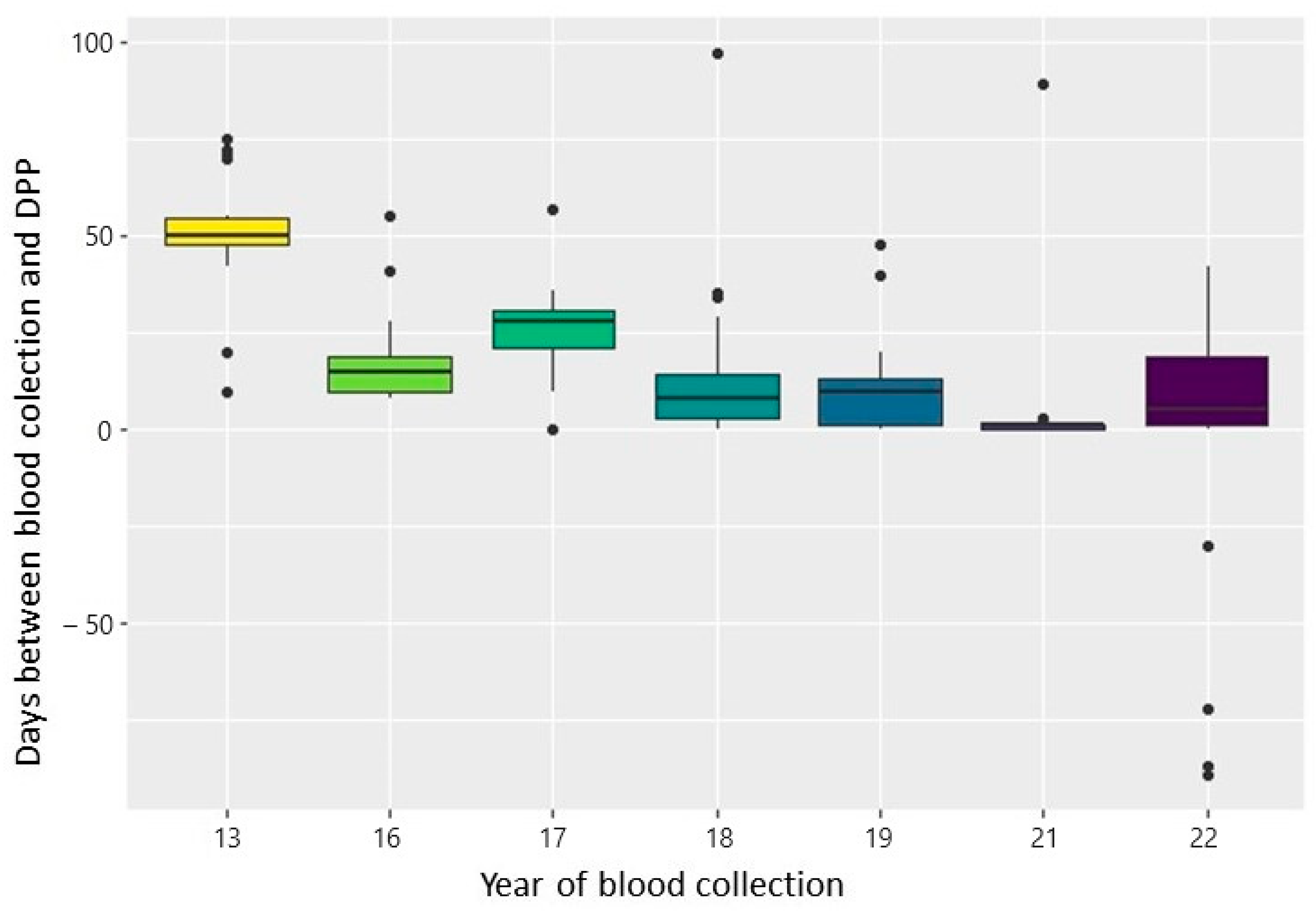

| Comparison Number | Year (A) | n (A) | Year (B) | n (B) | Median | Statistical Value (Ts) | Adjusted p Value |
|---|---|---|---|---|---|---|---|
| 1 | 2013 | 218 | 2016 | 230 | 50/20 | 48,672 | <0.001 |
| 2 | 2013 | 218 | 2017 | 312 | 50/28 | 65,174.5 | <0.001 |
| 3 | 2013 | 218 | 2018 | 872 | 50/10 | 185,140.5 | <0.001 |
| 4 | 2013 | 218 | 2019 | 99 | 50/15 | 21,464 | <0.001 |
| 5 | 2013 | 218 | 2021 | 436 | 50/3 | 94,830 | <0.001 |
| 6 | 2013 | 218 | 2022 | 1372 | 50/8 | 295,944.5 | <0.001 |
| 7 | 2016 | 230 | 2017 | 312 | 20/28 | 13,455 | <0.001 |
| 8 | 2016 | 230 | 2018 | 872 | 20/10 | 146,934 | <0.001 |
| 9 | 2016 | 230 | 2019 | 99 | 20/15 | 17,011 | <0.001 |
| 10 | 2016 | 230 | 2021 | 436 | 20/3 | 100,050 | <0.001 |
| 11 | 2016 | 230 | 2022 | 1372 | 20/8 | 209,675 | <0.001 |
| 12 | 2017 | 312 | 2018 | 872 | 28/10 | 246,174 | <0.001 |
| 13 | 2017 | 312 | 2019 | 99 | 28/15 | 29,122 | <0.001 |
| 14 | 2017 | 312 | 2021 | 436 | 28/3 | 135,056 | <0.001 |
| 15 | 2017 | 312 | 2022 | 1372 | 28/8 | 364,856.5 | <0.001 |
| 16 | 2018 | 872 | 2019 | 99 | 10/15 | 50,176 | 0.164 |
| 17 | 2018 | 872 | 2021 | 436 | 10/3 | 369,540.5 | <0.001 |
| 18 | 2018 | 872 | 2022 | 1372 | 10/8 | 658,831 | 0.001 |
| 19 | 2019 | 99 | 2021 | 436 | 15/3 | 35,423 | <0.001 |
| 20 | 2019 | 99 | 2022 | 1372 | 15/8 | 58,417 | 0.393 |
| 21 | 2021 | 436 | 2022 | 1372 | 3/8 | 87,374 | <0.001 |
| Negative | Positive | Odds Ratio | p Value | |||||
| n | (%) | n | (%) | Total | (CI 95%) | |||
| Age rate | Puppy | 224 | 95.3 | 11 | 4.7 | 235 | reference | |
| Young | 1066 | 92.1 | 91 | 7.9 | 1157 | 1.7 (0.89, 3.23) | 0.107 | |
| Adult | 1326 | 91.3 | 126 | 8.7 | 1452 | 1.92 (1.02, 3.62) | 0.043 | |
| Senior | 368 | 90.0 | 41 | 10.0 | 409 | 2.28 (1.15, 4.53) | 0.018 | |
| Geriatric | 196 | 89.5 | 23 | 10.5 | 219 | 2.39 (1.14, 5.03) | 0.022 | |
| Sex | Female | 2078 | 92.4 | 172 | 7.6 | 2250 | reference | |
| Male | 1511 | 90.7 | 155 | 9.3 | 1666 | 1.24 (0.99, 1.56) | 0.064 | |
| Number of dogs per residence | Up to 2 | 2598 | 51.3 | 2470 | 48.7 | 5068 | reference | |
| From 3 to 4 | 819 | 76.5 | 251 | 23.5 | 1070 | 0.32 (0.28, 0.37) | <0.001 | |
| From 5 to 7 | 209 | 87.1 | 31 | 12.9 | 240 | 0.16 (0.11, 0.23) | <0.001 | |
| From 8 to 15 | 22 | 84.6 | 4 | 15.4 | 26 | 0.19 (0.07, 0.56) | 0.002 | |
| Cluster Number | Prevalence (%) | Population (n) | Positive (n) | Relative Risk | p-Value | Radius (Km) |
|---|---|---|---|---|---|---|
| 1 | 84.2 | 2300 | 1936 | 1.44 | <0.001 | 3.20 |
| 2 | 97.6 | 626 | 611 | 1.57 | <0.001 | 66.92 |
| 3 | 99.3 | 447 | 444 | 1.63 | <0.001 | 1.63 |
| 4 | 98.9 | 272 | 269 | 1.56 | <0.001 | 0.93 |
| 5 | 97.6 | 125 | 122 | 1.53 | <0.001 | 0.43 |
| 6 | 97.6 | 84 | 82 | 1.53 | <0.001 | 0.29 |
| 7 | 100 | 50 | 50 | 1.56 | <0.001 | 0.60 |
| 8 | 100 | 37 | 37 | 1.56 | <0.001 | 0.29 |
| 9 | 96.1 | 51 | 49 | 1.50 | 0.0021 | 0.43 |
| 10 | 100 | 28 | 28 | 1.56 | 0.032 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.C.B.d.; Sevá, A.d.P.; Neto, J.A.B.C.; Lopes, U.d.L.; Bresciani, K.D.S. Finding Priority Areas in the Evaluation of Strategies for the Prevention of Leishmaniasis in an Endemic Municipality of Brazil. Trop. Med. Infect. Dis. 2024, 9, 115. https://doi.org/10.3390/tropicalmed9050115
Oliveira TCBd, Sevá AdP, Neto JABC, Lopes UdL, Bresciani KDS. Finding Priority Areas in the Evaluation of Strategies for the Prevention of Leishmaniasis in an Endemic Municipality of Brazil. Tropical Medicine and Infectious Disease. 2024; 9(5):115. https://doi.org/10.3390/tropicalmed9050115
Chicago/Turabian StyleOliveira, Talita Carolina Bragança de, Anaiá da Paixão Sevá, João Alfredo Biagi Camargo Neto, Uelio de Lima Lopes, and Katia Denise Saraiva Bresciani. 2024. "Finding Priority Areas in the Evaluation of Strategies for the Prevention of Leishmaniasis in an Endemic Municipality of Brazil" Tropical Medicine and Infectious Disease 9, no. 5: 115. https://doi.org/10.3390/tropicalmed9050115
APA StyleOliveira, T. C. B. d., Sevá, A. d. P., Neto, J. A. B. C., Lopes, U. d. L., & Bresciani, K. D. S. (2024). Finding Priority Areas in the Evaluation of Strategies for the Prevention of Leishmaniasis in an Endemic Municipality of Brazil. Tropical Medicine and Infectious Disease, 9(5), 115. https://doi.org/10.3390/tropicalmed9050115






