Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting, Data Source, and Ethics
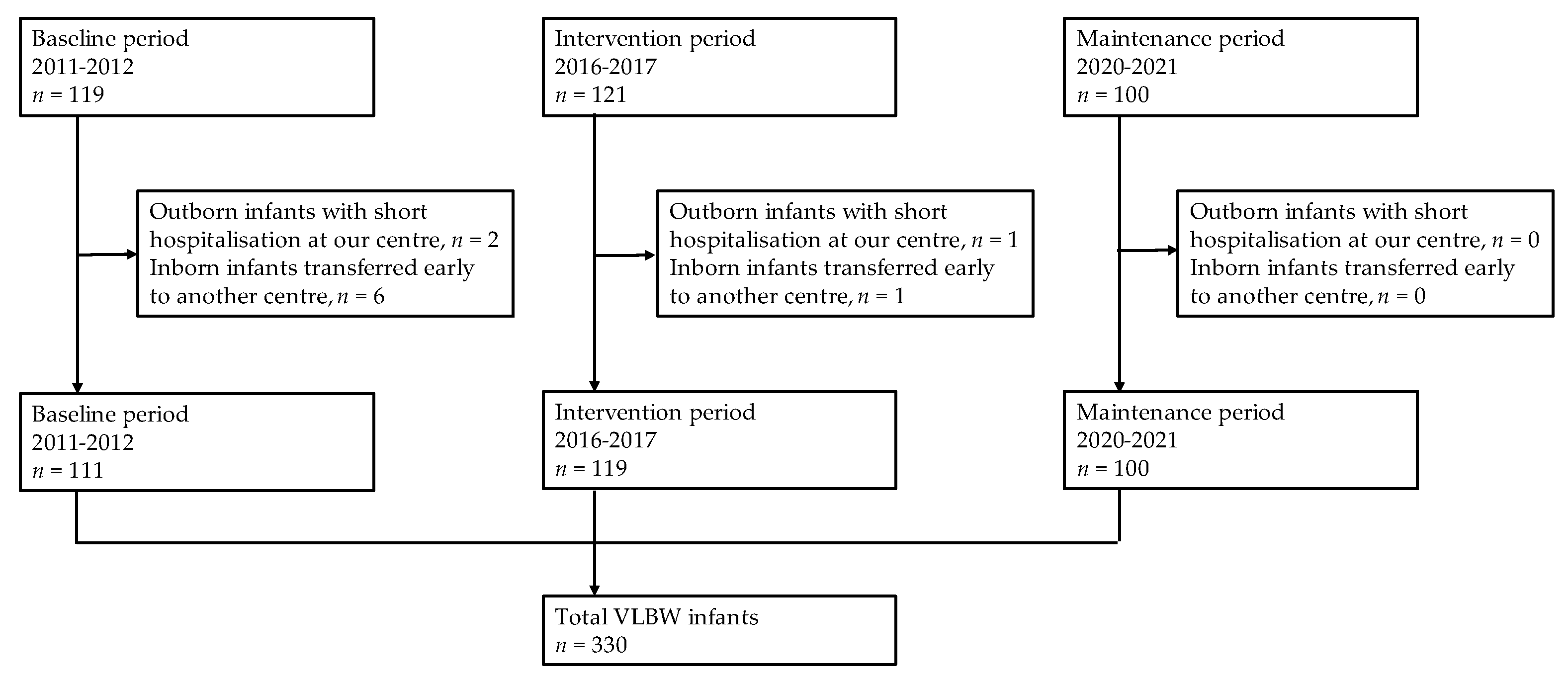
2.2. Study Design
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Definitions
2.6. Statistical Analyses
3. Results
3.1. Demographics of VLBW Infants
3.2. Overall Antibiotic Use in VLBW Infants and the Policy of 48-Hour Rule-Out Sepsis Antibiotic Course
3.3. Neonatal Outcomes and Infections
3.4. Antibiotic Use According to Birth Weight
3.5. Antibiotics Exposure of VLBW Infants without Culture-Proven Sepsis
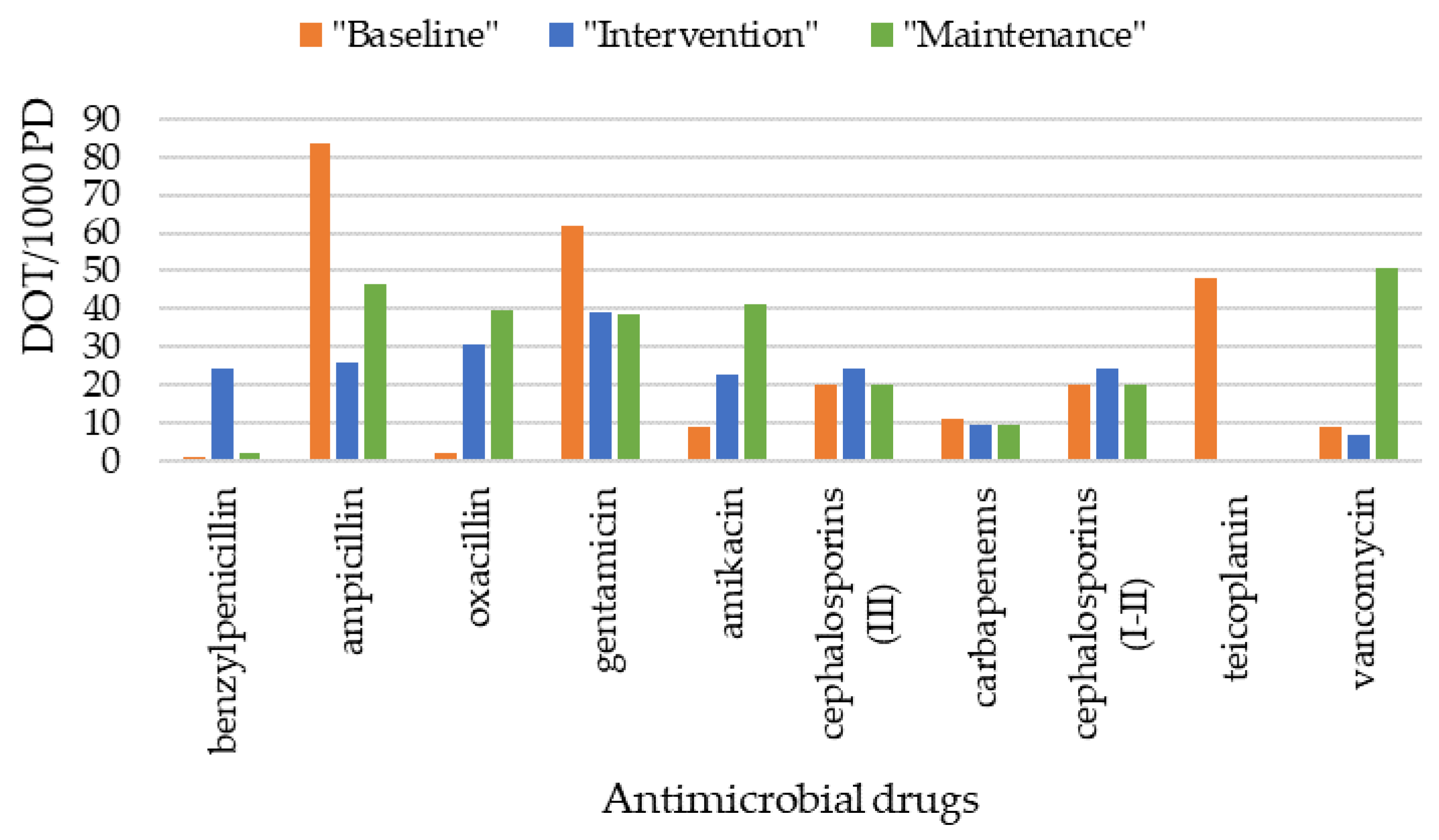
3.6. Antimicrobial Drugs Administered
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182894. [Google Scholar] [CrossRef]
- Berardi, A.; Zinani, I.; Rossi, C.; Spaggiari, E.; D’amico, V.; Toni, G.; Bedetti, L.; Lucaccioni, L.; Iughetti, L.; Lugli, L. Antibiotic Use in Very Low Birth Weight Neonates after an Antimicrobial Stewardship Program. Antibiotics 2021, 10, 411. [Google Scholar] [CrossRef]
- De Rose, D.U.; Santisi, A.; Ronchetti, M.P.; Martini, L.; Serafini, L.; Betta, P.; Maino, M.; Cavigioli, F.; Giuffré, M.; Bonanno, E.; et al. Decreased incidence of late-onset sepsis during the SARS-CoV-2 pandemic in Italy: A multicentric study on a cohort of infants requiring major surgery. Eur. J. Pediatr. 2023, 182, 4859–4866. [Google Scholar] [CrossRef]
- Laccetta, G.; Ciantelli, M.; Tuoni, C.; Sigali, E.; Miccoli, M.; Cuttano, A. Early-onset sepsis risk calculator: A review of its effectiveness and comparative study with our evidence-based local guidelines. Ital. J. Pediatr. 2021, 47, 73. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Zinani, I.; Bedetti, L.; Vaccina, E.; Toschi, A.; Toni, G.; Lecis, M.; Leone, F.; Monari, F.; Cozzolino, M.; et al. Should we give antibiotics to neonates with mild non-progressive symptoms? A comparison of serial clinical observation and the neonatal sepsis risk calculator. Front. Pediatr. 2022, 10, 882416. [Google Scholar] [CrossRef]
- Giannoni, E.; Schlapbach, L.J. Editorial: Sepsis in Neonates and Children. Front. Pediatr. 2020, 8, 621663. [Google Scholar] [CrossRef]
- Stocker, M.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; Dimopoulou, V.; et al. Less is more: Antibiotics at the beginning of life. Nat. Commun. 2023, 14, 2423. [Google Scholar] [CrossRef] [PubMed]
- Köstlin-Gille, N.; Serna-Higuita, L.M.; Bubser, C.; Arand, J.; Haag, L.; Schwarz, C.E.; Heideking, M.; Poets, C.F.; Gille, C. Early initiation of antibiotic therapy and short-term outcomes in preterm infants: A single-centre retrospective cohort analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 623–630. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Li, S.; Yan, W.; Bai, R.; Yang, Z.; Shi, J.; Yuan, J.; Yang, C.; Cai, W.; et al. Early Antibiotic Use and Neonatal Outcomes among Preterm Infants without Infections. Pediatrics 2023, 151, e2022059427. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Blumberg, R.S. Life at the beginning: Perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol. 2014, 15, 307–310. [Google Scholar] [CrossRef]
- Minotti, C.; Di Caprio, A.; Facchini, L.; Bedetti, L.; Miselli, F.; Rossi, C.; Muttini, E.D.C.; Lugli, L.; Luppi, L.; Ferrari, F.; et al. Antimicrobial Resistance Pattern and Empirical Antibiotic Treatments in Neonatal Sepsis: A Retrospective, Single-Center, 12-Year Study. Antibiotics 2023, 12, 1488. [Google Scholar] [CrossRef]
- Berardi, A.; Sforza, F.; Baroni, L.; Spada, C.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.; Carretto, E.; Ciccia, M.; et al. Epidemiology and complications of late-onset sepsis: An Italian area-based study. PLoS ONE 2019, 14, e0225407. [Google Scholar] [CrossRef]
- Huncikova, Z.; Stensvold, H.J.; Øymar, K.A.A.; Vatne, A.; Lang, A.M.; Støen, R.; Brigtsen, A.K.; Moster, D.; Eriksen, B.H.; Selberg, T.; et al. Variation in antibiotic consumption in very preterm infants-a 10 year population-based study. J. Antimicrob. Chemother. 2024, 79, 143–150. [Google Scholar] [CrossRef]
- Prusakov, P.; Goff, D.A.; Wozniak, P.S.; Cassim, A.; Scipion, C.E.; Urzúa, S.; Ronchi, A.; Zeng, L.; Ladipo-Ajayi, O.; Aviles-Otero, N.; et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study. eClinicalMedicine 2021, 32, 100727. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Dimopoulou, V.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; et al. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. JAMA Netw. Open 2022, 5, e2243691. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C.; ESGAP (ESCMID Study Group for Antimicrobial stewardshiP). What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Patel, S.J. Antimicrobial stewardship in the NICU. Infect. Dis. Clin. N. Am. 2014, 28, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Horbar, J.D. Metrics of neonatal antibiotic use. Semin. Perinatol. 2020, 44, 151329. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Custodio, H.; McCurley, C.; Whitehurst, R.; Gulati, R.; Jha, O.P.; Bhat, J.; Estrada, B.; Hill, A.; Eyal, F.; et al. Reducing antibiotic utilization rate in preterm infants: A quality improvement initiative. J. Perinatol. 2018, 38, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Takagi, K.; Arai, I.; Yasuhara, H.; Ebisu, R.; Ohgitani, A.; Kitagawa, D.; Oka, M.; Masuo, K.; Minowa, H. A simple and feasible antimicrobial stewardship pro-gram in a neonatal intensive care unit of a Japanese community hospital. J. Infect. Chemother. 2019, 25, 860–865. [Google Scholar] [CrossRef]
- Stritzke, A.; Tierney, A.; Keister, F.; Srivastava, A.M.; Dersch-Mills, D.B.P.; Hamilton, C.; Lodha, A.M.; Mehrem, A.A. Antimicrobial Stewardship at Birth in Preterm Infants: Not Just About a Decrease! Pediatr. Infect. Dis. J. 2022, 41, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.Y.; Paquette, V.; Ng, K.; Lisonkova, S.; Hait, V.B.; Shivanada, S.M.B.; Tilley, P.; Osiovich, H.; Roberts, A.M. Reduction of Inappropriate Antimicrobial Prescriptions in a Tertiary Neonatal Intensive Care Unit after Antimicrobial Stewardship Care Bundle Implementation. Pediatr. Infect. Dis. J. 2019, 38, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, Q.; Yuan, H.; Wang, L. Implementation of the Smart Use of Antibiotics Program to Reduce Unnecessary Antibiotic Use in a Neonatal ICU: A Prospective Interrupted Time-Series Study in a Developing Country. Crit. Care Med. 2019, 47, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Lee, J.H. Biomarkers for the Diagnosis of Neonatal Sepsis. Clin. Perinatol. 2021, 48, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Dhudasia, M.B.; Benitz, W.E.; Flannery, D.D.; Christ, L.; Rub, D.; Remaschi, G.; Puopolo, K.M.; Mukhopadhyay, S. Diagnostic Performance and Patient Outcomes with C-Reactive Protein Use in Early-Onset Sepsis Evaluations. J. Pediatr. 2023, 256, 98–104.e6. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sengupta, S.; Puopolo, K.M. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F327–F332. [Google Scholar] [CrossRef]
- Miselli, F.; Crestani, S.; Maugeri, M.; Passini, E.; Spaggiari, V.; Deonette, E.; Ćosić, B.; Rossi, K.; Roversi, M.F.; Bedetti, L.; et al. Late-Onset Sepsis Mortality among Preterm Infants: Beyond Time to First Antibiotics. Microorganisms. 2023, 11, 396. [Google Scholar] [CrossRef]
- Boverman, G.; Perez, C.; Vij, S.; Tgavalekos, K.; Ravindranath, S.; Antonescu, C.; Chambers-Hawk, B. Neonatal ICU antibiotic use trends within an integrated delivery network. Antimicrob. Resist. Infect. Control. 2022, 11, 21. [Google Scholar] [CrossRef]
- McCarthy, K.N.; Hawke, A.; Dempsey, E.M. Antimicrobial stewardship in the neonatal unit reduces antibiotic exposure. Acta Paediatr. 2018, 107, 1716–1721. [Google Scholar] [CrossRef]
- Cantey, J.B.; Wozniak, P.S.; Pruszynski, J.E.; Sánchez, P.J. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): A prospective interrupted time-series study. Lancet Infect. Dis. 2016, 16, 1178–1184. [Google Scholar] [CrossRef]
- Astorga, M.C.; Piscitello, K.J.; Menda, N.; Ebert, A.M.; Ebert, S.C.; Porte, M.P.; Kling, P.J. Antibiotic Stewardship in the Neonatal Intensive Care Unit: Effects of an Automatic 48-Hour Antibiotic Stop Order on Antibiotic Use. J. Pediatric Infect. Dis. Soc. 2019, 8, 310–316. [Google Scholar] [CrossRef]
- Desai, S.; Qin, H.; Rayburn, P.D.; Poon, G.; Murthy, K.; Ellsbury, D.L.; Chiruvolu, A.; Tolia, V.N. Implementation of an Automatic Stop Order and Initial Antibiotic Exposure in Very Low Birth Weight Infants. Am. J. Perinatol. 2017, 34, 105–110. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad between Efficient Sepsis Care and An-timicrobial Stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef]
- Rajar, P.; Saugstad, O.D.; Berild, D.; Dutta, A.; Greisen, G.; Lausten-Thomsen, U.; Mande, S.S.; Nangia, S.; Petersen, F.C.; Dahle, U.R.; et al. Antibiotic Stewardship in Premature Infants: A Systematic Review. Neonatology 2020, 117, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Ross, R.K.; Mukhopadhyay, S.; Tribble, A.C.; Puopolo, K.M.; Gerber, J.S. Temporal Trends and Center Variation in Early Antibiotic Use Among Premature Infants. JAMA Netw. Open 2018, 1, e180164. [Google Scholar] [CrossRef] [PubMed]
- Tagare, A.; Kadam, S.; Vaidya, U.; Pandit, A. Routine antibiotic use in preterm neonates: A randomised controlled trial. J. Hosp. Infect. 2010, 74, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Puopolo, K.M.; Hansen, N.I.; Gerber, J.S.; Sánchez, P.J.; Stoll, B.J. Antimicrobial Susceptibility Profiles Among Neonatal Early-onset Sepsis Pathogens. Pediatr. Infect. Dis. J. 2022, 41, 263–271. [Google Scholar] [CrossRef]
- Garber, S.J.; Dhudasia, M.B.; Flannery, D.D.; Passarella, M.R.; Puopolo, K.M.; Mukhopadhyay, S. Delivery-based criteria for empiric antibiotic administration among preterm infants. J. Perinatol. 2021, 41, 255–262. [Google Scholar] [CrossRef] [PubMed]
| All VLBW Infants (n = 330) | “Baseline” 2011–2012 (n = 111) | “Intervention” 2016–2017 (n = 119) | “Maintenance” 2020–2021 (n = 100) | p Value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Gender (male), n (%) | 169 (51) | 61 (55) | 67 (56) | 47 (47) | 0.84 |
| GA (weeks), median (IQR) | 29 (26–31) | 29 (26–31) | 29 (26–31) | 29 (27–31) | 0.67 |
| BW (g), median (IQR) | 1148 (844–1370) | 1146 (857–1346) | 1109 (851–1398) | 1188 (830–1339) | 0.94 |
| Twin birth, n (%) | 75 (23) | 28 (25) | 31 (26) | 16 (16) | 0.16 |
| 5 min Apgar score, median (IQR) | 8 (7–9) | 8 (7–9) | 8 (6–9) | 8 (7–9) | 0.27 |
| CRIB score, median (IQR) | 1 (0–4) | 1 (1–4) | 1 (0–4) | 1 (1–4) | 0.84 |
| Maternal indication for delivery, n (%) | 75 (23) | 28 (25) | 39 (33) | 35 (35) | 0.26 |
| Mode of delivery, n (%) Vaginal delivery CS in labour or with membrane rupture CS before labour and with intact membranes | 73 (22) 75 (23) 181 (55) | 21 (19) 30 (27) 60 (54) | 25 (21) 25 (21) 69 (58) | 27 (27) 20 (20) 52 (52) | 0.35 0.41 0.67 |
| Risk factors for EOS | |||||
| Histological chorioamnionitis, n (%) | 87 (26) | 29 (27) | 31 (26) | 27 (27) | 0.99 |
| Prolonged rupture of membranes (PROM >18 h), n (%) | 90 (27) | 29 (26) | 34 (29) | 27 (27) | 0.91 |
| Maternal fever in labour (T > 38 °C), n (%) | 14 (4) | 3 (3) | 6 (5) | 5 (5) | 0.61 |
| Positive maternal GBS screening, n (%) | 21 (6) | 8 (7) | 6 (5) | 7 (7) | 0.76 |
| Intrapartum antibiotic prophylaxis, n (%) No Adequate | 131 (40) 142 (43) | 68 (63) 30 (28) | 55 (48) 48 (40) | 8 (8) 64 (64) | <0.001 <0.001 |
| Exposures | |||||
| Length of hospital stay (days), median (IQR) | 47 (29–69) | 47 (29–75) | 46 (28–71) | 48 (30–64) | 0.97 |
| Duration of CVC placement (days), median (IQR) | 10 (4–25) | 11 (4–27) | 10 (4–28) | 9 (4–20) | 0.59 |
| Antibiotic use | |||||
| DOT/1000 PD, n | 267 | 302 | 215 | 291 | 0.09 |
| AUR/1000 PD, n | 162 | 192 | 136 | 160 | 0.07 |
| No antibiotic exposure, n (%) | 81 (25) | 26 (23) | 35 (29) | 20 (20) | 0.26 |
| Empirical antibiotics immediately after birth, n (%) | 203 (62) | 73 (66) | 68 (57) | 62 (62) | 0.40 |
| Duration of early empirical antibiotic treatment (days), median (IQR) | 5 (2–7) | 6 (4–7) | 3 (2–3) | 2 (2–3) | <0.001 |
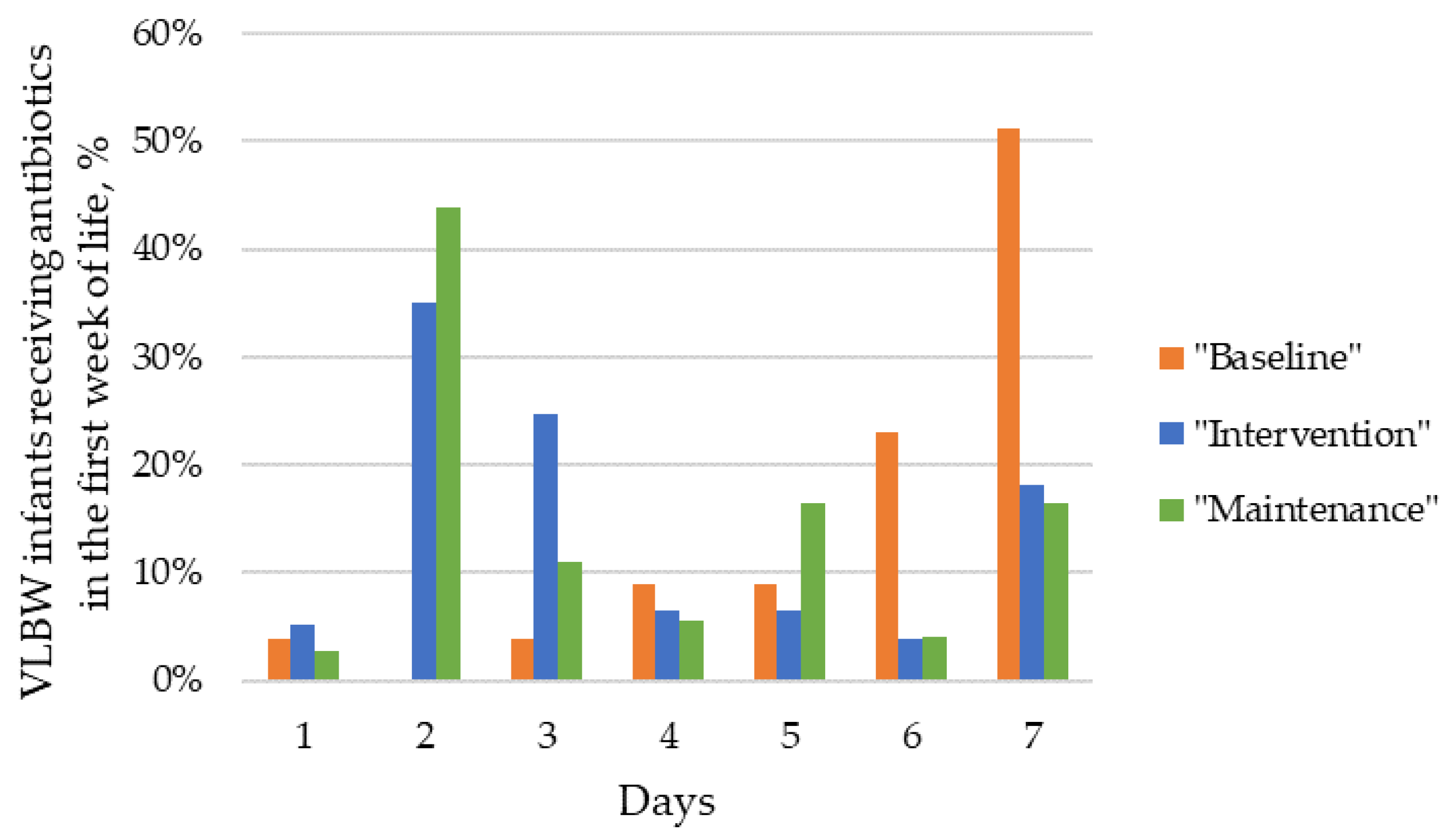
| Days of antibiotics in the first week of life, median (IQR) | 3 (0–7) | 6 (0–7) | 2 (0–5) | 2 (0–5) | <0.001 |
| Outcomes | |||||
| EOS, n (%) | 9 (3) | 3 (3) | 4 (4) | 2 (2) | 0.83 |
| LOS, n (%) | 32 (10) | 6 (5) | 14 (12) | 12 (12) | 0.17 |
| Sepsis with CoNS, n (%) | 27 (8) | 8 (7) | 2 (2) | 17 (17) | <0.001 |
| Culture-negative sepsis, n (%) | 43 (13) | 24 (22) | 13 (11) | 6 (6) | 0.002 |
| Surgically treated NEC, n (%) | 3 (1) | 1 (1) | 1 (1) | 1 (1) | 0.97 |
| Sepsis-related mortality, n (%) | 6 (2) | 3 (3) | 3 (3) | 0 (0) | 0.26 |
| In-hospital mortality, n (%) | 37 (11) | 11 (10) | 17 (14) | 9 (9) | 0.41 |
| BW < 1000 g (ELBW) | BW 1000–1499 g | |||||||
|---|---|---|---|---|---|---|---|---|
| “Baseline” 2011–2012 (n = 44) | “Intervention” 2016–2017 (n = 51) | “Maintenance” 2020–2021 (n = 35) | p Value | “Baseline” 2011–2012 (n = 67) | “Intervention” 2016–2017 (n = 68) | “Maintenance” 2020–2021 (n = 65) | p Value | |
| Risk factors for EOS | ||||||||
| Histological chorioamnionitis, n (%) | 20 (45) | 20 (39) | 12 (34) | 0.48 | 9 (13) | 11 (16) | 15 (23) | 0.32 |
| Positive maternal GBS screening, n (%) | 4 (6) | 4 (6) | 5 (8) | 0.89 | 4 (9) | 2 (4) | 2 (6) | 0.57 |
| Antibiotic use | ||||||||
| DOT/1000 PD, n | 367 | 269 | 449 | 0.62 | 237 | 154 | 182 | 0.02 |
| No antibiotic exposure, n (%) | 2 (5) | 5 (10) | 3 (9) | 0.62 | 24 (36) | 30 (44) | 17 (26) | 0.10 |
| Empirical antibiotics immediately after birth, n (%) | 36 (82) | 39 (76) | 21 (60) | 0.08 | 37 (55) | 29 (43) | 41 (63) | 0.06 |
| Days of antibiotics in the first week of life, median (IQR) | 7 (5–7) | 3 (2–4) | 2 (1–3) | <0.001 | 7 (5–7) | 2 (2–3) | 2 (1–3) | <0.001 |
| Outcomes | ||||||||
| EOS, n (%) | 3 (7) | 3 (6) | 2 (6) | 0.97 | 3 (3) | 4 (4) | 2 (2) | 0.83 |
| LOS, n (%) | 4 (9) | 10 (20) | 5 (14) | 0.35 | 6 (5) | 14 (12) | 12 (12) | 0.17 |
| Sepsis with CoNS, n (%) | 6 (14) | 2 (4) | 11 (31) | 0.002 | 8 (7) | 2 (2) | 17 (17) | <0.001 |
| Culture-negative sepsis, n (%) | 15 (34) | 9 (18) | 4 (11) | 0.02 | 24 (22) | 13 (11) | 6 (6) | 0.002 |
| Surgically treated NEC, n (%) | 2 (5) | 3 (6) | 0 (0) | 0.36 | 1 (1) | 1 (1) | 1 (1) | 0.97 |
| Sepsis-related mortality, n (%) | 10 (23) | 16 (31) | 9 (26) | 0.63 | 3 (3) | 3 (3) | 0 (0) | 0.26 |
| In-hospital mortality, n (%) | 3 (7) | 3 (6) | 2 (6) | 0.97 | 11 (10) | 17 (14) | 9 (9) | 0.41 |
| DOT/1000 PD | AUR/1000 PD | |||||||
|---|---|---|---|---|---|---|---|---|
| “Baseline” 2011–2012 | “Intervention” 2016–2017 | “Maintenance” 2020–2021 | p Value | “Baseline” 2011–2012 | “Intervention” 2016–2017 | “Maintenance” 2020–2021 | p Value | |
| VLBW infants without culture-proven sepsis | 251 | 134 | 166 | 0.01 | 158 | 85 | 91 | 0.006 |
| ELBW infants without culture-proven sepsis | 289 | 174 | 188 | 0.44 | 182 | 109 | 141 | 0.66 |
| 1000–1499 g BW infants without culture-proven sepsis | 223 | 98 | 115 | 0.004 | 140 | 63 | 70 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zini, T.; Miselli, F.; D’Esposito, C.; Fidanza, L.; Cuoghi Costantini, R.; Corso, L.; Mazzotti, S.; Rossi, C.; Spaggiari, E.; Rossi, K.; et al. Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants. Trop. Med. Infect. Dis. 2024, 9, 59. https://doi.org/10.3390/tropicalmed9030059
Zini T, Miselli F, D’Esposito C, Fidanza L, Cuoghi Costantini R, Corso L, Mazzotti S, Rossi C, Spaggiari E, Rossi K, et al. Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants. Tropical Medicine and Infectious Disease. 2024; 9(3):59. https://doi.org/10.3390/tropicalmed9030059
Chicago/Turabian StyleZini, Tommaso, Francesca Miselli, Chiara D’Esposito, Lucia Fidanza, Riccardo Cuoghi Costantini, Lucia Corso, Sofia Mazzotti, Cecilia Rossi, Eugenio Spaggiari, Katia Rossi, and et al. 2024. "Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants" Tropical Medicine and Infectious Disease 9, no. 3: 59. https://doi.org/10.3390/tropicalmed9030059
APA StyleZini, T., Miselli, F., D’Esposito, C., Fidanza, L., Cuoghi Costantini, R., Corso, L., Mazzotti, S., Rossi, C., Spaggiari, E., Rossi, K., Lugli, L., Bedetti, L., & Berardi, A. (2024). Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants. Tropical Medicine and Infectious Disease, 9(3), 59. https://doi.org/10.3390/tropicalmed9030059






