The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023
Abstract
1. Introduction
2. Materials and Methods
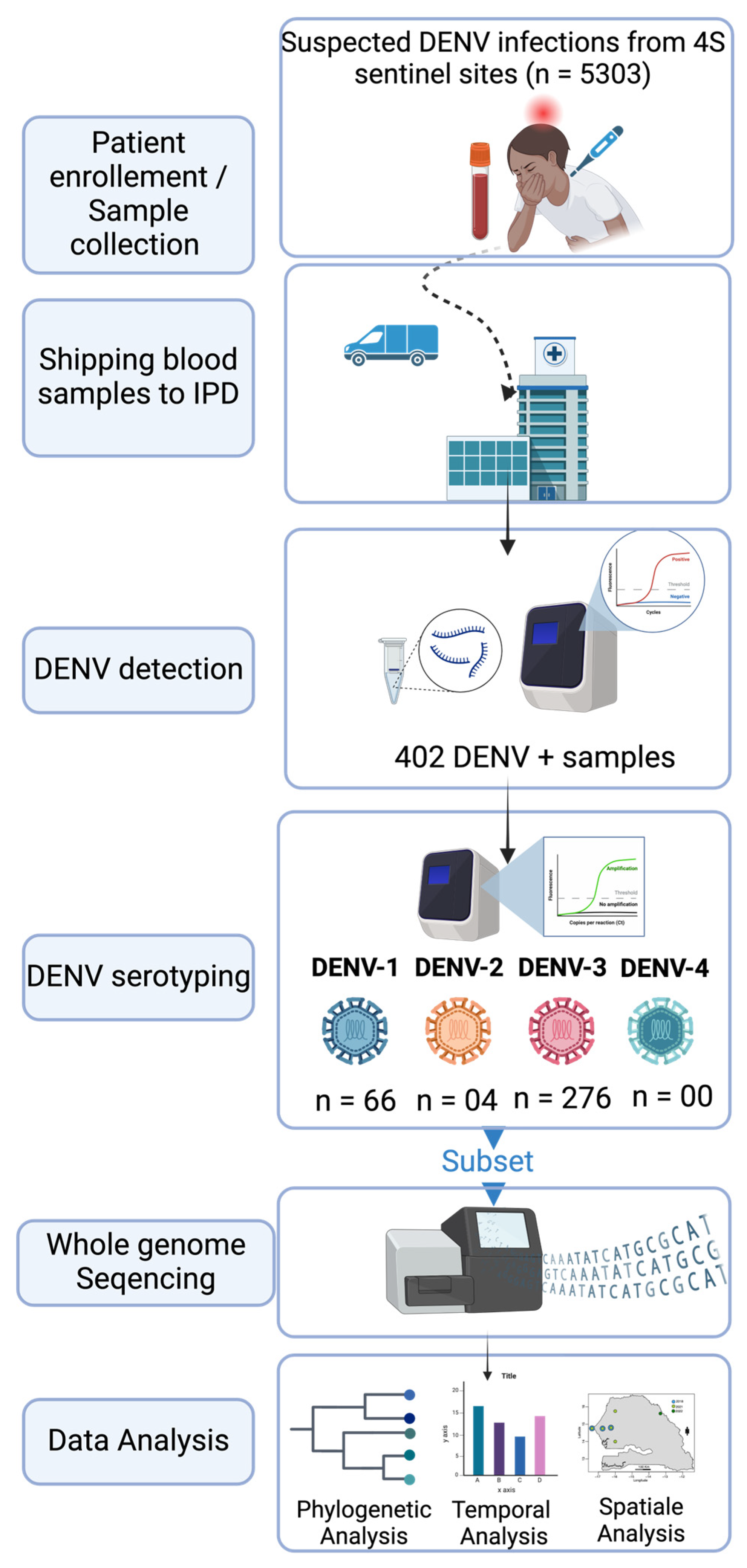
2.1. The Febrile Illnesses Surveillance System
2.2. Sample Shipping to WHO Collaborating Center for Arboviruses and Hemorrhagic Fevers at Institut Pasteur de Dakar
2.3. RNA Extraction
2.4. RT-qPCR DENV Detection
2.5. DENV Serotyping
2.6. cDNA Synthesis and Amplicons Generation
2.7. Library Preparation and Sequencing
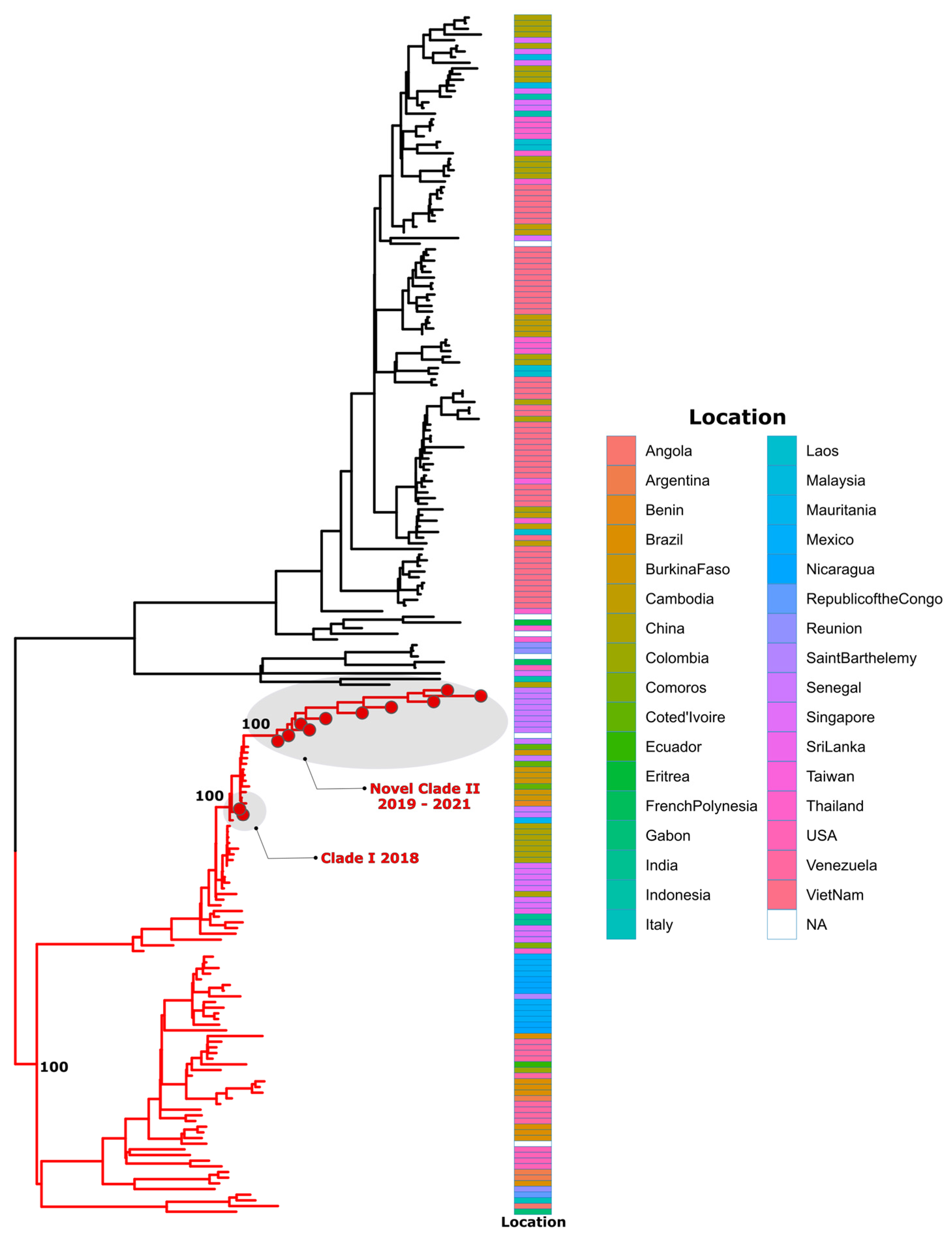
2.8. Dataset Construction and Phylogenetic Analysis
- (i)
- By using the Genome Detective dengue typing tool;
- (ii)
- By using a maximum likelihood (ML) phylogenetic analysis to put newly sequenced DENV strains in a global context and explore the relationship with other available global sequences.
2.9. Temporal Trend and Spatial Mapping of Detected Serotypes
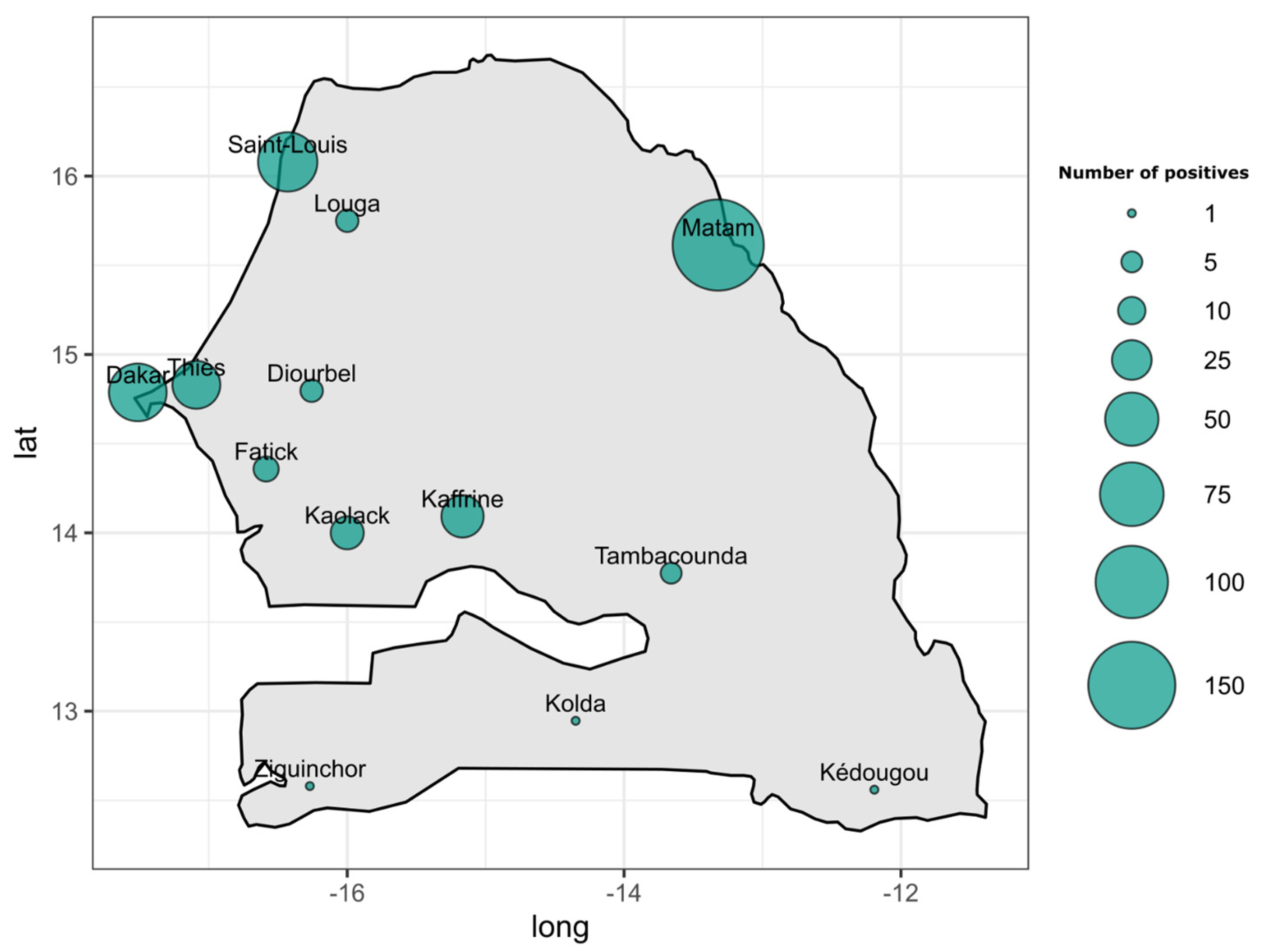
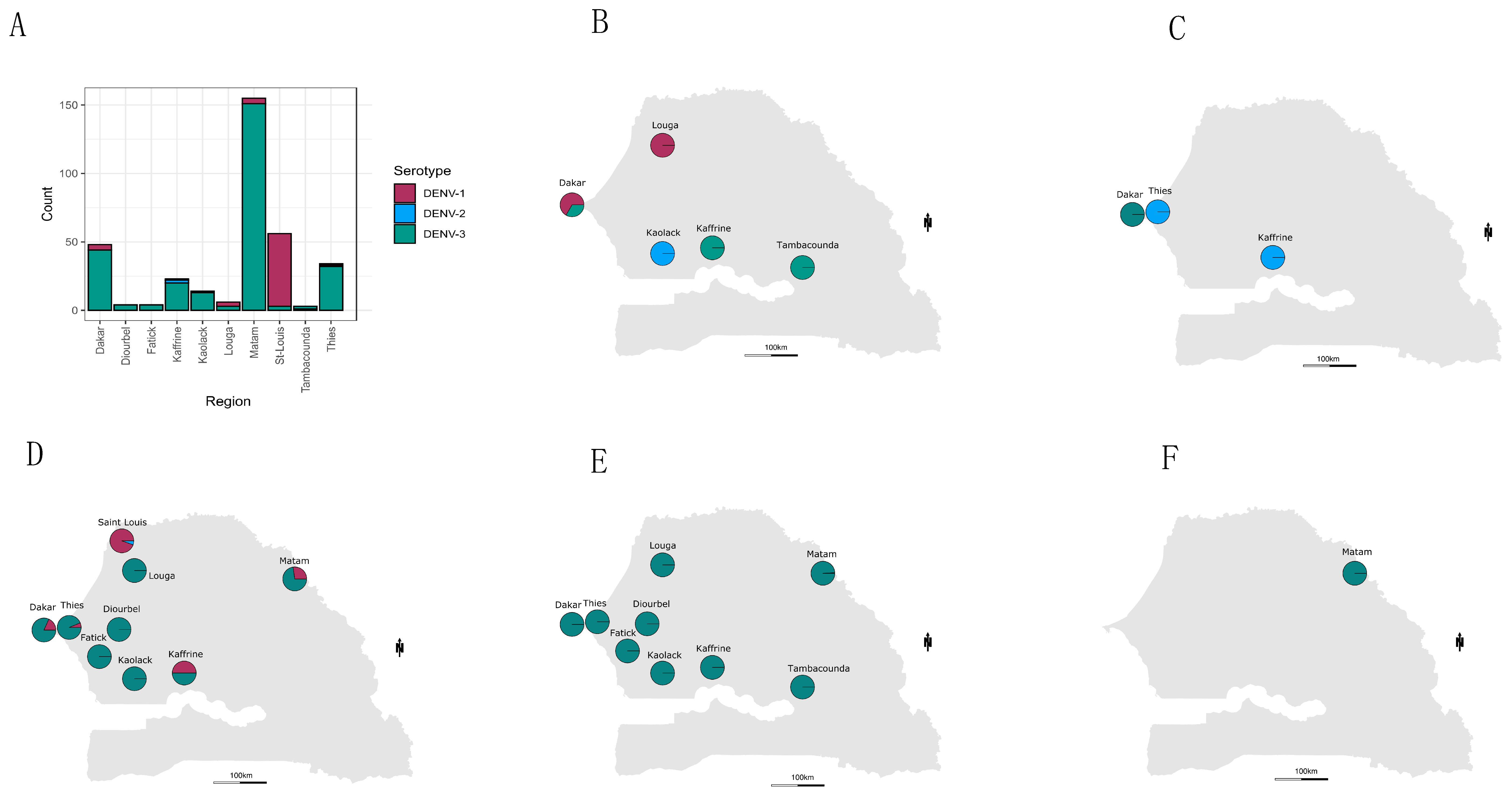
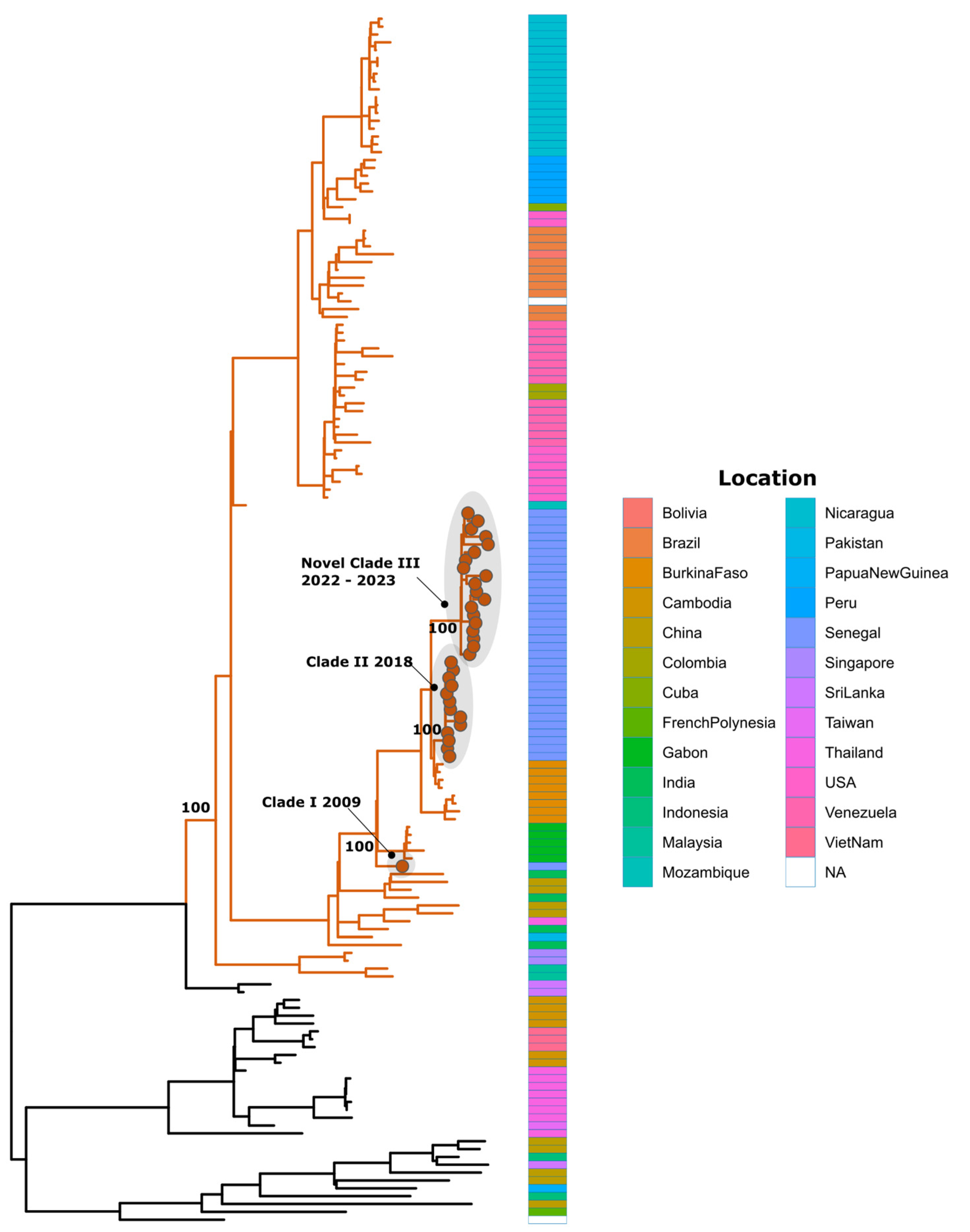
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Strategy for Dengue Prevention and Control, 2012–2020; World Health Organization: Geneva, Switzerland, 2012; Available online: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf (accessed on 12 September 2020).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- WHO. World Health Statistics 2012; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-156444-1. [Google Scholar]
- WHO/TDR. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control, New ed.; TDR/World Health Organization: Geneva, Switzerland, 2009; 147p. [Google Scholar]
- Fourié, T.; El Bara, A.; Dubot-Pérès, A.; Grard, G.; Briolant, S.; Basco, L.K.; Ouldabdallahi Moukah, M.; Leparc-Goffart, I. Emergence of dengue virus serotype 2 in Mauritania and molecular characterization of its circulation in West Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009829. [Google Scholar] [CrossRef]
- Amarasinghe, A.; Kuritsky, J.N.; Letson, G.W.; Margolis, H.S. Dengue Virus Infection in Africa. Emerg. Infect. Dis. 2011, 17, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Letizia, A.G.; Pratt, C.B.; Wiley, M.R.; Fox, A.T.; Mosore, M.; Agbodzi, B.; Yeboah, C.; Kumordjie, S.; Di Paola, N.; Assana, K.C.; et al. Retrospective Genomic Characterization of a 2017 Dengue Virus Outbreak, Burkina Faso. Emerg. Infect. Dis. 2022, 28, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Were, F. The dengue situation in Africa. Paediatr. Int. Child Health 2012, 32, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Ba, Y.; Sall, A.A.; Diop, O.M.; Ndione, J.A.; Mondo, M.; Girault, L.; Mathiot, C. Amplification of the Sylvatic Cycle of Dengue Virus Type 2, Senegal, 1999–2000: Entomologic Findings and Epidemiologic Considerations. Emerg. Infect. Dis. 2003, 9, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Diarra, M.; Diagne, M.M.; Faye, M.; Dior Ndione, M.H.; Ba, Y.; Diop, M.; Ndiaye, E.H.; Marinho de Andrade Zanotto, P.; Diop, B.; et al. Field Deployment of a Mobile Biosafety Laboratory Reveals the Co-Circulation of Dengue Viruses Serotype 1 and Serotype 2 in Louga City, Senegal, 2017. J. Trop. Med. 2021, 2021, 8817987. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Fall, C.; Barry, M.A.; Gaye, A.; Dia, N.; Ndione, M.H.D.; Fall, A.; Diop, M.; Sarr, F.D.; Ndiaye, O.; et al. Re-Emergence of Dengue Serotype 3 in the Context of a Large Religious Gathering Event in Touba, Senegal. IJERPH 2022, 19, 16912. [Google Scholar] [CrossRef]
- Gaye, A.; Ndiaye, T.; Sy, M.; Deme, A.B.; Thiaw, A.B.; Sene, A.; Ndiaye, C.; Diedhiou, Y.; Mbaye, A.M.; Ndiaye, I.; et al. Genomic investigation of a dengue virus outbreak in Thiès, Senegal, in 2018. Sci. Rep. 2021, 11, 10321. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Ndione, M.H.D.; Fall, C.; Diagne, M.M.; Diop, M.; Gaye, A.; Barry, M.A.; Diop, B.; Ndiaye, M.; Bousso, A.; et al. Multifoci and multiserotypes circulation of dengue virus in Senegal between 2017 and 2018. BMC Infect. Dis. 2021, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Ndiaye, M.; Ndione, M.H.; Sankhe, S.; Diagne, M.M.; Sagne, S.N.; Gaye, A.; Barry, A.; Fall, G.; Sall, A.A.; et al. Molecular Characterization of Circulating DENV-2 during Outbreak in Northern Senegal, Rosso 2018. Life Sci. 2021; preprints. [Google Scholar]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Twiddy, S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Usme-Ciro, J.A.; Méndez, J.A.; Laiton, K.D.; Páez, A. The relevance of dengue virus genotypes surveillance at country level before vaccine approval. Hum. Vaccines Immunother. 2014, 10, 2674–2678. [Google Scholar] [CrossRef]
- OhAinle, M.; Balmaseda, A.; Macalalad, A.R.; Tellez, Y.; Zody, M.C.; Saborío, S.; Nuñez, A.; Lennon, N.J.; Birren, B.W.; Gordon, A.; et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci. Transl. Med. 2011, 3, 114ra128. [Google Scholar] [CrossRef]
- Fried, J.R.; Gibbons, R.V.; Kalayanarooj, S.; Thomas, S.J.; Srikiatkhachorn, A.; Yoon, I.-K.; Jarman, R.G.; Green, S.; Rothman, A.L.; Cummings, D.A.T. Serotype-specific differences in the risk of dengue hemorrhagic fever: An analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl. Trop. Dis. 2010, 4, e617. [Google Scholar] [CrossRef]
- O’Connor, O.; Ou, T.P.; Aubry, F.; Dabo, S.; Russet, S.; Girault, D.; In, S.; Minier, M.; Lequime, S.; Hoem, T.; et al. Potential role of vector-mediated natural selection in dengue virus genotype/lineage replacements in two epidemiologically contrasted settings. Emerg. Microbes Infect. 2021, 10, 1346–1357. [Google Scholar] [CrossRef]
- Amoako, N.; Duodu, S.; Dennis, F.E.; Bonney, J.H.K.; Asante, K.P.; Ameh, J.; Mosi, L.; Hayashi, T.; Agbosu, E.E.; Pratt, D.; et al. Detection of Dengue Virus among Children with Suspected Malaria, Accra, Ghana. Emerg. Infect. Dis. 2018, 24, 1544–1547. [Google Scholar] [CrossRef]
- Ayolabi, C.I.; Olusola, B.A.; Ibemgbo, S.A.; Okonkwo, G.O. Detection of Dengue viruses among febrile patients in Lagos, Nigeria and phylogenetics of circulating Dengue serotypes in Africa. Infect. Genet. Evol. 2019, 75, 103947. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Sakamoto, T.; Sekizuka, T.; Kato, K.; Takasaki, T.; Kuroda, M. DGV: Dengue Genographic Viewer. Front. Microbiol. 2016, 7, 875. [Google Scholar] [CrossRef] [PubMed]
- Selhorst, P.; Lequime, S.; Dudas, G.; Proesmans, S.; Lutumba, P.; Katshongo, F.; Ramadan, K.; Micalessi, I.; Ahuka-Mundeke, S.; Vanlerberghe, V.; et al. Phylogeographic analysis of dengue virus serotype 1 and cosmopolitan serotype 2 in Africa. Int. J. Infect. Dis. 2023, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Tiraki, D.; Diwan, A.; Lalwani, S.K.; Modak, M.; Mishra, A.C.; Arankalle, V.A. Co-circulation of all the four dengue virus serotypes and detection of a novel clade of DENV-4 (genotype I) virus in Pune, India during 2016 season. PLoS ONE 2018, 13, e0192672. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Pattabiraman, C.; Sankaradoss, A.; Krishna, S.; Roy, R. Evolutionary dynamics of dengue virus in India. PLoS Pathog. 2023, 19, e1010862. [Google Scholar] [CrossRef] [PubMed]
- Poltep, K.; Phadungsombat, J.; Nakayama, E.E.; Kosoltanapiwat, N.; Hanboonkunupakarn, B.; Wiriyarat, W.; Shioda, T.; Leaungwutiwong, P. Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Trop. Med. 2021, 6, 162. [Google Scholar] [CrossRef]
- De Simone, T.S.; Nogueira, R.M.R.; Araújo, E.S.M.; Guimarães, F.R.; Santos, F.B.; Schatzmayr, H.G.; Souza, R.V.; Teixeira Filho, G.; Miagostovich, M.P. Dengue virus surveillance: The co-circulation of DENV-1, DENV-2 and DENV-3 in the State of Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 553–562. [Google Scholar] [CrossRef]
- Lim, J.K.; Carabali, M.; Camacho, E.; Velez, D.C.; Trujillo, A.; Egurrola, J.; Lee, K.-S.; Velez, I.D.; Osorio, J.E. Epidemiology and genetic diversity of circulating dengue viruses in Medellin, Colombia: A fever surveillance study. BMC Infect. Dis. 2020, 20, 466. [Google Scholar] [CrossRef]
- Dia, N.; Diene Sarr, F.; Thiam, D.; Faye Sarr, T.; Espié, E.; OmarBa, I.; Coly, M.; Niang, M.; Richard, V. Influenza-Like Illnesses in Senegal: Not Only Focus on Influenza Viruses. PLoS ONE 2014, 9, e93227. [Google Scholar] [CrossRef]
- Bob, N.S.; Barry, M.A.; Diagne, M.M.; Faye, M.; Ndione, M.H.D.; Diallo, A.; Diop, M.; Diop, B.; Faye, O.; Loucoubar, C.; et al. Detection of Rift Valley Fever Virus Lineage H From South Africa Through the Syndromic Sentinel Surveillance Network in Senegal. Open Forum Infect. Dis. 2022, 9, ofab655. [Google Scholar] [CrossRef]
- Wagner, D.; de With, K.; Huzly, D.; Hufert, F.; Weidmann, M.; Breisinger, S.; Eppinger, S.; Kern, W.V.; Bauer, T.M. Nosocomial Acquisition of Dengue. Emerg. Infect. Dis. 2004, 10, 1872–1873. [Google Scholar] [CrossRef]
- Dieng, I.; Cunha, M.D.P.; Diagne, M.M.; Sembène, P.M.; Zanotto, P.M.D.A.; Faye, O.; Faye, O.; Sall, A.A. Origin and Spread of the Dengue Virus Type 1, Genotype V in Senegal, 2015–2019. Viruses 2021, 13, 57. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and Clinical Performance of the CDC Real Time RT-PCR Assay for Detection and Typing of Dengue Virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Dieng, I.; Diallo, A.; Ndiaye, M.; Mhamadi, M.; Diagne, M.M.; Sankhe, S.; Ndione, M.H.D.; Gaye, A.; Sagne, S.N.; Heraud, J.M.; et al. Full genome analysis of circulating DENV-2 in Senegal reveals a regional diversification into separate clades. J. Med. Virol. 2022, 94, 5593–5600. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fall, A.; Dieng, I.; Touré, C.T.; Mhamadi, M.; Sadio, B.D.; Ndione, M.H.D.; Diagne, M.M.; Ndiaye, M.; Barry, M.A.; Diaw, Y.; et al. Institut Pasteur Dakar Mobile Lab: Part of the Solution to Tackle COVID Pandemic in Senegal, a Model to Be Exploited. COVID 2022, 2, 1509–1517. [Google Scholar] [CrossRef]
- Diagne, M.M.; Ndione, M.H.D.; Gaye, A.; Barry, M.A.; Diallo, D.; Diallo, A.; Mwakibete, L.L.; Diop, M.; Ndiaye, E.H.; Ahyong, V.; et al. Yellow Fever Outbreak in Eastern Senegal, 2020–2021. Viruses 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Mutai, N.; Heath, C.; Ndenga, B.; Labeaud, A.D. Dengue Viremia in Kenyan children With Acute Febrile Illness. Open Forum Infect. Dis. 2016, 3 (Suppl. S1), 597. [Google Scholar] [CrossRef]
- Alagarasu, K.; Patil, J.A.; Kakade, M.B.; More, A.M.; Yogesh, B.; Newase, P.; Jadhav, S.M.; Parashar, D.; Kaur, H.; Gupta, N.; et al. Serotype and genotype diversity of dengue viruses circulating in India: A multi-centre retrospective study involving the Virus Research Diagnostic Laboratory Network in 2018. Int. J. Infect. Dis. 2021, 111, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Hammon, W.M. Dengue Hemorrhagic Fever—Do We Know Its Cause? Am. J. Trop. Med. Hyg. 1973, 22, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Immune enhancement of viral infection. Prog. Allergy 1982, 31, 301–364. [Google Scholar] [PubMed]
- Cecilia, D.; Patil, J.A.; Kakade, M.B.; Walimbe, A.; Alagarasu, K.; Anukumar, B.; Abraham, A. Emergence of the Asian genotype of DENV-1 in South India. Virology 2017, 510, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Brunette, G.W.; Nemhauser, J.B. Travel-Related Infectious Diseases. In CDC Yellow Book 2020; Oxford University Press: Oxford, UK, 2019; pp. 169–394. ISBN 978-0-19-092893-3. [Google Scholar]
- Diagne, C.T.; Barry, M.A.; Ba, Y.; Faye, O.; Sall, A.A. Dengue epidemic in Touba, Senegal: Implications for the Grand Magal Pilgrimage for travellers. J. Travel Med. 2019, 26, tay123. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, A.C.; Fernandez, Z.; Ortiz, D.; Diallo, M.; Sall, A.; Hartman, S.; Davis, C.T.; Coffey, L.; Mathiot, C.C.; Tesh, R.B.; et al. Dengue Emergence and Adaptation to Peridomestic Mosquitoes. Emerg. Infect. Dis. 2004, 10, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Grard, G.; Paupy, C.; Mombo, I.M.; Bikie Bi Nso, B.; Kassa Kassa, F.R.; Nkoghe, D.; Leroy, E.M. First Evidence of Simultaneous Circulation of Three Different Dengue Virus Serotypes in Africa. PLoS ONE 2013, 8, e78030. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, H.; Moreno, K.; Lima, I.A.B.; Santos, C.S.; Costa, B.G.G.; De Almeida, B.L.; Dos Santos, R.A.; Francisco, M.V.L.D.O.; Sampaio, M.P.S.; De Lima, M.M.; et al. Phylogenetic Reconstructions Reveal the Circulation of a Novel Dengue Virus-1V Clade and the Persistence of a Dengue Virus-2 III Genotype in Northeast Brazil. Viruses 2023, 15, 1073. [Google Scholar] [CrossRef]
- Racherla, R.G.; Pamireddy, M.L.; Mohan, A.; Mudhigeti, N.; Mahalakshmi, P.A.; Nallapireddy, U.; Kalawat, U. Co-circulation of four dengue serotypes at South Eastern Andhra Pradesh, India: A prospective study. Indian J. Med. Microbiol. 2018, 36, 236–240. [Google Scholar] [CrossRef]
- Suzuki, K.; Phadungsombat, J.; Nakayama, E.E.; Saito, A.; Egawa, A.; Sato, T.; Rahim, R.; Hasan, A.; Lin, M.Y.-C.; Takasaki, T.; et al. Genotype replacement of dengue virus type 3 and clade replacement of dengue virus type 2 genotype Cosmopolitan in Dhaka, Bangladesh in 2017. Infect. Genet. Evol. 2019, 75, 103977. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; Van Den Hurk, A.F. An Explosive Epidemic of DENV-3 in Cairns, Australia. PLoS ONE 2013, 8, e68137. [Google Scholar] [CrossRef]
- Quintero-Gil, D.C.; Uribe-Yepes, A.; Ospina, M.; Díaz, F.J.; Martinez-Gutierrez, M. Differences in the replicative capacities of clinical isolates of dengue virus in C6/36 cells and in urban populations of Aedes aegypti from Colombia, South America. Braz. J. Infect. Dis. 2018, 22, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Holmes, E.C.; Zhang, C.; Mammen, M.P.; Nimmannitya, S.; Kalayanarooj, S.; Boots, M. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc. Natl. Acad. Sci. USA 2006, 103, 14234–14239. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Barry, M.A.; Talla, C.; Sow, B.; Faye, O.; Diagne, M.M.; Sene, O.; Ndiaye, O.; Diop, B.; Diagne, C.T.; et al. Analysis of a Dengue Virus Outbreak in Rosso, Senegal 2021. Trop. Med. Infect. Dis. 2022, 7, 420. [Google Scholar] [CrossRef]


| ID | Virus Type | Genotype | Region | Isolate | Sample Type | Collection Date | Depth of Coverage | Genome Coverage (%) |
|---|---|---|---|---|---|---|---|---|
| SH 377553 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 29 October 2021 | 1354.2 | 91.2 |
| SH 377552 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 29 October 2021 | 3207.7 | 91.2 |
| SH 377551 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 28 October 2021 | 162.9 | 58.3 |
| SH 377538 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 1 November 2021 | 2043.9 | 89.4 |
| SH 377555 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 29 October 2021 | 6465 | 93.7 |
| SH 377554 | DENV-1 | Genotype V | Saint-Louis | Human | Serum | 29 October 2021 | 3079 | 93.6 |
| SH 322838 | DENV-1 | Genotype V | Dakar | Human | Serum | 21 October 2019 | 1762.7 | 93.2 |
| SH 322765 | DENV-1 | Genotype V | Dakar | Human | Serum | 15 October 2021 | 2583.8 | 93.4 |
| SH 318479 | DENV-1 | Genotype V | Louga | Human | Serum | 24 January 2019 | 1045.8 | 88.8 |
| SH 318478 | DENV-1 | Genotype V | Louga | Human | Serum | 24 January 2019 | 2372.3 | 93.7 |
| SH 318267 | DENV-1 | Genotype V | Louga | Human | Serum | 10 January 2019 | 4266 | 93.6 |
| SH 326097 | DENV-2 | Genotype II | Kaffrine | Human | Serum | 30 September 2020 | 5691.0 | 75.6 |
| SH 323592 | DENV-2 | Genotype II | Thies | Human | Serum | 5 January 2020 | 4893.3 | 81.5 |
| SH 377545 | DENV-3 | Genotype III | Saint-Louis | Human | Serum | 27 October 2021 | 6991.1 | 94.7 |
| SH 377540 | DENV-3 | Genotype III | Saint-Louis | Human | Serum | 26 October 2021 | 2533.1 | 89.4 |
| SH 377524 | DENV-3 | Genotype III | Thies | Human | Serum | 28 October 2021 | 5318 | 98 |
| SH 377522 | DENV-3 | Genotype III | Thies | Human | Serum | 28 October 2021 | 4902.2 | 96.7 |
| SH 392244 | DENV-3 | Genotype III | NA | Human | Serum | NA | 2670 | 93.4 |
| SH 402404 | DENV-3 | Genotype III | Fatick | Human | Serum | 22 August 2022 | 4915 | 93.4 |
| SH 392518 | DENV-3 | Genotype III | Tambacounda | Human | Serum | 19 March 2022 | 5708.4 | 94.8 |
| SH 392408 | DENV-3 | Genotype III | Matam | Human | Serum | 15 March 2022 | 3506.2 | 93.4 |
| SH 392406 | DENV-3 | Genotype III | Matam | Human | Serum | 15 March 2022 | 3946.5 | 93.4 |
| SH 392265 | DENV-3 | Genotype III | Matam | Human | Serum | 25 February 2022 | 4319.5 | 95.7 |
| SH 392263 | DENV-3 | Genotype III | Matam | Human | Serum | 25 February 2022 | 3527.8 | 93.4 |
| SH 392260 | DENV-3 | Genotype III | Matam | Human | Serum | 23 February 2022 | 3906.5 | 96.4 |
| SH 392169 | DENV-3 | Genotype III | Matam | Human | Serum | 22 February 2022 | 4515 | 94.7 |
| SH 392168 | DENV-3 | Genotype III | Matam | Human | Serum | 21 February 2022 | 5482.1 | 95.9 |
| SH 330006 | DENV-3 | Genotype III | Dakar | Human | Serum | 3 November 2020 | 5740.9 | 93.6 |
| SH 330004 | DENV-3 | Genotype III | Dakar | Human | Serum | 3 November 2020 | 2271.8 | 71.7 |
| SH 329067 | DENV-3 | Genotype III | NA | Human | Serum | NA | 1966.4 | 91.6 |
| SH 327002 | DENV-3 | Genotype III | Dakar | Human | Serum | 12 October 2020 | 2597.6 | 89.8 |
| SH 392572 | DENV-3 | Genotype III | Matam | Human | Serum | 21 March 2022 | 6.4 | 52.4 |
| SH 392571 | DENV-3 | Genotype III | Matam | Human | Serum | 22 March 2022 | 5843.5 | 95.9 |
| SH 323342 | DENV-3 | Genotype III | Kaffrine | Human | Serum | 10 December 2019 | 10,262.1 | 95 |
| SH 322872 | DENV-3 | Genotype III | Kaffrine | Human | Serum | 28 October 2019 | 6151.3 | 94.4 |
| Year of Collection | Number of Suspected Cases | Number of Confirmed DENV Cases | Positivity Rate (%) |
|---|---|---|---|
| 2019 | 890 | 19 | 2.13 |
| 2020 | 862 | 20 | 2.32 |
| 2021 | 1353 | 131 | 9.68 |
| 2022 | 2089 | 216 | 10.33 |
| 2023 ** | 109 | 15 | 13.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieng, I.; Talla, C.; Barry, M.A.; Gaye, A.; Balde, D.; Ndiaye, M.; Kane, M.; Sagne, S.N.; Diagne, M.M.; Diop, B.; et al. The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023. Trop. Med. Infect. Dis. 2024, 9, 32. https://doi.org/10.3390/tropicalmed9020032
Dieng I, Talla C, Barry MA, Gaye A, Balde D, Ndiaye M, Kane M, Sagne SN, Diagne MM, Diop B, et al. The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023. Tropical Medicine and Infectious Disease. 2024; 9(2):32. https://doi.org/10.3390/tropicalmed9020032
Chicago/Turabian StyleDieng, Idrissa, Cheikh Talla, Mamadou Aliou Barry, Aboubacry Gaye, Diamilatou Balde, Mignane Ndiaye, Mouhamed Kane, Samba Niang Sagne, Moussa Moise Diagne, Boly Diop, and et al. 2024. "The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023" Tropical Medicine and Infectious Disease 9, no. 2: 32. https://doi.org/10.3390/tropicalmed9020032
APA StyleDieng, I., Talla, C., Barry, M. A., Gaye, A., Balde, D., Ndiaye, M., Kane, M., Sagne, S. N., Diagne, M. M., Diop, B., Diallo, B., Sall, A. A., Faye, O., Sow, A., Fall, G., Loucoubar, C., & Faye, O. (2024). The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023. Tropical Medicine and Infectious Disease, 9(2), 32. https://doi.org/10.3390/tropicalmed9020032







