Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador
Abstract
1. Introduction
2. Materials and Methods
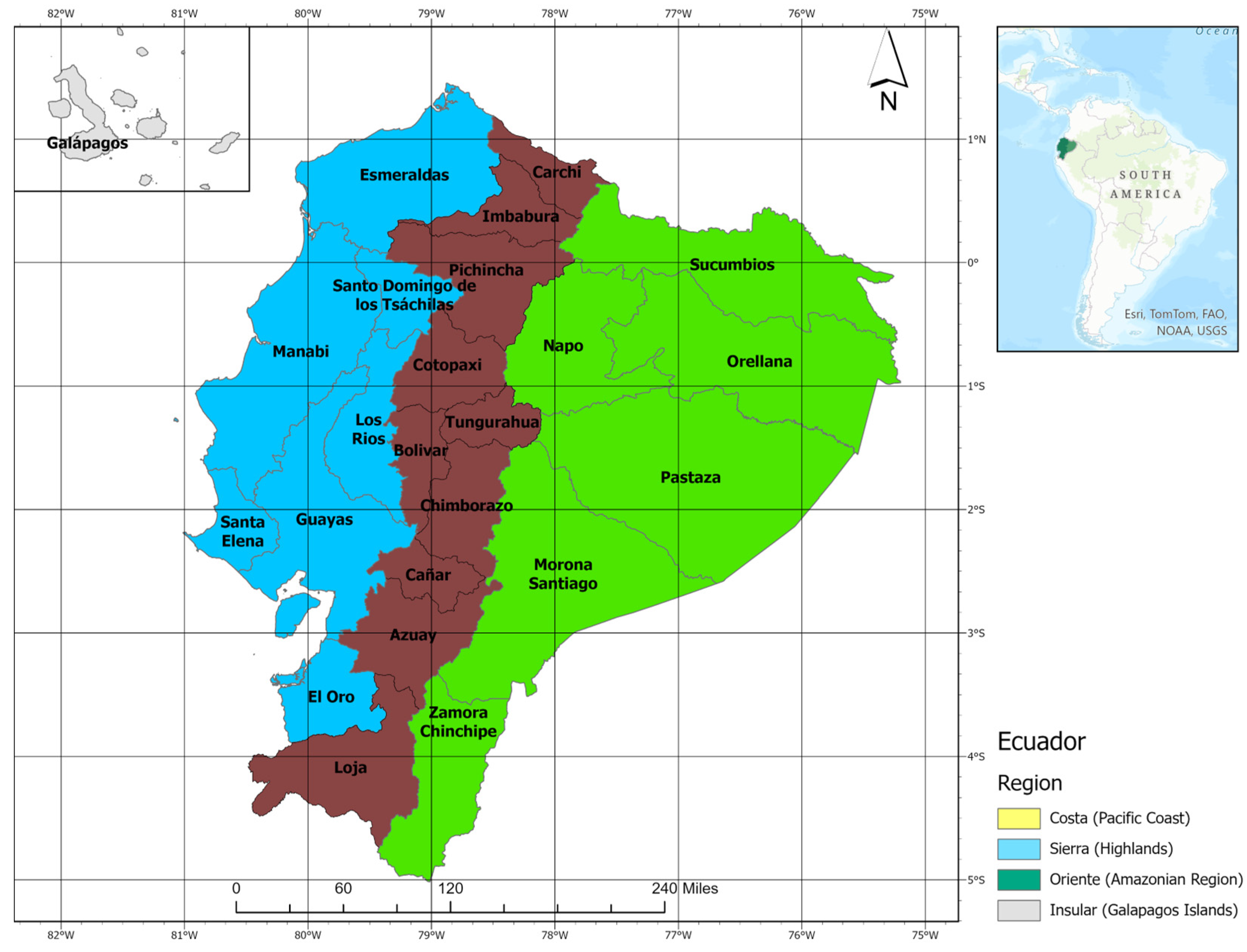
2.1. Study Area
2.2. Study Subjects
2.3. Statistical Analysis
2.4. Spatial and Spatiotemporal Analysis
2.5. Ethics Statement
3. Results
3.1. Study Population
3.2. Epidemiological Analysis
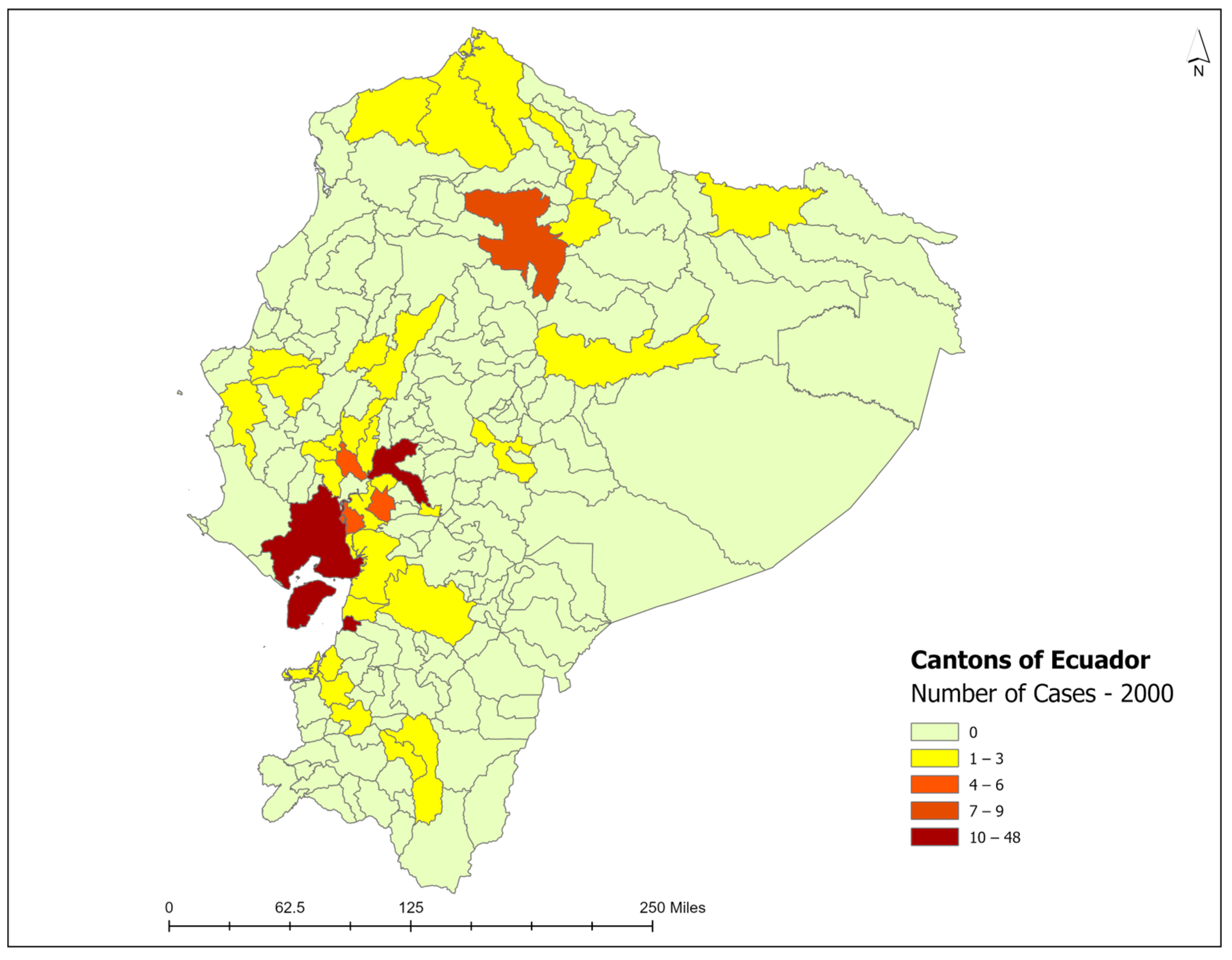
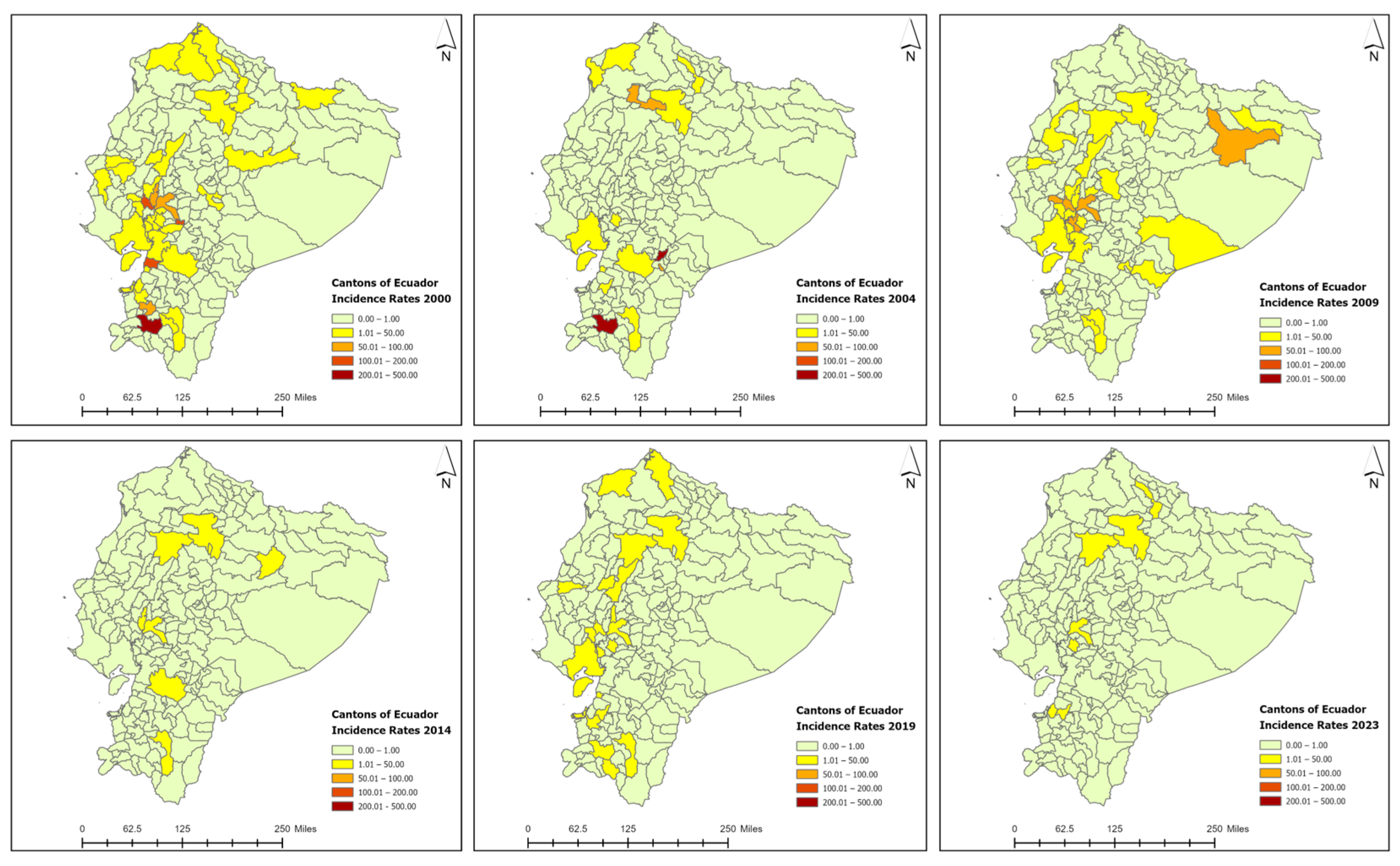
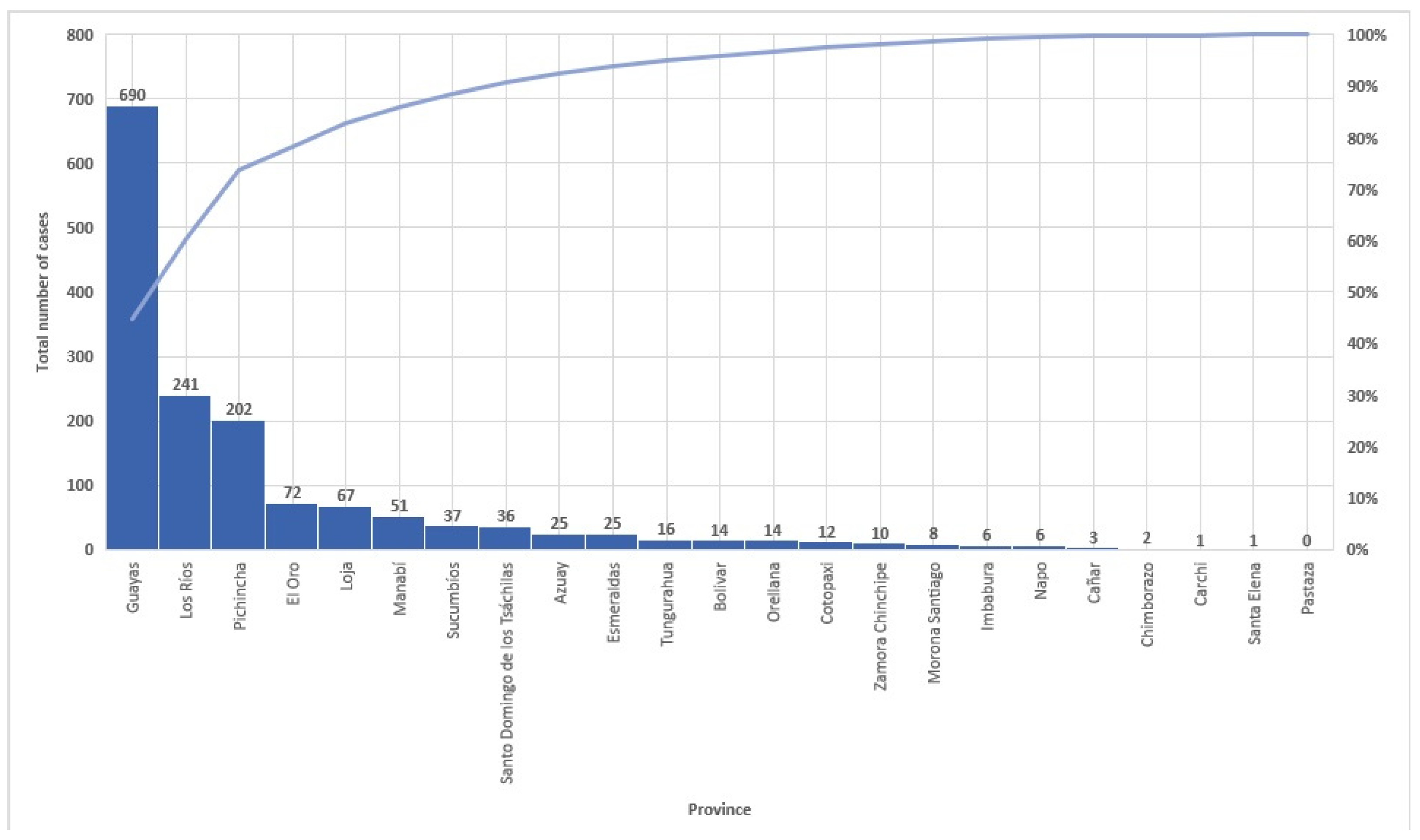
3.3. Spatiotemporal Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). About Hansen’s Disease (Leprosy); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/leprosy/about/index.html (accessed on 8 August 2024).
- World Health Organization (WHO). Leprosy; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 22 January 2022).
- Rodrigues, L.C.; Lockwood, D. Leprosy now: Epidemiology, progress, challenges, and research gaps. Lancet Infect. Dis. 2011, 11, 464–470. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Committee on Leprosy: Eight Report; WHO Technical Report Series 968; World Health Organization: Geneva, Switzerland, 2012; pp. 1–61. Available online: https://iris.who.int/handle/10665/75151 (accessed on 15 October 2023).
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Mycobact. Dis. 1966, 34, 255–273. [Google Scholar]
- World Health Organization (WHO). Guidelines for Diagnosis, Treatment and Prevention of Leprosy; World Health Organization, Regional Office of South-East Asia: New Delhi, India, 2018; Available online: https://www.who.int/publications/i/item/9789290226383 (accessed on 15 October 2023).
- Lastória, J.C.; Abreu, M.A. Leprosy: Review of the epidemiological, clinical, and etiopathogenic aspects—Part 1. An. Bras. Dermatol. 2014, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Leprosy (Hansen Disease) Update, 2020: Impact of COVID-19 on Global Leprosy Control 36; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/who-wer9636-421-444 (accessed on 26 October 2023).
- de Souza, E.A.; Fuentes Ferreira, A.; Heukelbach, J.; Nzundu Boigny, R.; Alencar, C.H.; Novaes Ramos, A., Jr. Epidemiology and Spatiotemporal Patterns of Leprosy Detection in the State of Bahia, Brazilian Northeast Region, 2001–2014. Trop. Med. Infect. Dis. 2018, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shui, T.J. The state of the leprosy epidemic in Yunnan, China 2011–2020: A spatial and spatiotemporal analysis, highlighting areas for intervention. PLoS Negl. Trop. Dis. 2021, 15, e0009783. [Google Scholar] [CrossRef]
- Freitas, L.R.S.; Duarte, E.C.; Garcia, L.P. Analysis of the epidemiological situation of leprosy in an endemic area in Brazil: Spatial distribution in the periods 2001–2003 and 2010–2012. Rev. Bras. Epidemiol. 2017, 20, 702–713. [Google Scholar] [CrossRef]
- Marciano, L.; Belone, A.F.F.; Rosa, P.S.; Coelho, N.M.B.; Ghidella, C.C.; Nardi, S.M.T.; Miranda, W.C.; Barrozo, L.V.; Lastoria, J.C. Epidemiological and geographical characterization of leprosy in a Brazilian hyperendemic municipality. Cad. Saude Publica 2018, 34, e00197216. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.; Balagon, M.V.; Orcullo, F.M.; Maghanoy, A.A.; Abellana, J.; Saunderson, P.R. A retrospective study of the epidemiology of leprosy in Cebu: An eleven-year profile. PLoS Negl. Trop. Dis. 2013, 7, e2444. [Google Scholar] [CrossRef]
- Matos, A.M.F.; Coelho, A.C.O.; Araujo, L.P.T.; Alves, M.J.M.; Baquero, O.S.; Duthie, M.S.; Teixeira, H.C. Assessing epidemiology of leprosy and socio-economic distribution of cases. Epidemiol. Infect. 2018, 146, 1750–1755. [Google Scholar] [CrossRef]
- Moet, F.J.; Pahan, D.; Schuring, R.P.; Oskam, L.; Richardus, J.H. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J. Infect. Dis. 2006, 193, 346–353. [Google Scholar] [CrossRef]
- Oktaria, S.; Hurif, N.S.; Naim, W.; Thio, H.B.; Nijsten, T.E.C.; Richardus, J.H. Dietary diversity and poverty as risk factors for leprosy in Indonesia: A case-control study. PLoS Negl. Trop. Dis. 2018, 12, e0006317. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, I.; van Muiden, L.; Alam, K.; Bowers, R.; Hossain, M.A.; Kispotta, K.; Richardus, J.H. Diet-related risk factors for leprosy: A case-control study. PLoS Negl. Trop. Dis. 2015, 9, e0003766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pontarollo, N.; Orellana, M.; Segovia, J. The Determinants of Subjective Well-Being in a Developing Country: The Ecuadorian Case. J. Happiness Stud. 2020, 21, 3007–3035. [Google Scholar] [CrossRef]
- Ministerio de Salud Pública del Ecuador. Manual De Procedimientos Del Subsistema Alerta Accion Sive—Alerta; Ministerio de Salud Pública del Ecuador: Quito, Ecuador, 2013; Available online: https://dspace.uniandes.edu.ec/bitstream/123456789/12315/1/Manual_de_procedimientos_del_subsistema_alerta_accion_sive.pdf (accessed on 26 April 2023).
- World Bank Group (World Bank). Ecuador; World Bank Group: Washington, DC, USA, 2024; Available online: https://data.worldbank.org/country/ecuador (accessed on 2 February 2024).
- Instituto Nacional de Estadísticas y Censos (INEC). Censo Ecuador Cuenta Conmigo; Instituto Nacional de Estadísticas y Censos (INEC): Quito, Ecuador, 2022; Available online: https://www.censoecuador.gob.ec/wp-content/uploads/2023/10/Presentacio%CC%81n_Nacional_1%C2%B0entrega-4.pdf (accessed on 21 November 2023).
- Ogunsumi, D.O.; Lal, V.; Puchner, K.P.; van Brakel, W.; Schwienhorst-Stich, E.M.; Kasang, C.; Chukwu, J.; Kreibich, S.; Parisi, S.; Richardus, J.H.; et al. Measuring endemicity and burden of leprosy across countries and regions: A systematic review and Delphi survey. PLoS Negl. Trop. Dis. 2021, 15, e0009769. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Griffith, D.; Chun, Y. Spatial Autocorrelation and Spatial Filtering. In Handbook of Regional Science; Fischer, M.M., Nijkamp, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1477–1507. [Google Scholar]
- Blum Gutiérrez, E. Program for the control of leprosy in Ecuador. Rev. Ecuat. Hig. Med. Trop. 1966, 23, 183–188. [Google Scholar]
- World Health Organization. Global Leprosy (Hansen Disease) Update, 2021: Moving Twards Interruption of Transmission; WER No. 36; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/who-wer9736-429-450 (accessed on 21 November 2023).
- Barreto, J.G.; Frade, M.A.C.; Bernardes Filho, F.; da Silva, M.B.; Spencer, J.S.; Salgado, C.G. Leprosy in Children. Curr. Infect. Dis. Rep. 2017, 19, 23. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). La lepran en las Américas. Bol. Epidemiol. 1983, 4, 1–5. [Google Scholar]
- Middleton, D.R. Migration and Urbanization in Ecuador: A View from the Coast. Urban Anthropol. 1979, 8, 313–332. [Google Scholar]
- Royuela, V.; Ordóñez, J. Internal migration in a developing country: A panel data analysis of Ecuador (1982–2010). Pap. Reg. Sci. 2018, 97, 345–368. [Google Scholar] [CrossRef]
- Schaub, R.; Avanzi, C.; Singh, P.; Paniz-Mondolfi, A.; Cardona-Castro, N.; Legua, P.; Crespo, L.; Sewpersad, K.; Dávila, J.J.; Barreto, J.; et al. Leprosy Transmission in Amazonian Countries: Current Status and Future Trends. Curr. Trop. Med. Rep. 2020, 7, 79–91. [Google Scholar] [CrossRef]
- Truman Richard, W.; Singh, P.; Sharma, R.; Busso, P.; Rougemont, J.; Paniz-Mondolfi, A.; Kapopoulou, A.; Brisse, S.; Scollard David, M.; Gillis Thomas, P.; et al. Probable Zoonotic Leprosy in the Southern United States. N. Engl. J. Med. 2011, 364, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Superina, M.; Loughry, W.J. Life on the Half-Shell: Consequences of a Carapace in the Evolution of Armadillos (Xenarthra: Cingulata). J. Mamm. Evol. 2012, 19, 217–224. [Google Scholar] [CrossRef]
- Pescarini, J.M.; Strina, A.; Nery, J.S.; Skalinski, L.M.; Andrade, K.V.; Penna, M.L.; Brickley, E.B.; Rodrigues, L.C.; Barreto, M.L.; Penna, G.O. Socioeconomic risk markers of leprosy in high-burden countries: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006622. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Reis, A.L.; Margalho, L.P.; Lopes, G.L.; Silva, A.R.; Moraes, N.S.; Xavier, M.B. Leprosy in elderly people and the profile of a retrospective cohort in an endemic region of the Brazilian Amazon. PLoS Negl. Trop. Dis. 2019, 13, e0007709. [Google Scholar] [CrossRef]
- Miranzi Sde, S.; Pereira, L.H.; Nunes, A.A. Epidemiological profile of leprosy in a Brazilian municipality between 2000 and 2006. Rev. Soc. Bras. Med. Trop. 2010, 43, 62–67. [Google Scholar] [CrossRef]
- Kaimal, S.; Thappa, D.M. Relapse in leprosy. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 126–135. [Google Scholar] [CrossRef]
- Ramu, G. Clinical features and diagnosis of relapses in leprosy. Indian J. Lepr. 1995, 67, 45–59. [Google Scholar]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [CrossRef]
- Arunraghav, P.; Herakal, K. Leprosy in Elderly and Children among New Cases—A 3-Year Retrospective Study. Indian Dermatol Online J. 2021, 12, 294–297. [Google Scholar] [CrossRef]
- United Nations Population Fund (UNFPA). El potencial y los desafíos de Ecuador; United Nations Population Fund: New York, NY, USA, 2024; Available online: https://ecuador.unfpa.org/es/el-potencial-y-los-desaf%C3%ADos-de-ecuador#:~:text=El%2064%25%20vive%20en%20el,36%25%20en%20el%20sector%20rural (accessed on 6 May 2024).
- Ministerio de Salud Pública del Ecuador (MSP). Sistema de Vigilancia en la Salud Pública Ecuador; Ministerio de Salud Pública del Ecuador: Quito, Ecuador, 2016; Available online: https://www.salud.gob.ec/direccion-nacional-de-vigilancia-epidemiologica/ (accessed on 2 January 2024).
- World Health Organization (WHO). Leprosy; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy#:~:text=Elimination%20of%20leprosy%20as%20a,in%20most%20countries%20by%202010 (accessed on 2 January 2024).
- Pan American Health Organization (PAHO). Action Plan to Further Advance Towards Leprosy Elimination in Latin America and the Caribbean: Action Lines for Reaching Regional Goals and Sustaining Achievements, 2012–2015; Pan American Health Organization: Washington, DC, USA, 2011; Available online: https://www.paho.org/en/documents/action-plan-further-advance-towards-leprosy-elimination-latin-america-and-caribbean (accessed on 2 January 2024).
- Pan American Health Organization. 49th Directing Council 61st Session of the Regional Committee, Elimination of Neglected Diseases and Other Poverty-Related Infections; World Health Organization: Washington, DC, USA, 2009. [Google Scholar]
- Pan American Health Organization (PAHO). Plan de Acción para Acelerar el Logro de la Eliminación de la Lepra en América Latina y el Caribe; Pan American Health Organization: Washington, DC, USA, 2011; Available online: https://www.paho.org/es/documentos/plan-accion-para-acelerar-logro-eliminacion-lepra-america-latina-caribe-2011 (accessed on 21 November 2023).
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki-Nakashimada, M.A.; Unzueta, A.; Berenise Gámez-González, L.; González-Saldaña, N.; Sorensen, R.U. BCG: A vaccine with multiple faces. Hum. Vaccines Immunother. 2020, 16, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud Pública (MSP). Lineamientos de la Campaña de Vacunación y Recuperación del Esquema Regular; Ministerio de Salud Pública: Quito, Ecuador, 2021; Available online: https://www.salud.gob.ec/wp-content/uploads/2022/04/Lineamiento_plan_recuperacion_de_vacunacion_version_30_09_2021-signed-signed-signed.pdf (accessed on 30 July 2024).
- World Health Organization (WHO). Towards Zero Leprosy; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789290228509 (accessed on 11 August 2024).
- Instituto Nacional de Estadísticas y Censos (INEC). Censo Ecuador; Instituto Nacional de Estadísticas y Censos: Quito, Ecuador, 2024; Available online: https://www.ecuadorencifras.gob.ec/estadisticas/ (accessed on 12 January 2024).
- Chaves, E.C.; Costa, S.V.; Flores, R.; Neves, E. Social deprivation index and leprosy in Pará State, Brazil, in 2013: Spatial analysis. Epidemiol. Serv. Saude 2017, 26, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Grantz, K.H.; Chabaari, W.; Samuel, R.K.; Gershom, B.; Blum, L.; Worden, L.; Ackley, S.; Liu, F.; Lietman, T.M.; Galvani, A.P.; et al. Spatial distribution of leprosy in India: An ecological study. Infect. Dis. Poverty 2018, 7, 20. [Google Scholar] [CrossRef]
- Silva, C.L.M.; Fonseca, S.C.; Kawa, H.; Palmer, D.O.Q. Spatial distribution of leprosy in Brazil: A literature review. Rev. Soc. Bras. Med. Trop. 2017, 50, 439–449. [Google Scholar] [CrossRef]
- Ortuño-Gutiérrez, N.; Mzembaba, A.; Ramboarina, S.; Andriamira, R.; Baco, A.; Braet, S.; Younoussa, A.; Cauchoix, B.; Salim, Z.; Amidy, M.; et al. Exploring clustering of leprosy in the Comoros and Madagascar: A geospatial analysis. Int. J. Infect. Dis. 2021, 108, 96–101. [Google Scholar] [CrossRef]
- Castro, S.S.; Santos, J.P.; Abreu, G.B.; Oliveira, V.R.; Fernandes, L.F. Leprosy incidence, characterization of cases and correlation with household and cases variables of the Brazilian states in 2010. An. Bras. Dermatol. 2016, 91, 28–33. [Google Scholar] [CrossRef]
- Phillips, D.A.; Ferreira, J.A.; Ansah, D.; Teixeira, H.S.; Kitron, U.; Filippis, T.; Alcântara, M.H.; Fairley, J.K. A tale of two neglected tropical infections: Using GIS to assess the spatial and temporal overlap of schistosomiasis and leprosy in a region of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2017, 112, 275–280. [Google Scholar] [CrossRef]


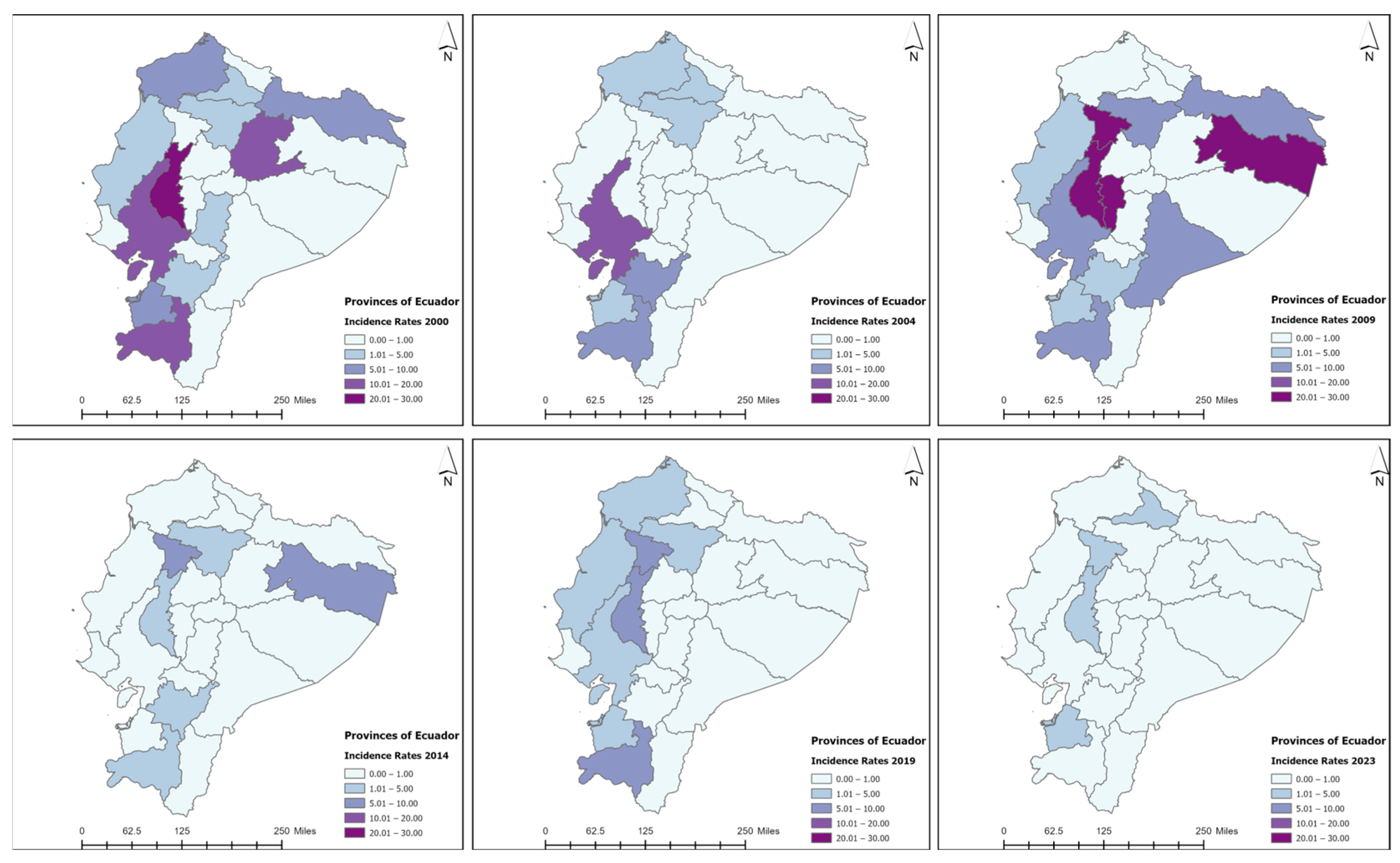
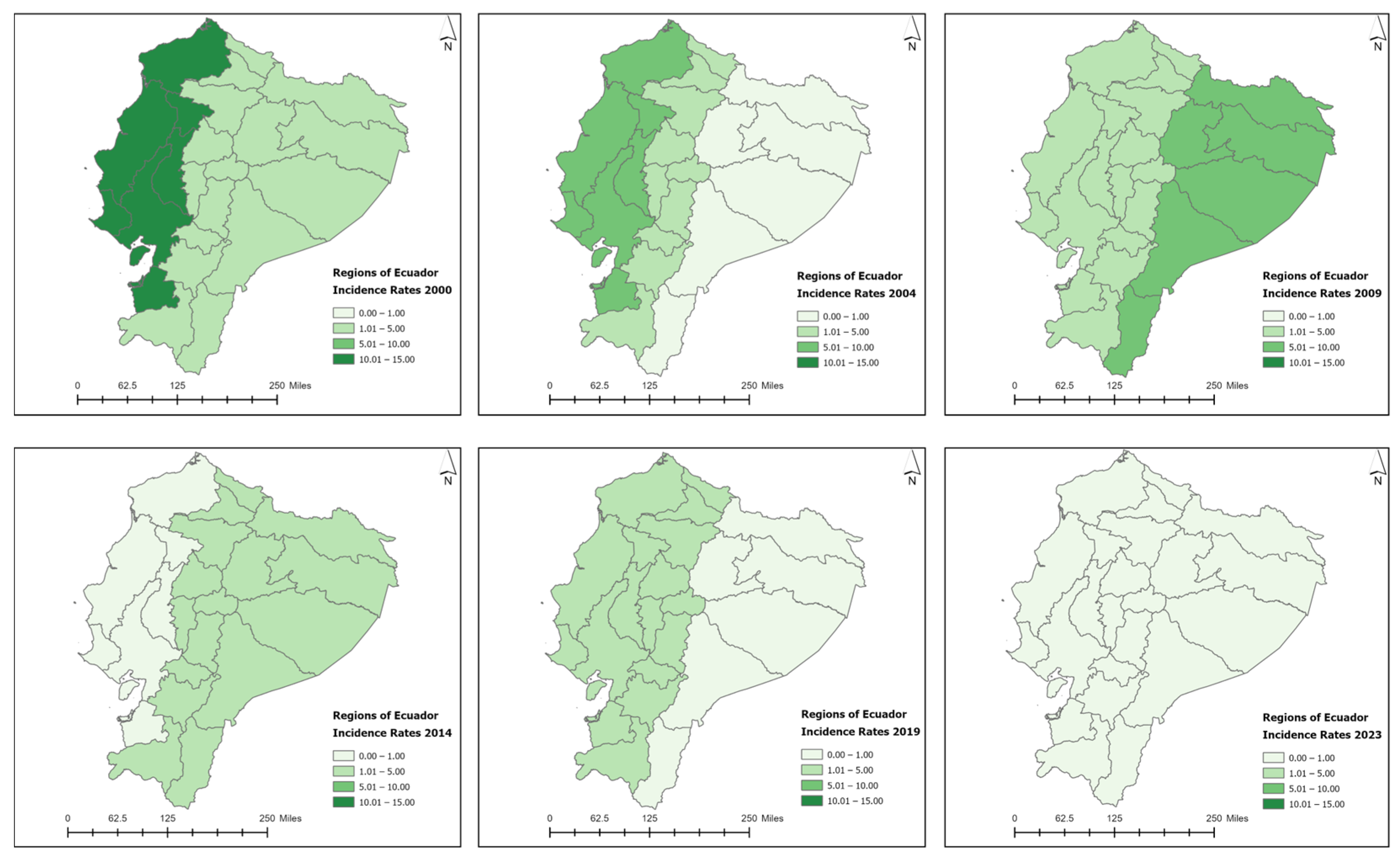
| Variable | Coast (N = 1070) | Sierra (N = 446) | Amazon (N = 23) | Total (N = 1539) | p-Value |
|---|---|---|---|---|---|
| Age * | 55 (18) | 53 (17) | 39 (25) | 54 (18) | <0.001 |
| Age group | <0.001 | ||||
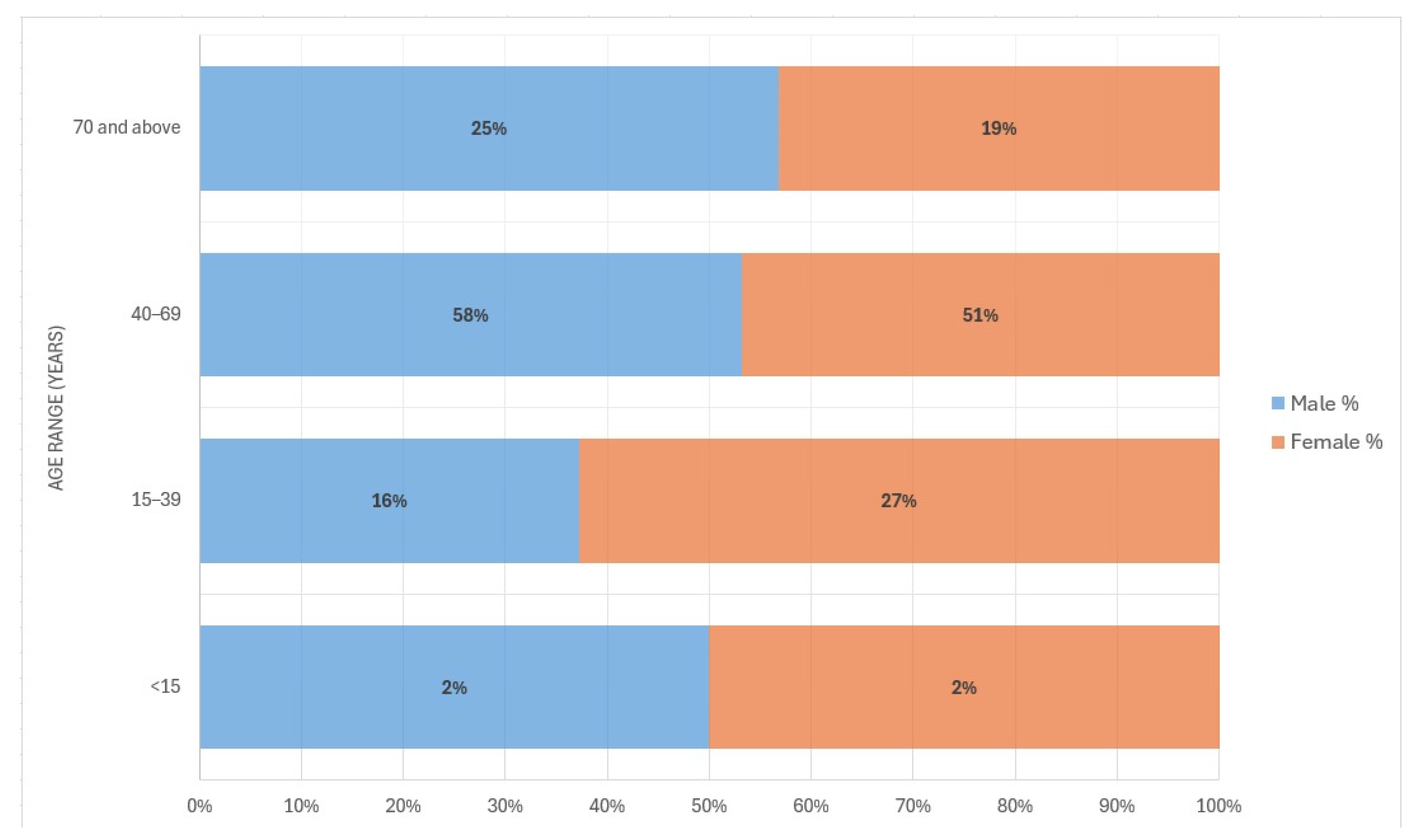
| <15 | 21 (2.0%) | 1 (0.2%) | 8 (34.8%) | 30 (1.9%) | |
| 15–39 | 185 (17%) | 107 (24%) | 2 (8.7%) | 294 (19.1%) | |
| 40–69 | 600 (56%) | 250 (56.1%) | 11 (47.8%) | 861 (56.0%) | |
| 70 and above | 264 (25%) | 88 (19.7%) | 2 (8.7%) | 354 (23.0%) | |
| Gender | 0.379 | ||||
| Female | 293 (27%) | 139 (31%) | 7 (30%) | 439 (28.5%) | |
| Male | 777 (73%) | 307 (69%) | 16 (70%) | 1100 (71.5%) | |
| Status | 0.585 | ||||
| Ambulatory (Outpatient) | 393 (37%) | 155 (35%) | 10 (43%) | 558 (36%) | |
| Hospitalized | 677 (63%) | 291 (65%) | 13 (57%) | 981 (64%) | |
| ICD Classification | <0.001 | ||||
| Borderline | 4 (0.4%) | 8 (1.8%) | 0 (0%) | 12 (0.8%) | |
| Borderline Lepromatous | 1 (0.1%) | 3 (0.7%) | 0 (0%) | 4 (0.3%) | |
| Borderline Tuberculoid | 8 (0.7%) | 10 (2.2%) | 0 (0%) | 18 (1.2%) | |
| Indeterminate | 175 (16%) | 69 (15%) | 5 (22%) | 249 (16.2%) | |
| Lepromatous | 15 (1.4%) | 101 (23%) | 0 (0%) | 116 (7.5%) | |
| Other | 3 (0.3%) | 0 (0%) | 0 (0%) | 3 (0.2%) | |
| Tuberculoid | 8 (0.7%) | 6 (1.3%) | 0 (0%) | 14 (0.9%) | |
| Unspecified | 856 (80%) | 249 (56%) | 18 (78%) | 1123 (73%) | |
| WHO Classification | <0.001 | ||||
| Paucibacillary (PB) | 1050 (98.1%) | 334 (74.9%) | 23 (100%) | 1407 (92%) | |
| Multibacillary (MB) | 20 (1.9%) | 112 (25.1%) | 0 (0%) | 132 (8%) |
| Year | New Cases Detected | NCDR per 1,000,000 Population. * | MB Proportion. n (%). * | Children under 15 Proportion. n (%) | Female Proportion. n (%) | |||
|---|---|---|---|---|---|---|---|---|
| 2000 | 106 | 8.50 | 10 | 9.4 | 0 | 0% | 35 | 33% |
| 2001 | 98 | 8.08 | 14 | 14.3 | 1 | 1.0% | 32 | 33% |
| 2002 | 82 | 6.36 | 12 | 14.6 | 0 | 0% | 30 | 37% |
| 2003 | 46 | 3.50 | 5 | 10.9 | 1 | 2.2% | 16 | 35% |
| 2004 | 70 | 5.24 | 16 | 22.9 | 2 | 2.9% | 18 | 26% |
| 2005 | 116 | 8.53 | 9 | 7.8 | 6 | 5.2% | 44 | 38% |
| 2006 | 95 | 6.86 | 4 | 4.2 | 1 | 1.1% | 36 | 38% |
| 2007 | 107 | 7.58 | 5 | 4.7 | 6 | 5.6% | 36 | 34% |
| 2008 | 93 | 6.48 | 4 | 4.3 | 0 | 0% | 22 | 24% |
| 2009 | 92 | 6.29 | 11 | 12.0 | 0 | 0% | 22 | 24% |
| 2010 | 134 | 9.29 | 12 | 9.0 | 7 | 5.2% | 16 | 12% |
| 2011 | 112 | 7.37 | 5 | 4.5 | 5 | 4.5% | 16 | 14% |
| 2012 | 86 | 5.56 | 17 | 19.8 | 0 | 0% | 33 | 38% |
| 2013 | 26 | 1.65 | 3 | 11.5 | 0 | 0% | 9 | 35% |
| 2014 | 23 | 1.44 | 2 | 8.7 | 0 | 0% | 11 | 48% |
| 2015 | 43 | 2.65 | 2 | 4.7 | 0 | 0% | 16 | 37% |
| 2016 | 34 | 2.07 | 0 | 0.0 | 0 | 0% | 3 | 8.8% |
| 2017 | 31 | 1.86 | 0 | 0.0 | 0 | 0% | 9 | 29% |
| 2018 | 35 | 2.06 | 1 | 2.9 | 0 | 0% | 8 | 23% |
| 2019 | 38 | 2.21 | 0 | 0.0 | 1 | 2.6% | 9 | 24% |
| 2020 | 17 | 0.98 | 0 | 0.0 | 0 | 0% | 4 | 24% |
| 2021 | 22 | 1.25 | 0 | 0.0 | 0 | 0% | 9 | 41% |
| 2022 | 21 | 1.24 | 0 | 0.0 | 0 | 0% | 4 | 19% |
| 2023 | 12 | 0.68 | 0 | 0.0 | 0 | 0% | 1 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Bojorge, S.; Gardellini, T.; Parikh, J.; Rupani, N.; Jacob, B.; Hoare, I.; Calvopiña, M.; Izurieta, R. Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador. Trop. Med. Infect. Dis. 2024, 9, 246. https://doi.org/10.3390/tropicalmed9100246
Hernandez-Bojorge S, Gardellini T, Parikh J, Rupani N, Jacob B, Hoare I, Calvopiña M, Izurieta R. Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador. Tropical Medicine and Infectious Disease. 2024; 9(10):246. https://doi.org/10.3390/tropicalmed9100246
Chicago/Turabian StyleHernandez-Bojorge, Santiago, Tatiana Gardellini, Jeegan Parikh, Neil Rupani, Benjamin Jacob, Ismael Hoare, Manuel Calvopiña, and Ricardo Izurieta. 2024. "Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador" Tropical Medicine and Infectious Disease 9, no. 10: 246. https://doi.org/10.3390/tropicalmed9100246
APA StyleHernandez-Bojorge, S., Gardellini, T., Parikh, J., Rupani, N., Jacob, B., Hoare, I., Calvopiña, M., & Izurieta, R. (2024). Ecuador Towards Zero Leprosy: A Twenty-Three-Year Retrospective Epidemiologic and Spatiotemporal Analysis of Leprosy in Ecuador. Tropical Medicine and Infectious Disease, 9(10), 246. https://doi.org/10.3390/tropicalmed9100246








