Abstract
Human pulmonary paragonimiasis, an emerging concern in North East India, frequently masquerades as pulmonary tuberculosis due to clinical and radiological similarities, leading to diagnostic challenges. This research aimed to harness the immunoblotting technique to discern immunodiagnostic protein antigens from both adult worm and excretory–secretory (ES) extracts of the prevalent Paragonimus westermani type 1 in Arunachal Pradesh, North East India. We studied the time kinetics of immunoreactive patterns in relation to the duration of infection in rodent models. Immunoblot analyses were also conducted using sera from ELISA-positive patients confirmed with paragonimiasis, facilitating the selection of antigenic extracts with diagnostic potential. Further, ES protein antigens were subjected to 2D immunoblot analysis and immunoreactive protein spots identified using MALDI-TOF MS. The immunoreactivity patterns of ES antigens with sera of paragonimiasis-positive patients were detailed, and specific immunoreactive protein antigens were pinpointed using peptide mass fingerprinting (MALDI-TOF). This work underscores the enhanced diagnostic accuracy when combining ELISA with immunoblotting for pulmonary paragonimiasis in regions like North East India, marked by co-existing helminth infections.
1. Introduction
Paragonimiasis, caused by trematodes of the genus Paragonimus, is a zoonotic disease posing a significant health threat, primarily affecting economically disadvantaged communities in Asia, Africa, and Latin America where freshwater crab consumption is common [1,2,3]. Considered a Neglected Tropical Disease (NTD) by the World Health Organization (WHO), paragonimiasis is alarmingly prevalent, with an estimated 20 million infections globally [4,5]. The South Asian region, including countries like China, the Philippines, and Vietnam, reports the highest incidence, although this likely represents only a fraction of the actual cases [6,7,8]. Therefore, understanding the disease’s epidemiology, transmission pathways, diagnostics, and treatment efficacy is crucial.
There are over 40 species of Paragonimus worldwide, and 16 of these are pathogenic to humans [1]. Noteworthy among them are P. westermani in Southeast Asia, P. africanus in West Africa, P. mexicanus in South America, and P. kellicotti in North America [9]. The first intermediate host for P. westermani is snails, while freshwater crabs, shrimps, and crayfish serve as second intermediate hosts. The definite hosts of Paragonimus include human, cats, and dogs. The human pulmonary paragonimiasis disease cycle begins with the consumption of inadequately cooked freshwater crabs, which harbor the larval form of the parasite known as metacercariae [10]. The metacercariae penetrate the gut, excyst their larvae in the small intestine, and migrate through the diaphragm to the pleural cavity of the lungs. In the lungs, cysts are formed, where larvae mature into adult worms and start releasing eggs. The eggs are discharged in the sputum and can be detected in feces. The symptoms of paragonimiasis may include chronic cough, hemoptysis, respiratory or gastrointestinal issues, fever, anemia, and weight loss. Although the infection can be effectively treated using praziquantel, the clinical symptoms of the disease can be misleading (often resembling tuberculosis or lung cancer), making diagnosis complex [5,11,12,13].
In the Indian subcontinent, the first case of paragonimiasis was identified in Manipur in 1981 [14]. Subsequent investigations have pinpointed other states in the northeastern (NE) regions of India as hotspots for this disease, with P. westermani and P. heterotremus as the predominant lung fluke species [8,15,16,17,18]. Molecular diversity suggests genetic diversity within the P. westermani species, potentially indicating a species complex. Notably, a study by Devi et al. [19] identified two distinct genotypes of P. westermani from NE Indian freshwater crab, with one genotype (type 1) differing from its East Asian counterparts. This diversity can impact the disease’s behavior, underscoring the need for a nuanced understanding of host–parasite interactions, especially in terms of the antibody response [20]. For the development of an immunodiagnostic test, the use of locally available species of lung fluke, Paragonimus westermani type 1, appears to be an important source of parasitic antigens because it can easily be procured locally and developed in rodent experimental models.
During their life cycle in the definite host, lung flukes produce a vast array of components, including excretory–secretory products, tegumental proteins, cyst wall proteins, cystic fluid proteins, egg-derived proteins, etc. [10]. These diverse sets of proteins are essential for parasite survival and involved in pathogenicity. Excretory–secretory (ES) products are mixture of molecules (proteins, glycans, and lipids) released by lung flukesinto the host’s biological fluids. These secreted and excreted molecules are presented at the host–parasite interface and elicit an immune response in the host; they are therefore considered a good source for antigens with serodiagnostic potential [21]. Apart from excreted–secreted proteins, adult worms contain many proteins that are important in performing various functions, including nutrition invasion, host immune response, and immune evasion [22]. The use of adult worm protein in serological assays has demonstrated high sensitivity and specificity in the detection of positive cases of human paragonimiasis [23]. Different antigenic extracts from helminth worms have their own specific protein components and the interaction mechanisms of these molecules with the host immune system are dissimilar. Advancements in proteomics tools have facilitated analysis of the proteins expressed during the different development stages of helminth parasites. Moreover, an immunoproteomics approach based on the integration of 1D or 2DE immunoblot analysis with proteomic techniques has been successful in the characterization of specific immunogenic proteins recognized by infected individuals and elucidating new protein biomarkers for the development of new immunoassays [24].
ELISA based on Paragonimus antigenic extracts are highly sensitive serological tests but are not free from the issues caused by cross-reactive antigens, which may yield false positive results [25]. Hence, ELISA should be combined with immunoblotting assays, which will help in the early and accurate diagnosis of the disease. Given the prevalence of pulmonary paragonimiasis in India’s NE regions, particularly in Manipur, Nagaland, Arunachal Pradesh, and Assam, our study focuses on investigating the antibody response to different antigenic extracts of adult lung flukes. In this study, we conducted immunoblot analyses using the sera from parasitologically confirmed paragonimiasis patients (ELISA-positive) to identify antigenic extracts with diagnostic value. Further, the ES antigenic proteins released by adult lung flukes were analyzed using 2DE immunoblotting coupled with mass spectrometry to identify the immunogenic proteins recognized in infected sera.
2. Materials and Methods
2.1. Biological Samples
The metacercariae of P. westermani type 1 were harvested from freshwater crabs (Maydelliathelphusalugubris) collected from Arunachal Pradesh, NE India, as detailed in previous work [26]. Identification of the metacercariae in the dissected crabs was carried out using a stereomicroscope. The metacercariae were mainly isolated from the hepatobiliary pancreas and muscles of the infected crabs. The isolated Paragonimus westermani type 1 metacercariae were cleaned in 1X PBS and identified under a light microscope before the infection of Wistar rats. Protein extraction from a pool of P. westermani metacercariae was performed in microcentrifuge tubes containing protein lysis buffer (8M urea) added with 1X Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany), and homogenization carried out using sonication in ice (30 s, times) and an ultrasonic probe. The lysate obtained was centrifuged at 16,000× g for 20 min at 20 °C to pellet the cell debris. After centrifugation, the supernatant was transferred into a clean tube and stored in aliquots at −80 °C.
Female Wistar rats (approximately 2 months old, n = 50) were fed with 20 metacercariae, obtained from infected crabs [27]. The rats were dissected weekly (up to 15th week post-infection) and their blood was collected via cardiac puncture. After clotting at room temperature for 1 h, the blood was centrifuged at 1000–2000 g for 10 min at 4 °C to obtain the sera. The sera was stored at −20 °C for subsequent use. Adult P. westermani type1 flukes were harvested by sacrificing the infected rats after 45 days of infection. The lungs were dissected and washed in 1X PBS (pH 7.4), and the cysts were separated from the lungs to avoid rupture of the cysts. The cysts were again washed in 1X PBS and the worms inside the cysts were taken out, cleaned, and placed in 1X PBS. The use of the Wistar rats for pulmonary paragonimiasis was approved by the Institutional Animal Ethics Committee, ICMR-RMRC, Dibrugarh, India.
This study included human serum samples from selected freshwater crab-eating communities of Arunachal Pradesh, NE India, which were collected and tested in the previous study by the authors [28]. All subjects gave their informed consent for inclusion before they participated in the study. Serum samples from 22 parasitologically confirmed patients and the pooled sera of 10 healthy subjects were analyzed with immunoblotting. Confirmed patients exhibited symptoms such as chronic cough with hemoptysis, with negative sputum samples for acid-fast bacilli (AFB, Mycobacterium tuberculosis) but positive sputum samples for lung fluke eggs. The anti-Paragonimus IgG antibodies were confirmed using an in-house ELISA kit (the first indigenously developed test from India) [28]. The sample was considered positive when the absorbance values were higher than 0.5. Ethical approval was obtained from the Institutional Ethics Committee, ICMR-Regional Medical Research Centre, Dibrugarh, Assam, India. All the experiments were performed in accordance with the relevant guidelines and regulations.
2.2. Preparation of Adult Worm Somatic Antigens and Immunoblotting
Live adult Paragonimus worms were homogenized to obtain soluble somatic antigens [28]. Briefly, live or frozen adult worms were homogenized by employing the Roche MagNALyser instrument (Roche Applied Science, Penzberg, Germany) in cold 45 mm phosphate buffered saline (PBS) (pH 7.2) containing protease inhibitor cocktail solution (1X). The lysate obtained was incubated overnight at 4 °C. Next, the lysate was centrifuged at 15,000 g for 1 h at 4 °C to obtain an adult worm (AW) soluble somatic antigen. The samples were stored in aliquots at −80 °C. The protein concentration of the AW extract was estimated using the Bradford method [29] and the homogeneity of extract was determined by running the samples in Tris-Glycine SDS-PAGE gels [30]. Prior to gel electrophoresis, the protein sample was reduced by adding β-mercaptoethanol and heating it at 95 °C for 5 min. The protein bands in the gels were observed using Coomassie and silver staining procedures [31]. For the silver staining, the gel was incubated for 1 h in fixing solution containing methanol, acetic acid, and milliQ at a ratio of 1:5:4, respectively. Subsequently, the gel was washed with 50% ethanol solution, treated with 0.06% sodium thiosulphate pentahydrate solution, and incubated for 30 min with silver nitrate solution. After incubation, the gel was developed to visualize the protein bands
Immunoblotting was performed using sera from infected rats collected over time. At first, the AW protein sample was resolved in 15% Tris-Glycine SDS-PAGE gels procured from Bio-Rad (Hercules, CA, USA). The electrotransfer of proteins from the gel to the polyvinylidene difluoride membrane (PVDF) was performed in a Bio-Rad Trans-Blot transfer cell for 90 min at 100 V. Next, the blotted membrane was incubated with blocking buffer containing 5% BSA in wash buffer (0.1% Tween20 in Tris-NaCl; TBST) for 1 h. The blotted PVDF was cut into vertical strips and stored overnight with sera collected weekly from infected rats. The rat sera were diluted in the ratio 1:100 with blocking buffer. An uninfected rat serum was taken as negative control group. Next, the membrane strips were washed in TBST and incubated in a solution containing the secondary antibody (dilution ratio 1:2500) HRP-conjugated anti-rat IgG (Promega Corporation, Madison, WI, USA). The membrane strips were then washed with TBST and the antibody–antigen reaction was observed using the Promega TMB (3,3′,5,5′-tetramethylbenzidine)chromogenic substrate.
2.3. Preparation of Excretory–Secretory Antigenic Proteins
Live adult worms collected from experimental rats were used for the preparation of excretory–secretory (ES) antigenic proteins [28]. The adult worms were first washed at least 10 times in 1X PBS (pH 7.4) and then soaked in 1 mL of 1X PBS (pH 7.4) for 3 h under shaking conditions. The adult lung flukes were removed and the spent culture was centrifuged at 15,000× g to eliminate eggs and debris. The supernatant was treated with a complete Roche Protease Inhibitor Cocktail solution and filtered through a 0.2 µm membrane to obtain the ES proteins. The protein concentration of the ES antigens was determined using Bradford assay. Subsequently, the ReadyPrep™ 2-D clean-up kit (Bio-Rad Laboratories Inc., Hercules, CA) was used for the quantitative precipitation of the sample proteins and removal of contaminants [32]. The dried pellet obtained after cleanup was suspended by adding 125 µL of rehydration buffer and stored at −80 °C. To check the quality of the antigenic extract, two replicates of the reduced ES sample (5 µg each) were resolved in Tris-Glycine SDS-PAGE and stained using Coomassie Brilliant BlueR250. The Bio-Rad Precision Plus (catalog no. 161–0377) protein marker was used for determining the molecular weights of the protein bands.
2.4. Immunoblot Analysis Using Human Serum
Immunoblotting was performed using AW and ES protein antigens and the pooled sera of confirmed pulmonary paragonimiasis patients. The protein antigens were reduced using β-mercaptoethanol and loaded on to SDS-PAGE gels to perform gel electrophoresis using a Bio-Rad Mini-PROTEAN Tetra cell (Hercules, CA, USA). After the completion of electrophoresis, a portion of the gel was cut and subjected to silver staining. The remaining part of the gel was used for the immunoblotting procedure as described above. The blotted membrane was incubated overnight at 4 °C with the sera of confirmed pulmonary paragonimiasis patients (diluted 1:100 ratio). For the negative control, the pooled sera of 10 uninfected individuals were used. The strips were incubated with secondary antibody (dilution ratio 1:1000). Immunoreactive proteins were visualized using a chromogenic substrate.
The ES protein samples were also separated in two-dimensional gel electrophoresis (2D-PAGE). At first, isoelectric focusing was carried out in 7 cm (pH 3–10) IPG strips (Bio-Rad, Hercules, CA, USA) under standardized running conditions [33]. Isoelectric focusing was performed in the Bio-Rad PROTEAN IEF Cell at 50 µA per strip; Step 1: 250 V for 20 min, Step 2: 4000 V for 2 h; Step 3: increasing voltage from 4,000 V for 3–4 h until1 4000 V-h. After focusing, the strips were equilibrated in two equilibration steps—10 min in Equilibration Buffer I containing DTT, followed by 10 min incubation in Equilibration Buffer II containing iodoacetamide. The equilibrated strips were applied to 12% SDS-PAGE gels and electrophoresis carried out. The gel was stained using Coomassie Brilliant Blue to visualize the protein spots, and selected protein spots were subjected to in-gel trypsin digestion.
Briefly, the stained gel was first washed with miliQ water twice followed by the excision of protein spots of interest. The gel pieces were diced into small pieces and further destained using an ammonium bicarbonate (NH4HCO3) solution and acetonitrile (1:1 ratio). Next, the gel pieces were dehydrated using acetonitrile until completely dry. The gel particles were rehydrated in freshly prepared 10 mM dithiothreitol (DTT) in 100 mM ammonium bicarbonate solution and incubated for 1 h at 56 °C in water bath. After incubation, the DTT was removed and the gel pieces treated with freshly prepared 55 mM iodoacetamide (IAA) in 100 mM of NH4HCO3 solution for 45 min. The supernatant was removed and the gel pieces were incubated with NH4HCO3 solution for 10 min. After the gel pieces had shrunk, the supernatant was removed and the gel was again dehydrated with acetonitrile for 10 min and vacuum-centrifuged until completely dry. Subsequently, 10 µL of freshly prepared trypsin solution (10–20 ng/µL in NH4HCO3 solution) was added to tubes containing the gel pieces and kept for overnight incubation at 37 °C. The solution was collected using centrifugation and transferred into fresh microcentrifuge tubes. The extraction buffer (acetonitrile and 0.1% trifluoroacetic acid = 1:1 ratio) was added to the gel pieces and sonicated for 20 min at room temperature. The fresh supernatant obtained using centrifugation was mixed with the previous supernatant. The supernatant collected was treated using a Speedvac until completely dry. The dried pepmix was resuspended in a solution containing acetonitrile and 0.1% trifluoroacetic acid (1:2 ratio). The peptides obtained were mixed with an α-Cyano-4-hydroxycinnamic acid MALDI matrix and the resulting 2 µL was spotted onto the MALDI plate. After air-drying the sample, it was analyzed on the MALDI-TOF mass spectrometry instrument and further analysis was undertaken with flex analysis software for obtaining the peptide mass fingerprint. The masses generated in the peptide mass fingerprint were submitted to MASCOT for protein identification against the Paragonimus Uniprot database [34].
The remaining gels were used for immunoblot analysis as described above. To study the interactions of the protein spots with the serum antibody, the blotted PVDF membrane was incubated with the pooled sera of confirmed patients (diluted 1:100).
3. Results
3.1. Protein Profiling of Different Antigenic Extracts
The majority of proteins in both the AW and ES extracts fell within the molecular mass range of 10 kDa to 50 kDa, with faint bands above 50 kDa in the AW extract (Figure 1; Figure 2). Prominent protein bands were observed in both extracts, with a cluster around 20–25 kDa and faints bands near 37 kDa observed in the SDS-PAGE gels.
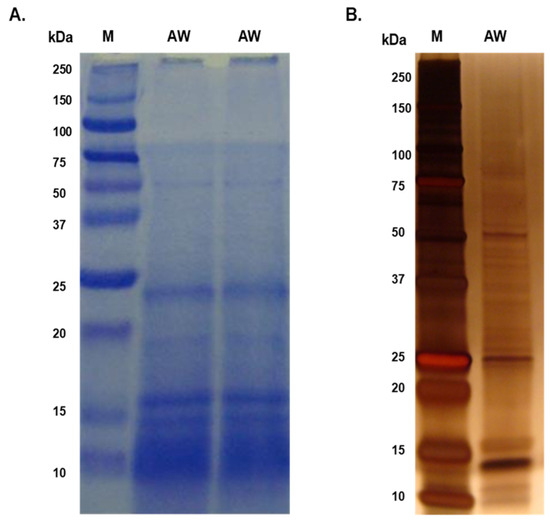
Figure 1.
Separation of adult worm (AW) soluble somatic antigenic proteins using SDS-PAGE. (A) 10 µg of AW was resolved in 15% SDS-PAGE and stained with Coomassie Brilliant Blue; (B) 1 µg of AW resolved in 4–12% SDS-PAGE gel and silver-stained.
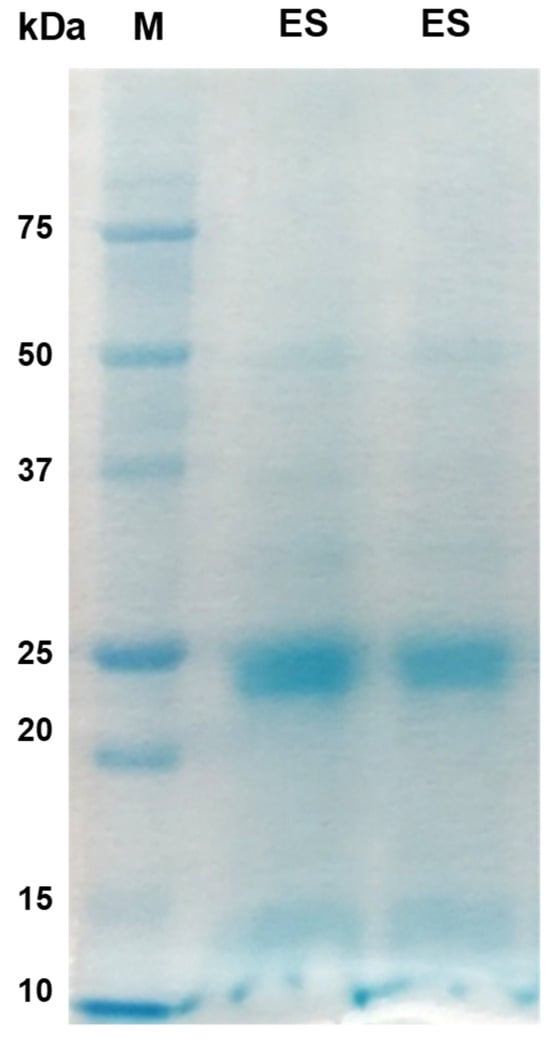
Figure 2.
Excretory–secretory (ES) protein antigens of P. westermani type 1 resolved in 12% Tris-Glycine SDS-PAGE.
3.2. Serum Antibody Responses in Experimental Rats
The rats infected with metacercariae developed juvenile worms in the peritoneal cavity, and later, adult worms in the lung cysts, with parasitic cysts also found in the liver (Figure 3). From the third week onward, the lungs developed black cysts while after the seventh to eighth week, parasitic cysts were observed in the liver. Using immunoblotting, we monitored IgG antibody development against AW antigens (Figure 4 and Table 1). Reactive fractions emerged from the first week, expanding up to 15 weeks. Protein bands of molecular masses ~100, ~75, ~14, and ~9 kDa began to react with the experimental sera at the first week. Subsequently, the protein bands displayed increased reactivity up to the 15th week post-infection. Additional protein bands of molecular masses ~25, ~22, and ~19 kDa displayed immune reactivity from the seventh week post-infection. The pre-infection sera from experimental rats were non-reactive to the AW antigens.
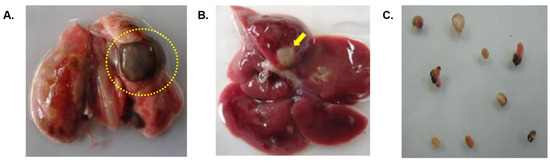
Figure 3.
(A) Infected lung of a Wistar rat with cysts (dotted circle) containing adult P. westermani type 1 lung flukes. (B) Cysts formed in liver of Wistar rats due to Paragonimus infection indicated by arrow. (C) Adult lung fluke worms in 1X PBS.
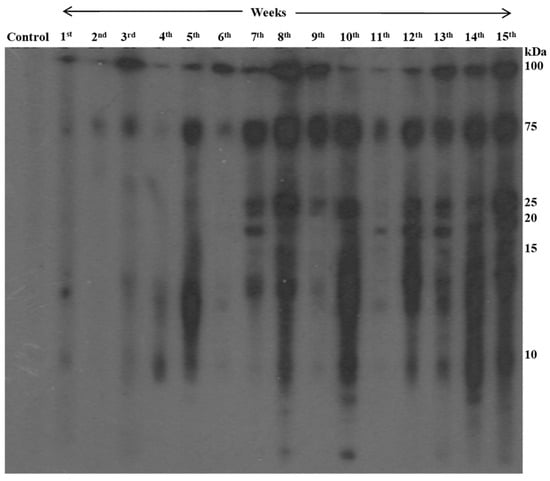
Figure 4.
Chronological changes in antibody reactivity against adult worm antigens of P. westermani type 1 analyzed using immunoblotting.

Table 1.
Immunoreactivity pattern of different protein bands at different points in time post-infection.
3.3. Immunoreactivity of Human Sera with Antigenic Extracts
The serum antibodies from confirmed paragonimiasis patients reacted with specific protein bands, including ~34 and ~25 kDa of the adult worm antigens (Figure 5). In addition, the infected human sera displayed slightly weak immunoreactivity against ~75 and ~15 kDa AW protein antigens. The protein antigens of the P. westermani metacercariae (pre-adult stage) were also reactive with the infected sera. The reactivity profiles of the metacercarial proteins and AW proteins were found to be similar. Two major protein bands (~34 and ~25 kDa) of the ES antigens showed strong immunoreactivity with the pooled lung-fluke-infected human sera.
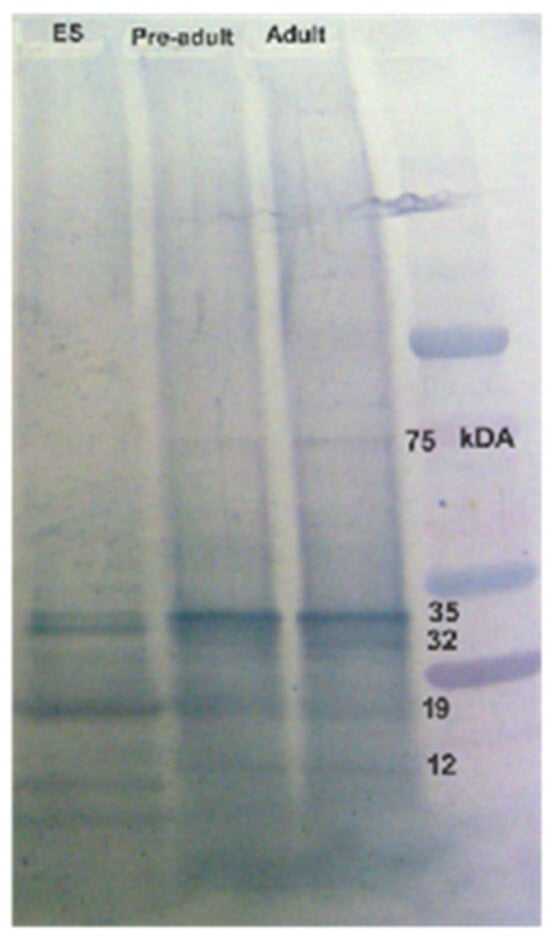
Figure 5.
Immunoreactivity of human sera (pooled) from confirmed paragonimiasis patients of NE India against lung fluke antigens (P. westermani type 1).
Further, we studied the immunoreactive patterns of the individual serum of confirmed paragonimiasis patients (n = 7) with ES proteins using immunoblotting (Figure 6). Particularly, the protein bands of size 25 and 35 kDa were found to be highly immunoreactive.
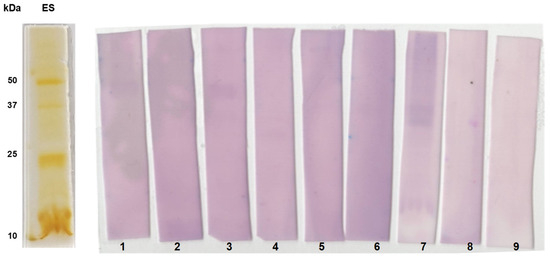
Figure 6.
1D immunoblot analysis using excretory–secretory protein antigens of P. westermani type 1. Silver-stained gel showing banding patterns of ES antigen. Blotted membranes were incubated with individual human serum of ELISA-positive samples (Lanes 1–7) and known negative control samples (Lane 8 and 9).
3.4. 2D Immunoblot Analysis of ES Antigens
Numerous protein spots were identified within molecular weights and pI ranges of 25 to 50 kDa and pI 3–6, respectively (Figure 7A). Seven protein spots were positively identified using mass spectrometry, with matches predominantly from P. westermani and some from other related species (Table 2). Seven peptides of protein spot 1 were found to match with the globin family profile domain-containing protein of P. westermani upon MASCOT search. Cathepsin F, a cysteine protease, was identified in spot 9. The other proteins identified in this study were carboxylesterase B, nucleosome assembly protein 1-like 1, 5′-Nucleotidase C-terminal domain protein, Ras and EF-hand domain-containing protein, and SET domain-containing protein. These proteins were previously not reported from ES extracts of P. westermani. Furthermore, the ES antigenic extracts were tested against the sera of confirmed paragonimiasis patients. The major immunoreactive clusters were found in the molecular mass region of 25–50 kDa and pI range 4–8. The MS-identified proteins were effectively recognized by the host humoral immune response and therefore determined to be antigenic with serodiagnostic potential.
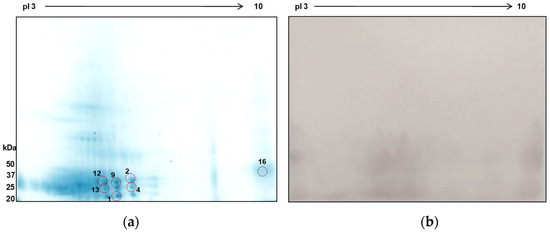
Figure 7.
2D-PAGE of total ES protein antigens of P. westermani type 1 and corresponding immunoblot. (a) 2D gel profile of ES protein extract. Red circle indicates the protein spots positively identified by mass spectrometry. (b) 2D immunoblot profile using the pooled sera of paragonimiasis ELISA-positive cases.

Table 2.
Seven protein spots identified by MALDI-TOF mass spectrometry.
4. Discussion
Human pulmonary paragonimiasis disease is a pressing public health concern in the northeastern (NE) states of India, primarily caused by Paragonimus species such as P. westermani and P. heterotremus. Notably, the NE Indian form of P. westermani type 1 has distinct characteristics from its East Asian counterparts [26]. While prior studies have reported on the immunodominant antigens of different antigenic extracts of lung flukes [34,35], the protein profile and immunoreactivity patterns of P. westermani type 1 of NE Indian origin remain elusive. Understanding the protein composition of antigenic extracts is crucial, as it offers insights into the biologically active molecules involved in the infection’s pathophysiology. In this context, we investigated the protein profiles of adult worm (AW) somatic and excretory–secretory (ES) protein extracts of P. westermani type 1 using 1D SDS-PAGE. The presence of a doublet protein band at 21/23 kDa and a diffuse band at 35 kDa supports exposure to Paragonimus species. These bands are known to contain highly immunogenic antigens [22,34,36]. Notably, a ~15 kDa in the ES sample aligns with the previous identification of host-tissue-derived hemoglobin [34]. The presence of host proteins in the ES extracts could indicate symbiotic interactions, aiding the parasite’s survival and infection process [37].
Further, we aimed to unveil the pathology and dynamics of the antibody response following infection with P. westermani type 1. In order to infect definite hosts, metacercaria should excyst into the host intestine. The cyst wall of metacercariae contains proteins that protect the juveniles and help them to evade the host immune system [38,39]. The appearance of parasitic cysts in the experimental animals correlated with detectable antigen-specific antibodies. The antibody response varied based on the maturation stage of the lung flukes, suggesting a dynamic interaction. Similar observations were also reported previously [27].
Laboratory diagnosis of human pulmonary paragonimiasis typically relies on detecting parasite eggs in clinical samples [28,40]. However, this method is not always feasible, especially at early stages or extra-pulmonary infections [41,42,43]. In these cases, serological assays are valuable, particularly in regions where paragonimiasis coexists with other infections like tuberculosis. ELISA and immunoblot assay are common approaches to detecting parasite-specific antibodies [44,45,46,47]. Several studies have evaluated the serodiagnostic potential of adult worm somatic extracts, excretory–secretory antigens, and recombinant proteins of Paragonimus species using ELISA and Western blot for the screening of human paragonimiasis [28,35,36,48]. Taking into consideration the genetically and geographically diverse complex of P. westermani populations found in Asia [49,50,51], it is crucial to identify and characterize the repertoire of antigenic proteins present in different samples. During their life cycles in the host, the quantity and type of molecules secreted/excreted at different developmental stages by lung flukes may greatly vary. Also, the type of immune response triggered in the host and interaction of parasite-derived components with the host molecules depends on their stage of development. Therefore, the present study evaluated different antigenic extracts of P. westermani type 1 (pre-adult/juvenile, adult worms and excretory-secretory) for their potential as serodiagnostic antigens using the sera of parasitologically confirmed pulmonary paragonimiasis human patients. The results obtained in this study are in line with previous studies [35]. The immunoreactive antigens of adult P. westermani were identified to be cysteine proteases of molecular weights between 27 and 35 kDa that largely reacted with the sera of paragonimiasis patients [34]. Although the banding pattern varied among the samples, it could recognize the different proteins of molecular size 10–75 kDa. The developmental stage of parasites responsible for infection in patients could be one of the explanations for the reactivity pattern variation. The antigenic determinants of proteins are lost during the denaturation process in immunoblot assay, while the same protein in its native form may expose relevant epitopes in an ELISA test. Studies on parasitic diseases have suggested the use of Western blotting and ELISA as confirmatory tests for positive cases in order to distinguish false positive sera from true positive sera [52,53,54]. In summary, combining ELISA and immunoblotting could enhance the serodiagnosis of human pulmonary paragonimiasis in endemic areas.
Identification of Paragonimus-species-derived proteins has been possible due to advancements in proteomics, and such studies have provided valuable insights into the biology of parasites and the identification of drug targets. Moreover, proteomics-based studies on diverse parasite antigenic preparations has facilitated unveiling s repertoire of antigens involved in host–parasite interactions [31,55]. A recent study explored the proteome of extracellular vesicles in excretory–secretory products released by P. kellicotti adult worms, providing new insights into the biology of host–parasite interactions and its potential implications in the development of novel diagnostic methods [56]. Excretory–secretory proteins of helminth parasitic worms are known to be ideal candidates for serodiagnostic protein antigens due to host–parasite interactions [21]. A two-dimensional gel electrophoresis (2DE)-based immunoblotting approach combined with mass spectrometry is the most popular research technique to obtain an overview of the specific protein antigens recognized in human patient sera [57,58]. Hence, to gain insight into the proteins present, we analyzed the ES extracts of P. westermani type 1 worms using 2D-PAGE, followed by protein identification. Cathepsin F and globins were identified in this study. The high content of globins in adult lung flukes was reported previously, making it another diagnostic candidate, but it requires further exploration [21,22]. Cysteine proteases, which have implications in pathogenesis and immune modulation [21,49], are known to share high sequence similarity among many Paragonimus species. Selecting unique regions of these cysteine proteases and the use of recombinant technology might prove useful for designing species-specific diagnostic kits. Lately, the cathepsin F gene expression has been detected at different developmental stages of P. westermani worms, and the recombinant protein expressed was found to be highly immunoreactive with paragonimiasis patient sera [59]. This study supports cysteine proteases as promising diagnostic targets. The carboxylesterase B domain-containing protein identified in this study may have a role in the metabolism of parasitic helminths and might be associated with resistance against anthelmintics [60]. Overall, the findings in this study provide a foundation for identifying potential diagnostic antigens. However, more comprehensive proteomics studies using high-throughput technologies are warranted to fully characterize the proteins of various antigenic preparations involved in the host–parasite interaction. The identification of potential diagnostic immunogenic proteins may assist in the development of more specific diagnostic tools that may eliminate the requirement for crude antigenic extracts. In recent years, there has been growing research interest in understanding glycoproteins and glycolipids of parasitic helminths, which have the potential to contribute to the development of novel intervention tools for helminth diseases [61,62]. The highly immunogenic glycans are widely shared among helminths and often lead to high cross-reactivity. The glycosylated moieties of proteins can greatly influence their immunogenicity, and hence the elimination of glycan structures via periodate oxidation increases the selection of more specific candidates for diagnostic tests [63,64,65]. Thus, exploring the glycoprotein profile for different life stages of the Paragomius parasite using a mass-spectrometry-based glycomic approach requires immediate attention that may contribute to understanding the importance of glycobiology in parasite development and the role of glycans in host–parasite interaction.
5. Conclusions
Our study has successfully identified specific antigenic fractions with immunodiagnostic potential for paragonimiasis. By using an experimental infection model involving laboratory rats and sera from confirmed human paragonimiasis cases, we have pinpointed antigenic markers that hold promise for accurate diagnosis. Moreover, we have unraveled the temporal evolution of immunoreactivity in response to the duration of infection in rodent models of paragonimiasis. These findings are anticipated to significantly contribute to the diagnosis of chronic stages of paragonimiasis. Importantly, this investigation marks the first of its kind in India, shedding light on the reactivity patterns between P. westermani protein antigens and sera from ELISA-positive patients using immunoblotting. Our results underscore the value of combining ELISA and immunoblot assays to effectively screen for paragonimisis in endemic regions. Of particular significance is the identification of cathepsin F using MALDI-TOF MS analysis, representing a highly promising serodiagnostic antigen. The potential of cathepsin F, along with other antigenic candidates, merits further exploration for the development of diagnostic kits that offer enhanced sensitivity and specificity for paragonimiasis. Overall, this study contributes valuable insights to the realm of paragonimiasis diagnostics and provides a foundation for continued advancements in the field.
Author Contributions
Conceptualization, K.N. and K.R.D.; methodology, A.D., K.R.D., and K.N.; validation, K.N., K.R.D., and A.D.; formal analysis, A.D., D.M., K.N., H.K., and K.R.D.; investigation, K.N and K.R.D.; resources, K.N. and K.R.D.; data curation, K.N and K.R.D.; writing—original draft preparation, A.D. and D.M.; writing—review and editing, A.D., D.M., K.N., and K.R.D.; visualization, K.N. and K.R.D.; supervision, K.N., H.K., and K.R.D.; project administration, K.N., H.K., and K.R.D.; funding acquisition, K.N. and K.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Indian Council of Medical Research, the Govt. of India: grant number Y110118/1/2020-21-(199).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of ICMR-Regional Medical Research Centre, Dibrugarh, India (RMRC/Dib/IEC (Human)/2017-18/3483; date of approval:7 March 2018). The animal study protocol was approved by the Institutional Animal Ethics Committee, ICMR-RMRC, Dibrugarh, India (approval code: 4 (2022); date of approval: 16 December 2022).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was funded by the Indian Council of Medical Research, New Delhi, India. The authors thank all the project staff for their help in the field and laboratory work. AD acknowledges the Indian Council of Medical Research, the Govt. of India, for an ICMRResearch Associate Fellowship (sanction no. 2021-8051/PROTEOMICS-BMS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yoshida, A.; Doanh, P.N.; Maruyama, H. Paragonimus and paragonimiasis in Asia: An update. Acta Trop. 2019, 199, 105074. [Google Scholar] [CrossRef] [PubMed]
- Rabone, M.; Wiethase, J.; Clark, P.F.; Rollinson, D.; Cumberlidge, N.; Emery, A.M. Endemicity of Paragonimus and paragonimiasis in Sub-Saharan Africa: A systematic review and mapping reveals stability of transmission in endemic foci for a multi-host parasite system. PLoS Negl. Trop. Dis. 2021, 15, e0009120. [Google Scholar] [CrossRef] [PubMed]
- Cumberlidge, N.; Rollinson, D.; Vercruysse, J.; Tchuem Tchuente, L.A.; Webster, B.; Clark, P.F. Paragonimus and paragonimiasis in West and Central Africa: Unresolved questions. Parasitology 2018, 145, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Keiser, J.; Utzinger, J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 210–221. [Google Scholar] [CrossRef]
- World Health Organization. Control of Foodborne Trematode Infections: Report of a WHO Study Group; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1995; Volume 849, pp. 1–157. [Google Scholar]
- Betson, M.; Alonte, A.J.; Ancog, R.C.; Aquino, A.M.; Belizario, V.Y.; Bordado, A.M.; Clark, J.; Corales, M.; Dacuma, M.G.; Divina, B.P.; et al. Chapter Two—Zoonotic transmission of intestinal helminths in southeast Asia: Implications for control and elimination. In Advances in Parasitology; Rollinson, D., Stothard, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 47–131. [Google Scholar]
- Zhou, X.J.; Yang, Q.; Tan, Q.H.; Zhang, L.Y.; Shi, L.B.; Zou, J.X. Paragonimus and its hosts in China: An update. Acta Trop. 2021, 223, 106094. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.S.; Sugiyama, H.; Rangsiruji, A. Paragonimus & paragonimiasis in India. Indian J. Med. Res. 2012, 136, 192–204. [Google Scholar]
- Castilla, E.A.; Jessen, R.; Sheck, D.N.; Procop, G.W. Cavitary mass lesion and recurrent pneumothoraces due to Paragonimus kellicotti infection: North American paragonimiasis. Am. J. Surg. Pathol. 2003, 27, 1157–1160. [Google Scholar] [CrossRef]
- Blair, D. Paragonimiasis. Adv. Exp. Med. Biol. 2019, 1154, 105–138. [Google Scholar]
- Lane, M.A.; Barsanti, M.C.; Santos, C.A.; Yeung, M.; Lubner, S.J.; Weil, G.J. Human paragonimiasis in North America following ingestion of raw crayfish. Clin. Infect. Dis. 2009, 49, e55–e61. [Google Scholar] [CrossRef]
- Sharma, D.C. Paragonimiasis causing diagnostic confusion with tuberculosis. Lancet Infect. Dis. 2005, 5, 538. [Google Scholar] [CrossRef]
- Jeon, K.; Koh, W.J.; Kim, H.; Kwon, O.J.; Kim, T.S.; Lee, K.S.; Han, J. Clinical features of recently diagnosed pulmonary paragonimiasis in Korea. Chest 2005, 128, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.I.; Singh, N.B.; Devi, S.S.; Singh, Y.M.; Razaque, M. Pulmonary paragonimiasis in Manipur. Indian J. Chest Dis. Allied Sci. 1982, 24, 304–306. [Google Scholar]
- Singh, T.S.; Sugiyama, H.; Lepcha, C.; Khanna, S.K. Massive pleural effusion due to paragonimiasis: Biochemical, cytological, and parasitological findings. Indian J. Pathol. Microbiol. 2014, 57, 492. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.S.; Sugiyama, H.; Umehara, A.; Hiese, S.; Khalo, K. Paragonimusheterotremus infection in Nagaland: A new focus of Paragonimiasis in India. Indian J. Med. Microbiol. 2009, 27, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Das, P.P.; Borah, A.K.; Das, J.K. Paragonimiasis in a Child from Assam, India. J. Clin. Diagn. Res. 2016, 10, DD06–DD07. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.S.; Hiromu, S.; Devi, K.R.; Singh, W.A. First case of Paragonimus westermani infection in a female patient in India. Indian J. Med. Microbiol. 2015, 33, 156–159. [Google Scholar] [CrossRef]
- Rekha, D.K.; Narain, K.; Mahanta, J.; Nirmolia, T.; Blair, D.; Saikia, S.P.; Agatsuma, T. Presence of three distinct genotypes within the Paragonimus westermani complex in northeastern India. Parasitology 2013, 140, 76–86. [Google Scholar]
- Dreyfuss, G.; Rondelaud, D. Biodiversity of flukes. Parasite 2008, 15, 282–285. [Google Scholar] [CrossRef][Green Version]
- Yeshi, K.; Ruscher, R.; Loukas, A.; Wangchuk, P. Immunomodulatory and biological properties of helminth-derived small molecules: Potential applications in diagnostics and therapeutics. Front. Parasitol. 2022, 1, 984152. [Google Scholar] [CrossRef]
- McNulty, S.N.; Fischer, P.U.; Townsend, R.R.; Curtis, K.C.; Weil, G.J.; Mitreva, M. Systems biology studies of adult paragonimus lung flukes facilitate the identification of immunodominant parasite antigens. PLoS Negl. Trop. Dis. 2014, 8, e3242. [Google Scholar] [CrossRef]
- Maleewong, W.; Pariyanonda, S.; Wongkham, C.; Intapan, P.; Daenseegaew, W.; Morakote, N. Comparison of adult somatic and excretory-secretory antigens in enzyme-linked immunosorbent assay for serodiagnosis of human infection with Paragonimus heterotremus. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Pothong, K.; Komalamisra, C.; Kalambaheti, T.; Watthanakulpanich, D.; Yoshino, T.P.; Dekumyoy, P. ELISA based on a recombinant Paragonimus heterotremus protein for serodiagnosis of human paragonimiasis in Thailand. Parasites Vectors 2018, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.G.; Nakamura-Uchiyama, F.; Nawa, Y.; Itoh, M. A tool for mass-screening of paragonimiasis: An enzyme-linked immunosorbent assay with urine samples. Trop. Med. Health 2016, 44, 19. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.R.; Narain, K.; Agatsuma, T.; Blair, D.; Nagataki, M.; Wickramasinghe, S.; Mahanta, J. Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Trop. 2010, 116, 31–38. [Google Scholar] [CrossRef]
- Narain, K.; Rekha, D.K.; Mahanta, J. A rodent model for pulmonary paragonimiasis. Parasitol. Res. 2003, 91, 517–519. [Google Scholar] [CrossRef]
- Narain, K.; Devi, K.R.; Mahanta, J. Development of enzyme-linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J. Med. Res. 2005, 121, 739. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- De Kostha, Y.S.; Pathirana, S.L.; Handunnetti, S.M.; Gunawardena, S. Characterization of antigens of Enterobius vermicularis (pinworm) eggs. Sci. Rep. 2022, 12, 14414. [Google Scholar] [CrossRef]
- Morassutti, A.L.; Levert, K.; Pinto, P.M.; da Silva, A.J.; Wilkins, P.; Graeff-Teixeira, C. Characterization of Angiostrongylus cantonensis excretory–secretory proteins as potential diagnostic targets. Exp. Parasitol. 2012, 130, 26–31. [Google Scholar] [CrossRef]
- Deka, A.; Reza, M.A.; Hoque KM, F.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Na, B.K.; Bae, Y.A.; Kim, S.H.; Je, E.Y.; Ju, J.W.; Kong, Y. Identification of immunodominant excretory–secretory cysteine proteases of adult Paragonimus westermani by proteome analysis. Proteomics 2006, 6, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.U.; Curtis, K.C.; Folk, S.M.; Wilkins, P.P.; Marcos, L.A.; Weil, G.J. Serological diagnosis of North American Paragonimiasis by Western blot using Paragonimus kellicotti adult worm antigen. Am. J. Trop. Med. Hyg. 2013, 88, 1035. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Cho, S.Y.; Kong, Y.; Gan, X.X.; Hong, S.J. Recombinant Paragonimus westermani yolk ferritin is a useful serodiagnostic antigen. J. Infect. Dis. 2012, 185, 1373–1375. [Google Scholar]
- Dagenais, M.; Tritten, L. Hidden in plain sight: How helminths manage to thrive in host blood. Front. Parasitol. 2023, 2, 1128299. [Google Scholar] [CrossRef]
- Lou, Y.S.; Fujino, T.; Morita, K.; Ishii, Y. A comparative ultrastructural and histochemical study of the metacercarial cyst walls of four species of Paragonimus (Troglotrematidae: Trematoda). Parasitol. Res. 1992, 78, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.P.; Moon, S.U.; Na, B.K.; Kim, S.H.; Cho, S.H.; Lee, H.W.; Kim, T.S. Paragonimus westermani: Biochemical and immunological characterizations of paramyosin. Exp. Parasitol. 2007, 115, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, P.; Veasna, D.; Zhang, W.; Vannavong, N.; Phrommala, S.; Habe, S.; Strobel, M. Rapid identification of paragonimiasis foci by lay informants in Lao People’s Democratic Republic. PLoS Negl. Trop. Dis. 2009, 3, e521. [Google Scholar] [CrossRef]
- Kim, K.E.; Jung, S.S.; Park, H.S.; Lee, J.E.; Chung, C.; Lee, S.I.; Park, D. The first case report of Paragonimus westermani infection diagnosed by transbronchial lung cryobiopsy. Int. J. Infect. Dis. 2023, 128, 184–186. [Google Scholar] [CrossRef]
- Poudyal, B.S.; Paudel, B.; Bista, B.; Shrestha, G.S.; Pudasaini, P. Clinical, Laboratory and Radiological Features of Paragonimiasis Misdiagnosed as Pulmonary Tuberculosis. Iran. J. Parasitol. 2022, 17, 410. [Google Scholar] [CrossRef]
- Hwang, K.E.; Song, H.Y.; Jung, J.W.; Oh, S.J.; Yoon, K.H.; Park, D.S.; Kim, H.R. Pleural fluid characteristics of pleuropulmonary paragonimiasis masquerading as pleural tuberculosis. Korean J. Intern. Med. 2015, 30, 56. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Y.; Sadjjadi, S.M.; Jafari, S.H.; NikoupourDeilami, H.; Mardani, P.; Solgi, R. Application and evaluation of native antigen B from Echinococcus granulosus sensu stricto and E. canadensis alone or mixture for serodiagnosis of human G1-G3 and G6/G7 genotypes cystic echinococcosis sera, using ELISA and Western blotting. Parasitol. Res. 2023, 122, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Medina-Rojas, R.; Zuñiga-Sanchez, H.A.; Castillo-Coaquira, I.E.; Sucari-Turpo, W.G.; Hoces-La-Rosa, Z.P.; Sarayasi-Alencastre, Y. Western Blot for the diagnosis of the acute and chronic phase of animal and human fasciolosis, using different antigens of Fasciola hepatica. J. Surv. Fish. Sci. 2023, 10, 1362–1373. [Google Scholar]
- Suescún-Carrero, S.H.; Tadger, P.; Sandoval Cuellar, C.; Armadans-Gil, L.; Ramirez Lopez, L.X. Rapid diagnostic tests and ELISA for diagnosing chronic Chagas disease: Systematic revision and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010860. [Google Scholar] [CrossRef] [PubMed]
- Meftahi, G.H.; Bahari, Z.; Zarei Mahmoudabadi, A.; Iman, M.; Jangravi, Z. Applications of western blot technique: From bench to bedside. Biochem. Mol. Biol. Educ. 2021, 49, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Ahn, C.S.; Kang, I.; Shin, J.W.; Jeong, H.B.; Nawa, Y.; Kong, Y. Cerebral paragonimiasis: Clinicoradiological features and serodiagnosis using recombinant yolk ferritin. PLoS Negl. Trop. Dis. 2022, 16, e0010240. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.A.; Choi, Y.J.; McNulty, S.N.; Jung, H.; Martin, J.; Agatsuma, T.; Mitreva, M. Comparative genomics and transcriptomics of 4 Paragonimus species provide insights into lung fluke parasitism and pathogenesis. GigaScience 2020, 9, giaa073. [Google Scholar] [CrossRef]
- Biswal, D.K.; Chatterjee, A.; Bhattacharya, A.; Tandon, V. The mitochondrial genome of Paragonimus westermani (Kerbert, 1878), the Indian isolate of the lung fluke representative of the family Paragonimidae (Trematoda). PeerJ 2014, 2, e484. [Google Scholar] [CrossRef]
- Doanh, N.P.; Tu, A.L.; Bui, T.D.; Loan, T.H.; Nonaka, N.; Horii, Y.; Nawa, Y. Molecular and morphological variation of Paragonimus westermani in Vietnam with records of new second intermediate crab hosts and a new locality in a northern province. Parasitology 2016, 143, 1639–1646. [Google Scholar] [CrossRef]
- Persichetti, M.F.; Solano-Gallego, L.; Vullo, A.; Masucci, M.; Marty, P.; Delaunay, P.; Pennisi, M.G. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasites Vectors 2017, 10, 119. [Google Scholar] [CrossRef]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Blaga, R.; Zivojinovic, M.; Ribicich, M.; Pozio, E. A distinctive Western blot pattern to recognize Trichinella infections in humans and pigs. Int. J. Parasitol. 2012, 42, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Shiguekawa KY, M.; Mineo, J.R.; de Moura, L.P.; Costa-Cruz, J.M. ELISA and western blotting tests in the detection of IgG antibodies to Taenia solium metacestodes in serum samples in human neurocysticercosis. Trop. Med. Int. Health 2000, 5, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, L.S.; Curtis, K.C.; Erdmann-Gilmore, P.; Sprung, R.S.; Townsend, R.R.; Weil, G.J.; Fischer, P.U. Comparative proteomics of adult Paragonimus kellicotti excretion/secretion products released in vitro or present in the lung cyst nodule. PLoS Negl. Trop. Dis. 2022, 16, e0010679. [Google Scholar] [CrossRef]
- Di Maggio, L.S.; Fischer, K.; Yates, D.; Curtis, K.C.; Rosa, B.A.; Martin, J.; Fischer, P.U. The proteome of extracellular vesicles of the lung fluke Paragonimus kellicotti produced in vitro and in the lung cyst. Sci. Rep. 2023, 13, 13726. [Google Scholar] [CrossRef]
- Becerro-Recio, D.; González-Miguel, J.; Ucero, A.; Sotillo, J.; Martínez-Moreno, Á.; Pérez-Arévalo, J.; Siles-Lucas, M. Recognition pattern of the Fasciola hepatica excretome/secretome during the course of an experimental infection in sheep by 2D Immunoproteomics. Pathogens 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, S.; Stachyra, A.; Stefaniak, J.; Mrówka, K.; Moskwa, B.; Bień-Kalinowska, J. Immunoproteomic analysis of Trichinella spiralis and Trichinella britovi excretory-secretory muscle larvae proteins recognized by sera from humans infected with Trichinella. PLoS ONE 2020, 15, e0241918. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Na, B.K.; Chung, D.L.; Kim, J.G.; Kim, J.T.; Kong, Y. Expression characteristics and specific antibody reactivity of diverse cathepsin F members of Paragonimus westermani. Parasitol. Int. 2015, 64, 37–42. [Google Scholar] [CrossRef]
- Pedroza-Gómez, Y.J.; Cossio-Bayugar, R.; Aguilar-Díaz, H.; Scarcella, S.; Reynaud, E.; Sanchez-Carbente, M.D.R.; Miranda-Miranda, E. Transcriptome-Based Identification of a Functional Fasciola Hepatica Carboxylesterase B. Pathogens 2021, 10, 1454. [Google Scholar] [CrossRef]
- Hokke, C.H.; van Diepen, A. Helminth glycomics–glycan repertoires and host-parasite interactions. Mol. Biochem. Parasitol. 2017, 215, 47–57. [Google Scholar] [CrossRef]
- Smit, C.H.; van Diepen, A.; Nguyen, D.L.; Wuhrer, M.; Hoffmann, K.F.; Deelder, A.M.; Hokke, C.H. Glycomic Analysis of Life Stages of the Human Parasite Schistosoma Mansoni Reveals Developmental Expression Profiles of Functional and Antigenic Glycan Motifs. Mol. Cell. Proteom. 2015, 14, 1750–1769. [Google Scholar] [CrossRef]
- Meevissen, M.H.; Wuhrer, M.; Doenhoff, M.J.; Schramm, G.; Haas, H.; Deelder, A.M.; Hokke, C.H. Structural characterization of glycans on omega-1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J. Proteome Res. 2010, 9, 2630–2642. [Google Scholar] [CrossRef] [PubMed]
- Silva-Moraes, V.; Shollenberger, L.M.; Castro-Borges, W.; Rabello, A.L.T.; Harn, D.A.; Medeiros, L.C.S.; Jeremias, W.D.J.; Siqueira, L.M.V.; Pereira, C.S.S.; Pedrosa, M.L.C.; et al. Serological proteomic screening and evaluation of a recombinant egg antigen for the diagnosis of low-intensity Schistosoma mansoni infections in endemic area in Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0006974. [Google Scholar] [CrossRef] [PubMed]
- de Noya, B.A.; Colmenares, C.; Lanz, H.; Caracciolo, M.A.; Losada, S.; Noya, O. Schistosoma mansoni: Immunodiagnosis is improved by sodium metaperiodate which reduces cross-reactivity due to glycosylated epitopes of soluble egg antigen. Exp. Parasitol. 2000, 95, 106–112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).