Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Location
2.3. Study Population
2.4. Inclusion and Exclusion Criteria
2.5. Identification of HH Contacts for TPT
2.6. Initiation of TPT with 3HP
2.7. Treatment Support and Monitoring of Adverse Events
2.8. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics
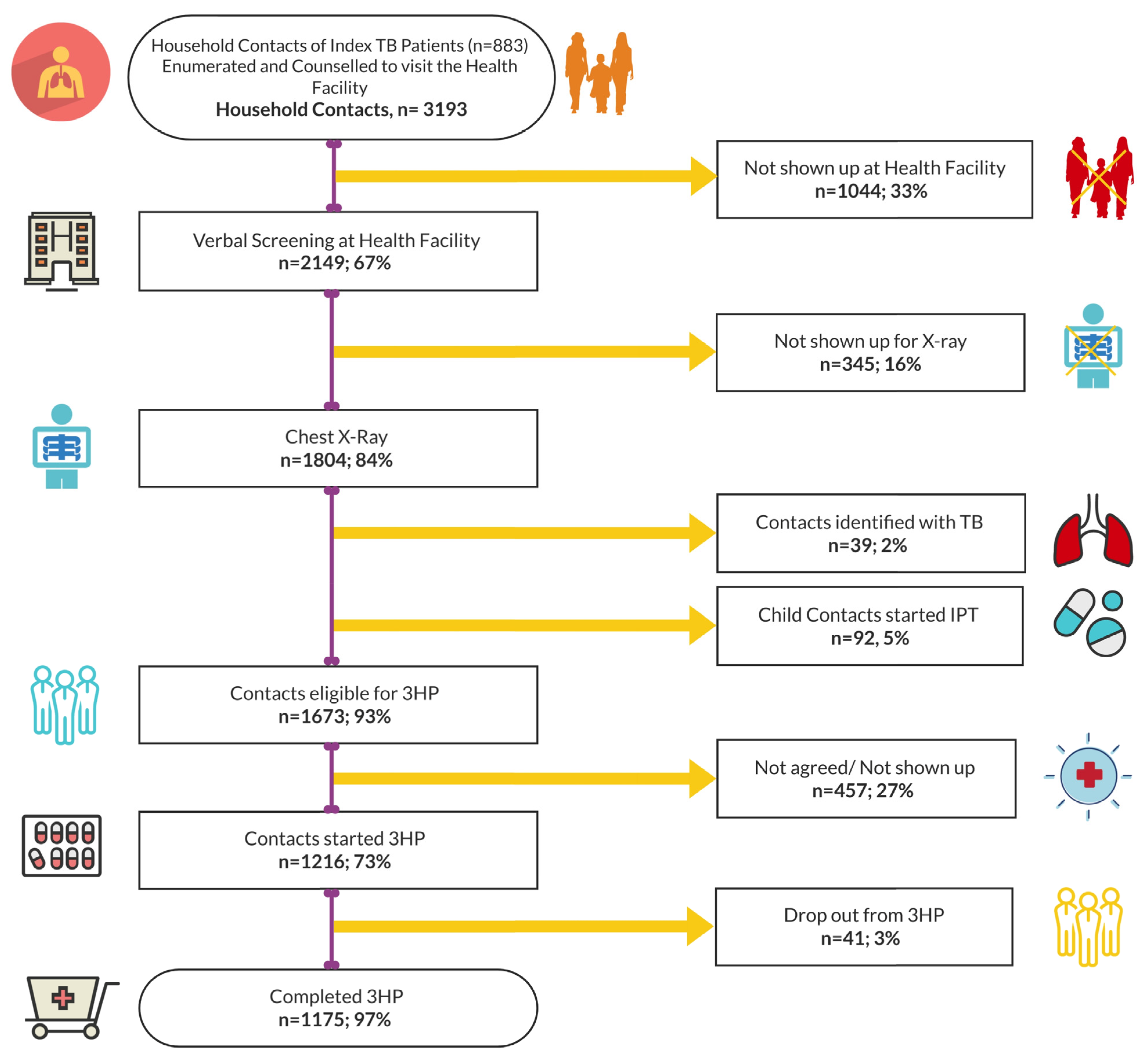
3.2. Evaluation, Initiation, and Completion of TPT among HH Contacts
3.3. Reported Adverse Events
3.4. Factors Associated with TPT Completion with the 3HP Regimen
3.5. Intervention Approaches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G. Getahun H: WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Latent TB Infection: Updated and Consolidated Guidelines for Programmatic Management; World Health Organization: Geneva, Switzerland, 2018.
- Styblo, K. The relationship between the risk of tuberculous infection and the risk of developing infectious tuberculosis. Bull IUAT 1985, 60, 117–119. [Google Scholar]
- Dye, C.; Glaziou, P.; Floyd, K.; Raviglione, M. Prospects for tuberculosis elimination. Annu. Rev. Public Health 2013, 34, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Gutierrez, S.M.B.; Bruchfeld, J.; Burhan, E. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
- National TB Control Program. Tuberculosis Control in Bangladesh: Annual Report 2019; Directorate General of Health Services: Dhaka, Bangladesh, 2019.
- Stuurman, A.L.; Noordegraaf-Schouten, M.V.; van Kessel, F.; Oordt-Speets, A.M.; Sandgren, A.; van der Werf, M.J. Interventions for improving adherence to treatment for latent tuberculosis infection: A systematic review. BMC Infect. Dis. 2016, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Martinson, N.A.; Barnes, G.L.; Moulton, L.H.; Msandiwa, R.; Hausler, H.; Ram, M.; McIntyre, J.A.; Gray, G.E.; Chaisson, R.E. New regimens to prevent tuberculosis in adults with HIV infection. N. Engl. J. Med. 2011, 365, 11–20. [Google Scholar] [CrossRef]
- Sterling, T.R.; Villarino, M.E.; Borisov, A.S.; Shang, N.; Gordin, F.; Bliven-Sizemore, E.; Hackman, J.; Hamilton, C.D.; Menzies, D.; Kerrigan, A. Three months of rifapentine and isoniazid for latent tuberculosis infection. N. Engl. J. Med. 2011, 365, 2155–2166. [Google Scholar] [CrossRef]
- Villarino, M.E.; Scott, N.A.; Weis, S.E.; Weiner, M.; Conde, M.B.; Jones, B.; Nachman, S.; Oliveira, R.; Moro, R.N.; Shang, N. Treatment for preventing tuberculosis in children and adolescents: A randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015, 169, 247–255. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, A.; Kadhiravan, T.; Tharyan, P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Evid. Based Child Health A Cochrane Rev. J. 2014, 9, 169–294. [Google Scholar] [CrossRef]
- Njie, G.J.; Morris, S.B.; Woodruff, R.Y.; Moro, R.N.; Vernon, A.A.; Borisov, A.S. Isoniazid-rifapentine for latent tuberculosis infection: A systematic review and meta-analysis. Am. J. Prev. Med. 2018, 55, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Zenner, D.; Beer, N.; Harris, R.J.; Lipman, M.C.; Stagg, H.R.; Van Der Werf, M.J. Treatment of latent tuberculosis infection: An updated network meta-analysis. Ann. Intern. Med. 2017, 167, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Semitala, F.C.; Kadota, J.L.; Musinguzi, A.; Nabunje, J.; Welishe, F.; Nakitende, A.; Akello, L.; Bishop, O.; Patel, D.; Sammann, A. Completion of isoniazid–rifapentine (3HP) for tuberculosis prevention among people living with HIV: Interim analysis of a hybrid type 3 effectiveness–implementation randomized trial. PLoS Med. 2021, 18, e1003875. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.M.; Majidulla, A.; Jaswal, M.; Safdar, N.; Malik, A.A.; Khan, A.J.; Becerra, M.C.; Keshavjee, S.; Lu, C.; Hussain, H. Cost of delivering 12-dose isoniazid and rifapentine versus 6 months of isoniazid for tuberculosis infection in a high-burden setting. Clin. Infect. Dis. 2021, 73, e1135–e1141. [Google Scholar] [CrossRef] [PubMed]
- Challenge TB Project, Contextual Factors Affecting Implementation and Uptake of Preventive Treatment in Urban Settings: A Qualitative Study; IRD, MSH, KNCV: Dhaka, Bangladesh, 2018.
- Hamada, Y.; Ford, N.; Schenkel, K.; Getahun, H. Three-month weekly rifapentine plus isoniazid for tuberculosis preventive treatment: A systematic review. Int. J. Tuberc. Lung Dis. 2018, 22, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Harries, A.D.; Kumar, A.M.; Satyanarayana, S.; Takarinda, K.C.; Timire, C.; Dlodlo, R.A. Treatment for latent tuberculosis infection in low-and middle-income countries: Progress and challenges with implementation and scale-up. Expert Rev. Respir. Med. 2020, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Pease, C.; Hutton, B.; Yazdi, F.; Wolfe, D.; Hamel, C.; Quach, P.; Skidmore, B.; Moher, D.; Alvarez, G.G. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: A systematic review with network meta-analyses. BMC Infect. Dis. 2017, 17, 256. [Google Scholar] [CrossRef]
- Sandul, A.L.; Nwana, N.; Holcombe, J.M.; Lobato, M.N.; Marks, S.; Webb, R.; Wang, S.-H.; Stewart, B.; Griffin, P.; Hunt, G. High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine for latent Mycobacterium tuberculosis infection. Clin. Infect. Dis. 2017, 65, 1085–1093. [Google Scholar] [CrossRef]
- Belknap, R.; Holland, D.; Feng, P.; Millet, J.; Caylà, J.; Martinson, N.; Wright, A.; Chen, M.; Moro, R.; Scott, N. TB Trials Consortium iAdhere Study Team Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: A randomized trial. Ann. Intern. Med. 2017, 167, 689–697. [Google Scholar] [CrossRef]
- Haas, M.K.; Aiona, K.; Erlandson, K.M.; Belknap, R.W. Higher Completion Rates with Self-administered Once-weekly Isoniazid-Rifapentine versus Daily Rifampin in Adults with Latent Tuberculosis. Clin. Infect. Dis. 2021, 73, e3459–e3467. [Google Scholar] [CrossRef]
- Cruz, A.T.; Starke, J.R. Completion rate and safety of tuberculosis infection treatment with shorter regimens. Pediatrics 2018, 141, e20172838. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, Y.; Hu, Z.-d.; Xia, L.; Liu, X.-h.; Yu, X.; Ma, J.-y.; Li, T.; Lu, S.-h. High rate of completion for weekly rifapentine plus isoniazid treatment in Chinese children with latent tuberculosis infection—A single center study. PLoS ONE 2021, 16, e0253159. [Google Scholar] [CrossRef] [PubMed]
- Surey, J.; Stagg, H.R.; Yates, T.A.; Lipman, M.; White, P.J.; Charlett, A.; Muñoz, L.; Gosce, L.; Rangaka, M.X.; Francis, M. An open label, randomised controlled trial of rifapentine versus rifampicin based short course regimens for the treatment of latent tuberculosis in England: The HALT LTBI pilot study. BMC Infect. Dis. 2021, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Stennis, N.L.; Burzynski, J.N.; Herbert, C.; Nilsen, D.; Macaraig, M. Treatment for tuberculosis infection with 3 months of isoniazid and rifapentine in New York City health department clinics. Clin. Infect. Dis. 2016, 62, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Lee, M.-R.; Cheng, M.-H.; Lu, P.-L.; Huang, C.-K.; Sheu, C.-C.; Lai, P.-C.; Chen, T.-C.; Wang, J.-Y.; Chong, I.-W. Impact of age on outcome of rifapentine-based weekly therapy for latent tuberculosis infection. Clin. Infect. Dis. 2021, 73, e1064–e1071. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.E.; Bass, S.; Srinivas, P.; Miranda, C.; Johnson, L.; Pallotta, A.M. Evaluation of 3 months of once-weekly rifapentine and isoniazid for latent tuberculosis infection. Ann. Pharmacother. 2020, 54, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.-L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Huang, Y.-W.; Huang, W.-C.; Chang, L.-Y.; Chan, P.-C.; Chuang, Y.-C.; Ruan, S.-Y.; Wang, J.-Y.; Wang, J.-T. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: A multicentre randomised controlled trial in Taiwan. Tuberculosis 2018, 111, 121–126. [Google Scholar] [CrossRef]
- Malik, A.A.; Farooq, S.; Jaswal, M.; Khan, H.; Nasir, K.; Fareed, U.; Shahbaz, S.; Amanullah, F.; Safdar, N.; Khan, A.J. Safety and feasibility of 1 month of daily rifapentine plus isoniazid to prevent tuberculosis in children and adolescents: A prospective cohort study. Lancet Child Adolesc. Health 2021, 5, 350–356. [Google Scholar] [CrossRef]
- Schmit, K.M.; Wortham, J.M.; Ho, C.S.; Powell, K.M. Analysis of severe adverse events reported among patients receiving isoniazid-rifapentine treatment for latent Mycobacterium tuberculosis infection—United States, 2012–2016. Clin. Infect. Dis. 2020, 71, 2502–2505. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Tsao, S.-M.; Yang, W.-T.; Huang, W.-C.; Lin, C.-H.; Chen, W.-W.; Yang, S.-F.; Chiou, H.-L.; Huang, Y.-W. Association of drug metabolic enzyme genetic polymorphisms and adverse drug reactions in patients receiving rifapentine and isoniazid therapy for latent tuberculosis. Int. J. Environ. Res. Public Health 2020, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- Pease, C.; Hutton, B.; Yazdi, F.; Wolfe, D.; Hamel, C.; Barbeau, P.; Skidmore, B.; Alvarez, G.G. A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiol. Drug Saf. 2018, 27, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, J.; Coleman, K.J.; Simon, G.; Nebeker, C. The role of community health workers (CHWs) in health promotion research: Ethical challenges and practical solutions. Health Promot. Pract. 2011, 12, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.K.; Harris-Roxas, B.; Yadav, U.N.; Shabnam, S.; Rawal, L.B.; Harris, M.F. Community health workers can provide psychosocial support to the people during COVID-19 and beyond in low-and middle-income countries. Front. Public Health 2021, 800, 666753. [Google Scholar] [CrossRef]
| Variables | Household Contacts Verbally Screened (n = 2149) | Household Contacts Who Initiated TPT with 3HP (n = 1216) |
|---|---|---|
| Age—mean age (± SD) | 21.2 (±17.5) | 27.4 (±23.8) |
| <5 | 65 (3.0%) | 40 (3.3%) |
| 5–<15 | 484 (22.5%) | 272 (22.4%) |
| 15 and above | 1600 (74.5%) | 904 (74.3%) |
| Female | 1178 (54.8%) | 675 (56.0%) |
| Schooling in year | ||
| No schooling | 323 (15.0%) | 146 (12.0%) |
| 1–5 years | 726 (33.8%) | 383 (31.5%) |
| 6–10 years | 710 (33.0%) | 345 (28.4%) |
| 10+ years | 390 (18.1%) | 342 (28.1%) |
| Household income/month in BDT (mean ± SD) * | 14,532 ± 9853 | 15,251 ± 10,235 |
| ≤5000 | 14 (0.7%) | 7 (0.5%) |
| 5001–10,000 | 321 (14.9%) | 165 (13.6%) |
| 10,001–20,000+ | 1814 (84.4%) | 1044 (85.9%) |
| Original residence | ||
| Permanent resident of Dhaka | 677 (31.5%) | 398 (32.7%) |
| Tenant (rural-to-urban migrant) | 1472 (68.5%) | 818 (67.3%) |
| Current dwelling status | ||
| Living in slums | 1027 (47.8%) | 589 (48.4%) |
| Non-slum households | 1122 (52.2%) | 627 (51.6%) |
| Occupation | ||
| Student and dependent child | 543 (25.3%) | 361 (29.7%) |
| Day labor/garments/factory work | 605 (28.2%) | 234 (19.2%) |
| Self-employed and business | 359 (16.7%) | 138 (11.3%) |
| Public/private service | 246 (11.4%) | 132 (10.9%) |
| Homemaker | 380 (19.9%) | 341 (28.0%) |
| Unemployed | 16 (0.7%) | 10 (0.8%) |
| Current or past smoker | 892 (41.4%) | 483 (39.7%) |
| Comorbidity | ||
| No comorbidity | 1372 (68.5%) | 1194 (98.2%) |
| Diabetes mellitus | 95 (4.4%) | 16 (1.3%) |
| Hypertension | 409 (19.0%) | 04 (0.3%) |
| Asthma | 14 (0.7%) | 01 (0.1%) |
| Thyroid dysfunction | 159 (7.4%) | 01 (0.1%) |
| Experienced any adverse events | NA | 65 (5.3%) |
| Experienced Adverse Events after Any 3HP Dose by Type * | Frequency ** with Grading *** | |||
|---|---|---|---|---|
| Not Graded | Grade 1 | Grade 2 | Grades 3, 4, and 5 | |
| Gastrointestinal—nausea/vomiting | - | 20 (1.64%) | 01 (0.08%) | - |
| Neurological symptoms | - | 01 (0.08%) | - | - |
| Muscle pain | - | 02 (0.16%) | - | - |
| Hepatotoxicity | - | 00 (0.00%) | - | - |
| Flu-like symptoms | - | 10 (0.82%) | 01 (0.08%) | - |
| Dermal—itching/skin rash | 01 (0.08%) | 09 (0.74%) | - | - |
| Respiratory symptoms | - | 02 (0.16%) | - | - |
| Fatigue | - | 07 (0.58%) | - | - |
| Headache | - | 06 (0.49%) | - | - |
| Other symptoms ‡ | - | 05 (0.41%) | - | - |
| Total experiencing adverse events | 01 (0.08%) | 62 (5.09%) | 02 (0.16%) | 00 (0.00%) |
| Variables | Bivariate Model | Multivariable Model * | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Current age (r = age < 15 years) | 2.1 (1.8–2.4) | 0.004 | 1.5 (1.1–2.0) | 0.043 |
| Female (r = male) | 2.5 (1.7–2.8) | 0.002 | 1.7 (1.3–2.1) | 0.009 |
| Schooling in year (r = 0) | 2.2 (1.7–2.5) | 0.004 | 1.4 (1.1–1.9) | 0.044 |
| Monthly household income > BDT 10,000 (r = ≤ BDT 10,000) a | 1.7 (1.3–2.1) | 0.005 | 1.5 (1.0–2.1) | 0.047 |
| Permeant urban resident (r = rural-to-urban migrant with temporary settlement) | 1.2 (0.8–2.3) | 0.098 | NA | |
| Lives in non-slum household (r = lives in slum) | 1.6 (1.3–2.1) | 0.049 | 1.1 (0.7–2.4) | 0.088 |
| Occupation: non-manual work (r = manual work) | 1.1 (0.7–2.7) | 0.106 | NA | |
| No comorbidities (r = have had any comorbidities) | 2.3 (1.8–2.5) | 0.008 | 1.7 (1.1–2.2) | 0.046 |
| Experienced no adverse events (r = experienced any adverse events) | 1.8 (1.3–2.4) | 0.003 | 1.6 (1.2–2.1) | 0.009 |
| Intervention Approaches | ||||
| Reminder phone calls + treatment counseling (r = reminder phone calls only for the next dose schedule) | 2.7 (2.1–3.2) | 0.002 | 1.9 (1.5–2.4) | 0.007 |
| Reminder phone calls + treatment counseling + follow-up home visit by health workers (r = reminder phone calls only) | 3.2 (2.7–3.6) | 0.001 | 2.1 (1.5–2.7) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.T.; Hossain, F.; Banu, R.S.; Islam, M.S.; Alam, S.; Faisel, A.J.; Salim, H.; Cordon, O.; Suarez, P.; Hussain, H.; et al. Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study. Trop. Med. Infect. Dis. 2024, 9, 4. https://doi.org/10.3390/tropicalmed9010004
Rahman MT, Hossain F, Banu RS, Islam MS, Alam S, Faisel AJ, Salim H, Cordon O, Suarez P, Hussain H, et al. Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study. Tropical Medicine and Infectious Disease. 2024; 9(1):4. https://doi.org/10.3390/tropicalmed9010004
Chicago/Turabian StyleRahman, Md. Toufiq, Farzana Hossain, Rupali Sisir Banu, Md. Shamiul Islam, Shamsher Alam, Abu Jamil Faisel, Hamid Salim, Oscar Cordon, Pedro Suarez, Hamidah Hussain, and et al. 2024. "Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study" Tropical Medicine and Infectious Disease 9, no. 1: 4. https://doi.org/10.3390/tropicalmed9010004
APA StyleRahman, M. T., Hossain, F., Banu, R. S., Islam, M. S., Alam, S., Faisel, A. J., Salim, H., Cordon, O., Suarez, P., Hussain, H., & Roy, T. (2024). Uptake and Completion of Tuberculosis Preventive Treatment Using 12-Dose, Weekly Isoniazid–Rifapentine Regimen in Bangladesh: A Community-Based Implementation Study. Tropical Medicine and Infectious Disease, 9(1), 4. https://doi.org/10.3390/tropicalmed9010004








