Spectrum of TB Disease and Treatment Outcomes in a Mobile Community Based Active Case Finding Program in Yogyakarta Province, Indonesia
Abstract
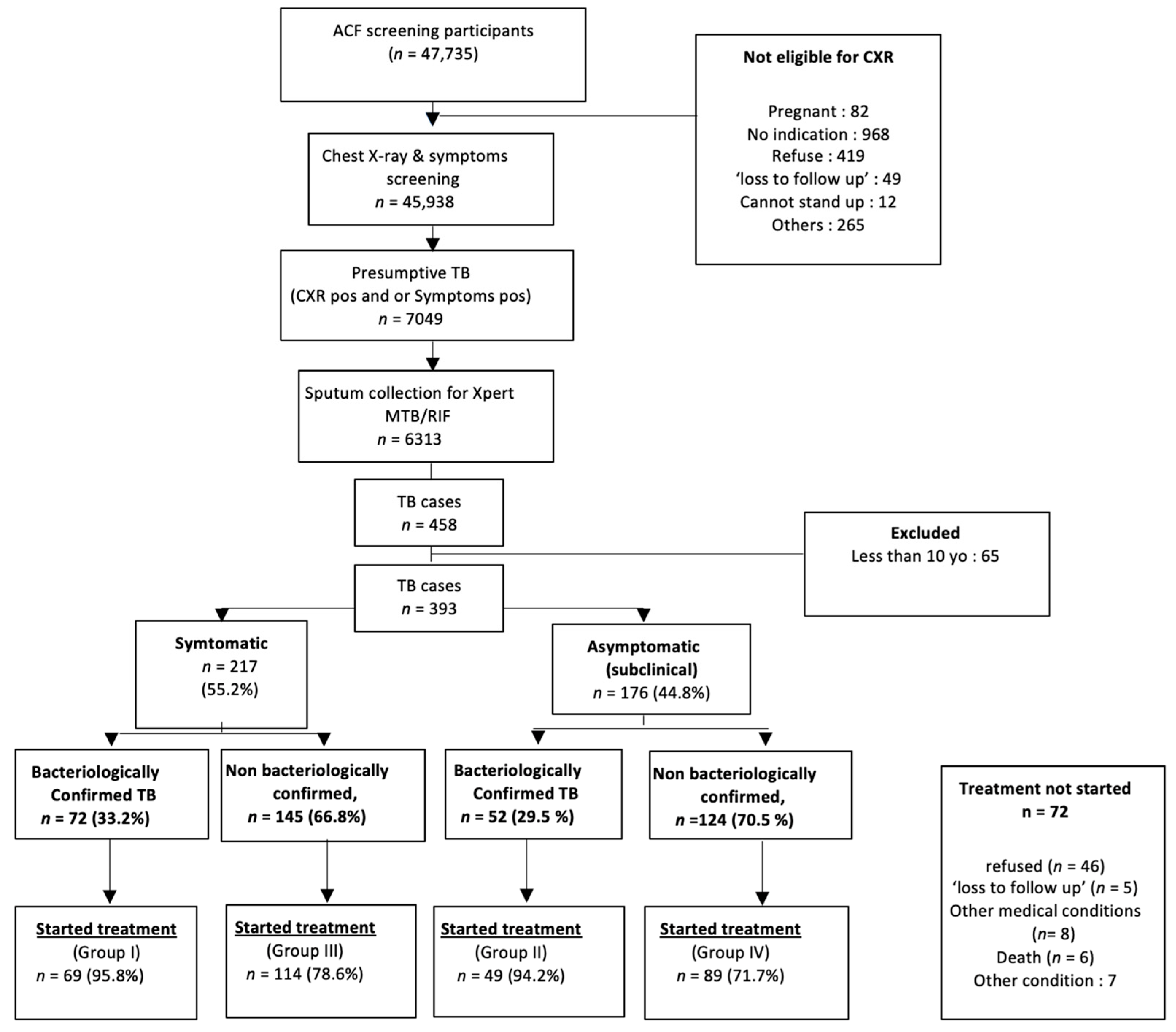
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Study Population
2.4. Data Management and Statistics
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. Available online: http://www.nature.com/articles/nrmicro2236 (accessed on 4 August 2022). [CrossRef] [PubMed]
- Wong, E.B. It Is Time to Focus on Asymptomatic Tuberculosis. Clin. Infect. Dis. 2021, 72, e1044–e1046. Available online: https://academic.oup.com/cid/article/72/12/e1044/6024996 (accessed on 25 July 2023). [CrossRef]
- Ku, C.C.; MacPherson, P.; Khundi, M.; Nzawa Soko, R.H.; Feasey, H.R.A.; Nliwasa, M.; Horton, K.C.; Corbett, E.L.; Dodd, P.J. Durations of asymptomatic, symptomatic, and care-seeking phases of tuberculosis disease with a Bayesian analysis of prevalence survey and notification data. BMC Med. 2021, 19, 298. Available online: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-02128-9 (accessed on 25 July 2023). [CrossRef]
- Drain, P.K.; Bajema, K.L.; Dowdy, D.; Dheda, K.; Naidoo, K.; Schumacher, S.G.; Ma, S.; Meermeier, E.; Lewinsohn, D.M.; Sherman, D.R. Incipient and Subclinical Tuberculosis: A Clinical Review of Early Stages and Progression of Infection. Clin. Microbiol. Rev. 2018, 31, e00021-18. Available online: https://journals.asm.org/doi/10.1128/CMR.00021-18 (accessed on 3 August 2022). [CrossRef] [PubMed]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. Available online: http://breathe.ersjournals.com/lookup/doi/10.1183/20734735.0079-2021 (accessed on 4 August 2022). [CrossRef] [PubMed]
- Esmail, H.; Dodd, P.J.; Houben, R.M.G.J. Tuberculosis transmission during the subclinical period: Could unrelated cough play a part? Lancet Respir. Med. 2018, 6, 244–246. Available online: https://linkinghub.elsevier.com/retrieve/pii/S221326001830105X (accessed on 4 August 2022). [CrossRef] [PubMed]
- Frascella, B.; Richards, A.S.; Sossen, B.; Emery, J.C.; Odone, A.; Law, I.; Onozaki, I.; Esmail, H.; Houben, R.M.G.J. Subclinical Tuberculosis Disease—A Review and Analysis of Prevalence Surveys to Inform Definitions, Burden, Associations, and Screening Methodology. Clin. Infect. Dis. 2021, 73, e830–e841. Available online: https://academic.oup.com/cid/article/73/3/e830/5906549 (accessed on 4 August 2022). [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on Tuberculosis Module 2: Screening; WHO: Geneva, Switzerland, 2020; 99p.
- WHO. Chest Radiography in Tuberculosis Detection. In Summary of Current WHO Recommendations and Guidance on Programmatic Approaches; WHO: Geneva, Swistzerland, 2016. [Google Scholar]
- Keshavjee, S.; Dowdy, D.; Swaminathan, S. Stopping the body count: A comprehensive approach to move towards zero tuberculosis deaths. Lancet 2015, 386, e46–e47. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673615003207 (accessed on 10 February 2023). [CrossRef] [PubMed]
- Indonesia’s Ministry of Health. Inventory Study; Indonesia’s Ministry of Health: Jakarta, Indonesia, 2023.
- Engle, E.; Gabrielian, A.; Long, A.; Hurt, D.E.; Rosenthal, A. Performance of Qure.ai automatic classifiers against a large annotated database of patients with diverse forms of tuberculosis. PLoS ONE 2020, 15, e0224445. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1532046408001226 (accessed on 10 February 2023). [CrossRef] [PubMed]
- World Health Organization. Tuberculosis Country Profile 2021 Indonesia; World Health Organization: Geneva, Switzerland, 2021; Volume 2.
- Okoya, F.; Huang, C.C.; Zhang, Z.; Lecca, L.; Calderón, R.; Contreras, C.; Yataco, R.; Galea, J.; Becerra, M.; Murray, M. Culture-negative TB: Clinical characteristics, risk factors and treatment outcomes. Int. J. Tuberc. Lung Dis. 2023, 27, 557–563. Available online: https://www.ingentaconnect.com/content/10.5588/ijtld.22.0554 (accessed on 26 July 2023). [CrossRef] [PubMed]
- JEMM Review Team 2020. The Republic of Indonesia Joint External Monitoring Mission for Tuberculosis. Available online: https://tbindonesia.or.id/wp-content/uploads/2021/06/INDONESIA-JEMM-2020-Eng-1.pdf (accessed on 10 February 2023).
- Min, J.; Chung, C.; Jung, S.S.; Park, H.K.; Lee, S.S.; Lee, K.M. Subclinical tuberculosis disease and its treatment outcomes: A prospective cohort study in South Korea. In Tuberculosis; European Respiratory Society: Lausanne, Switzerland, 2020; p. 516. Available online: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.congress-2020.516 (accessed on 10 February 2023).
- Kendall, E.A.; Shrestha, S.; Dowdy, D.W. The Epidemiological Importance of Subclinical Tuberculosis. A Critical Reappraisal. Am. J. Respir. Crit. Care Med. 2021, 203, 168–174. Available online: https://www.atsjournals.org/doi/10.1164/rccm.202006-2394PP (accessed on 10 February 2023). [CrossRef] [PubMed]
- Burke, R.M.; Nliwasa, M.; Feasey, H.R.A.; Chaisson, L.H.; Golub, J.E.; Naufal, F.; E Shapiro, A.; Ruperez, M.; Telisinghe, L.; Ayles, H.; et al. Community-based active case-finding interventions for tuberculosis: A systematic review. Lancet Public Health 2021, 6, e283–e299. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Mapping evidence on tuberculosis active case finding policies, strategies, and interventions for tuberculosis key populations: A systematic scoping review protocol. Syst. Rev. 2019, 8, 162. [Google Scholar] [CrossRef] [PubMed]
| Total | Symptom (+) & Bacteriology (+) | Symptom (+) & Bacteriology (−) | Symptom (−) & Bacteriology (+) | Symptom (−) & Bacteriology (−) | p-Value | ||
|---|---|---|---|---|---|---|---|
| n = 321 | n = 69 | n = 114 | n = 49 | n = 89 | |||
| Sex | Male | 219 (68.2%) | 45 (65.2%) | 76 (66.7%) | 38 (77.6%) | 60 (67.4%) | 0.49 |
| Female | 102 (31.8%) | 24 (34.8%) | 38 (33.3%) | 11 (22.4%) | 29 (32.6%) | ||
| Age | 52.9 (18.7) | 44.9 (17.6) | 56.0 (18.4) | 52.9 (17.3) | 55.1 (19.2) | <0.001 | |
| Age Group | 10–<18 | 8 (2.5%) | 2 (2.9%) | 2 (1.8%) | 0 (0.0%) | 4 (4.5%) | <0.001 |
| 18–<60 | 174 (54.2%) | 53 (76.8%) | 49 (43.0%) | 29 (59.2%) | 43 (48.3%) | ||
| ≥60 | 139 (43.3%) | 14 (20.3%) | 63 (55.3%) | 20 (40.8%) | 42 (47.2%) | ||
| Screening location | Rural | 112 (37.8%) | 21 (33.3%) | 51 (49.5%) | 8 (16.7%) | 32 (39.0%) | 0.001 |
| Urban | 184 (62.2%) | 42 (66.7%) | 52 (50.5%) | 40 (83.3%) | 50 (61.0%) | ||
| Nutritional Status | BMI ≥18.5 to <23 | 120 (37.4%) | 27 (39.1%) | 36 (31.6%) | 17 (34.7%) | 40 (44.9%) | 0.28 |
| BMI < 18.5 | 133 (41.4%) | 29 (42.0%) | 55 (48.2%) | 17 (34.7%) | 32 (36.0%) | ||
| BMI > 23 | 68 (21.2%) | 13 (18.8%) | 23 (20.2%) | 15 (30.6%) | 17 (19.1%) | ||
| Previous TB treatment | New | 280 (90.0%) | 54 (81.8%) | 104 (94.5%) | 39 (83.0%) | 83 (94.3%) | 0.008 |
| Previously treated | 31 (10.0%) | 12 (18.2%) | 6 (5.5%) | 8 (17.0%) | 5 (5.7%) | ||
| Smoking Status | Never smoked | 165 (51.4%) | 34 (49.3%) | 57 (50.0%) | 26 (53.1%) | 48 (53.9%) | 0.92 |
| Current or previously smoked | 156 (48.6%) | 35 (50.7%) | 57 (50.0%) | 23 (46.9%) | 41 (46.1%) | ||
| HIV Status | Negative or unknown | 288 (89.7%) | 66 (95.7%) | 96 (84.2%) | 48 (98.0%) | 78 (87.6%) | 0.015 |
| Positive | 33 (10.3%) | 3 (4.3%) | 18 (15.8%) | 1 (2.0%) | 11 (12.4%) | ||
| Diabetes Mellitus Status | Negative or unknown | 288 (89.7%) | 60 (87.0%) | 104 (91.2%) | 41 (83.7%) | 83 (93.3%) | 0.26 |
| Positive | 33 (10.3%) | 9 (13.0%) | 10 (8.8%) | 8 (16.3%) | 6 (6.7%) | ||
| Cavity on Chest X-ray | no cavity | 273 (85.0%) | 51 (73.9%) | 101 (88.6%) | 44 (89.8%) | 77 (86.5%) | 0.031 |
| cavity | 48 (15.0%) | 18 (26.1%) | 13 (11.4%) | 5 (10.2%) | 12 (13.5%) | ||
| Rifampicin resistance | Rifampicin susceptible | 110 (93.2%) | 65 (94.2%) | 45 (91.8%) | 0.49 | ||
| Rifampicin resistant | 7 (5.9%) | 3 (4.4%) | 4 (8.2%) | ||||
| Indeterminate or missing | 1 (0.8%) | 1 (1.4%) | 0 (0%) |
| Total | Symptom (+) & Bacteriology (+) | Symptom (+) & Bacteriology (−) | Symptom (−) & Bacteriology (+) | Symptom (−) & Bacteriology (−) | ||
|---|---|---|---|---|---|---|
| n = 321 | n = 69 | n = 114 | n = 49 | n = 89 | ||
| Treatment outcome | successful | 227 (70.7%) | 54 (78.3%) | 84 (73.7%) | 33 (67.3%) | 56 (62.9%) |
| Unsuccessful | 94 (29.3%) | 15 (21.7%) | 30 (26.3%) | 16 (32.7%) | 33 (37.1%) | |
| Treatment outcome (detailed) | cure | 76 (23.7%) | 42 (60.9%) | 0 (0.0%) | 30 (61.2%) | 0 (0.0%) |
| completed | 151 (47.0%) | 12 (17.4%) | 84 (73.7%) | 3 (6.1%) | 56 (62.9%) | |
| LTFU | 29 (9%) | 5 (7.2%) | 12 (10.5%) | 3 (6.1%) | 9 (10.1%) | |
| failure | 2 (0.6%) | 0 (0.0%) | 0 (0.0%) | 2 (4.1%) | 0 (0.0%) | |
| death | 8 (2.5%) | 2 (2.9%) | 2 (1.8%) | 1 (2.0%) | 3 (3.4%) | |
| Not evaluated | 55 (17.1%) | 8 (11.6%) | 16 (14.0%) | 10 (20.4%) | 21 (23.6%) |
| Total | Successful | Unsuccessful | Odds Ratio | 95% CI | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| n = 321 | n = 227 | n = 94 | ||||||
| Age | 52.9 (18.7%) | 51.1 (19.0%) | 57.2 (17.4%) | 1.02 | (1.00, 1.03) | 1.01 | [1.00, 1.03] | |
| Sex | Male | 219 (68.2%) | 160 (70.5%) | 59 (62.8%) | Reference | Reference | ||
| Female | 102 (31.8%) | 67 (29.5%) | 35 (37.2%) | 1.42 | (0.85, 2.35) | 1.29 | [0.75, 2.22] | |
| Nutritional Status | BMI ≥ 18.5 to <23 | 120 (37.4%) | 82 (36.1%) | 38 (40.4%) | Reference | Reference | ||
| BMI < 18.5 | 133 (41.4%) | 96 (42.3%) | 37 (39.4%) | 0.83 | (0.48, 1.43) | 0.83 | [0.47, 1.48] | |
| BMI > 23 | 68 (21.2%) | 49 (21.6%) | 19 (20.2%) | 0.84 | (0.43, 1.61) | 0.85 | [0.43, 1.70] | |
| Previous TB treatment | New | 280 (90.0%) | 195 (88.6%) | 85 (93.4%) | Reference | Reference | ||
| Previously treated | 31 (10.0%) | 25 (11.4%) | 6 (6.6%) | 0.55 | (0.22, 1.39) | 0.63 | [0.24, 1.66] | |
| HIV Status | Negative or unknown | 288 (89.7%) | 199 (87.7%) | 89 (94.7%) | Reference | Reference | ||
| Positive | 33 (10.3%) | 28 (12.3%) | 5 (5.3%) | 0.40 | (0.15, 1.07) | 0.44 | [0.13, 1.47] | |
| Diabetes Mellitus Status | Negative or unknown | 288 (89.7%) | 207 (91.2%) | 81 (86.2%) | Reference | Reference | ||
| Positive | 33 (10.3%) | 20 (8.8%) | 13 (13.8%) | 1.66 | (0.79, 3.50) | 1.40 | [0.63, 3.13] | |
| Cavity on Chest X-ray | no cavity | 273 (85.0%) | 193 (85.0%) | 80 (85.1%) | Reference | Reference | ||
| cavity | 48 (15.0%) | 34 (15.0%) | 14 (14.9%) | 0.99 | (0.51, 1.95) | 1.11 | [0.54, 2.28] | |
| Symptom status | Asymptomatic | 138 (43.0%) | 89 (39.2%) | 49 (52.1%) | Reference | Reference | ||
| Symptomatic | 183 (57.0%) | 138 (60.8%) | 45 (47.9%) | 0.59 | (0.36, 0.96) | 0.60 | [0.36, 1.00] | |
| Bacteriological Confirmation | Bacteriologically negative | 203 (63.2%) | 140 (61.7%) | 63 (67.0%) | Reference | Reference | ||
| Bacteriologically positive | 118 (36.8%) | 87 (38.3%) | 31 (33.0%) | 0.79 | (0.48, 1.31) | 0.84 | [0.48, 1.48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananda, N.R.; Triasih, R.; Dwihardiani, B.; Nababan, B.; Hidayat, A.; Chan, G.; Cros, P.d. Spectrum of TB Disease and Treatment Outcomes in a Mobile Community Based Active Case Finding Program in Yogyakarta Province, Indonesia. Trop. Med. Infect. Dis. 2023, 8, 447. https://doi.org/10.3390/tropicalmed8090447
Ananda NR, Triasih R, Dwihardiani B, Nababan B, Hidayat A, Chan G, Cros Pd. Spectrum of TB Disease and Treatment Outcomes in a Mobile Community Based Active Case Finding Program in Yogyakarta Province, Indonesia. Tropical Medicine and Infectious Disease. 2023; 8(9):447. https://doi.org/10.3390/tropicalmed8090447
Chicago/Turabian StyleAnanda, Nur Rahmi, Rina Triasih, Bintari Dwihardiani, Betty Nababan, Arif Hidayat, Geoff Chan, and Philipp du Cros. 2023. "Spectrum of TB Disease and Treatment Outcomes in a Mobile Community Based Active Case Finding Program in Yogyakarta Province, Indonesia" Tropical Medicine and Infectious Disease 8, no. 9: 447. https://doi.org/10.3390/tropicalmed8090447
APA StyleAnanda, N. R., Triasih, R., Dwihardiani, B., Nababan, B., Hidayat, A., Chan, G., & Cros, P. d. (2023). Spectrum of TB Disease and Treatment Outcomes in a Mobile Community Based Active Case Finding Program in Yogyakarta Province, Indonesia. Tropical Medicine and Infectious Disease, 8(9), 447. https://doi.org/10.3390/tropicalmed8090447









