Surgical Antibiotic Prophylaxis Administration Improved after introducing Dedicated Guidelines: A Before-and-After Study from Dhulikhel Hospital in Nepal (2019–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Site
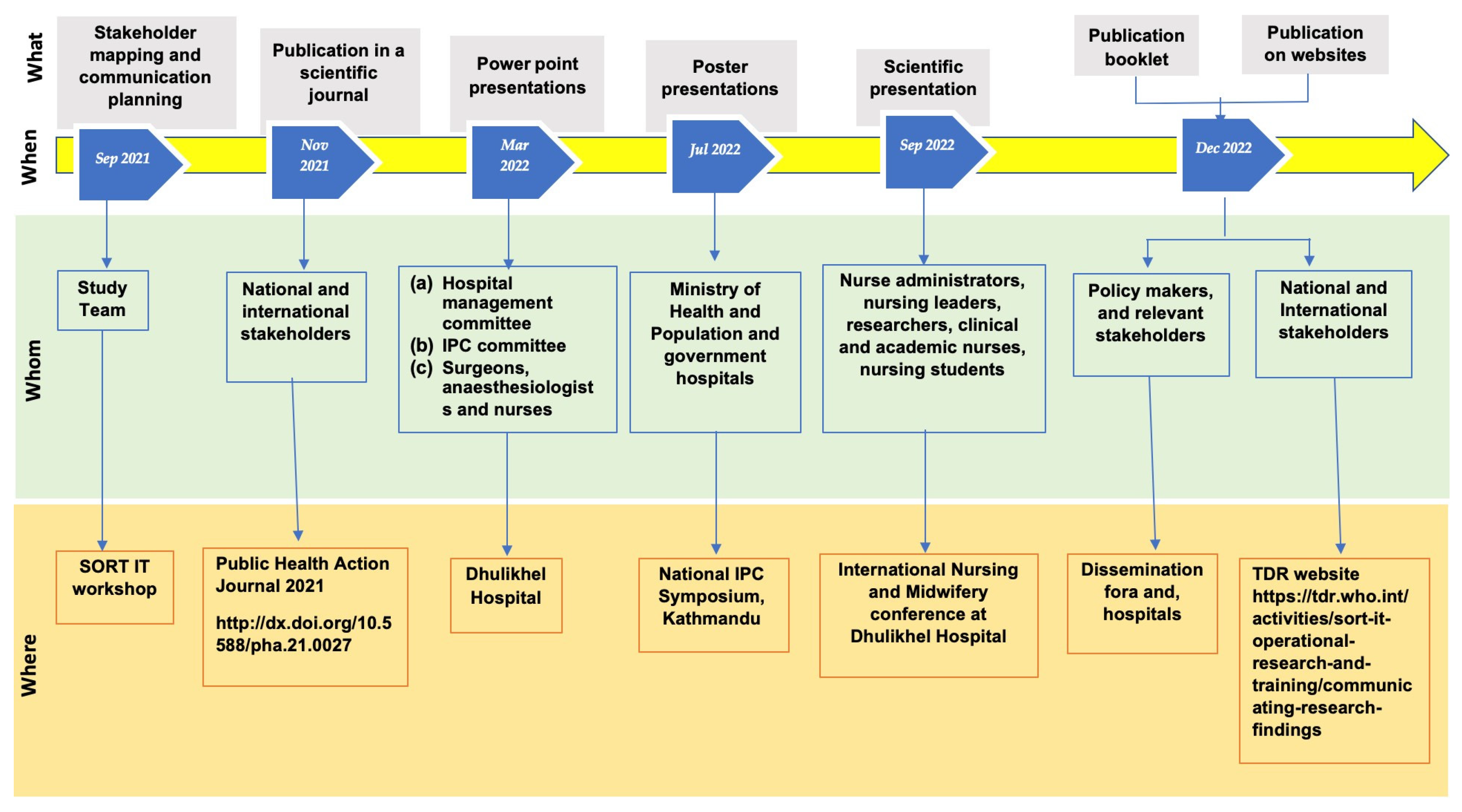
2.4. Dissemination Activities, Recommendations and Actions Taken
2.5. Development of Dedicated Guidelines for SAP
2.6. Study Population and Periods
2.7. Data Collection and Validation
2.8. Sample Size Calculation
2.9. Data Analysis and Statistics
3. Results
3.1. Demographic and Clinical Characteristics of Surgical Patients
3.2. Overall Proportion of Patients Who Received SAP in Compliance with the Guidelines
3.3. The Proportion of Eligible and Non-Eligible Patients Who Received Initial and Redosing of SAP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance-the Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.J.; Peacock, S.J.; Limmathurotsakul, D. Epidemiology and Burden of Multidrug-Resistant Bacterial Infection in a Developing Country. eLife 2016, 5, e18082. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. World Bank Rep. 2017, 2, 1–3. [Google Scholar]
- Antibiotic Resistance. Prev. Control 2010, 2005–2006. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/ (accessed on 1 January 2023).
- Cecchini, M.; Langer, J.; Slawomirski, L. Antimicrobial Resistance in G7 Countries and Beyond: Economic Issues, Policies and Options for Action; Director of the OECD Directorate for Employment, Labour and Social Affairs; OECD: Paris, France, 2015. [Google Scholar]
- Munckhof, W. Antibiotics for Surgical Prophylaxis. Aust. Prescr. 2005, 28, 38–40. [Google Scholar] [CrossRef]
- World Health Organization. WHO Drug Information; World Health Organization: Geneva, Switzerland, 1992; Volume 6. [Google Scholar]
- Hagel, S.; Scheuerlein, S.H. Perioperative Antibiotic Prophylaxis and Antimicrobial Therapy of Intra-Abdominal Infections. Visz. Gastrointest. Med. Surg. 2014, 30, 310–316. [Google Scholar] [CrossRef]
- Santana, R.S.; Viana, A.C.; Santiago, J.S.; Menezes, M.S.; Lobo, I.M.F.; Marcellini, P.S. The Cost of Excessive Postoperative Use of Antimicrobials: The Context of a Public Hospital. Rev. Do Colégio Bras. De Cir. 2014, 41, 149–154. [Google Scholar] [CrossRef]
- Tarchini, G.; Liau, K.H.; Solomkin, J.S. Antimicrobial Stewardship in Surgery: Challenges and Opportunities. Clin. Infect. Dis. 2017, 64, 112–114. [Google Scholar] [CrossRef]
- Van Disseldorp, J.; Slingenberg, E.J.; Matute, A.; Delgado, E.; Hak, E.; Hoepelman, I.M. Application of Guidelines on Preoperative Antibiotic Prophylaxis in León, Nicaragua. Neth. J. Med. 2006, 64, 411–416. [Google Scholar]
- Musmar, S.M.; Baba, H.; Owais, A. Adherence to Guidelines of Antibiotic Prophylactic Use in Surgery: A Prospective Cohort Study in North West Bank, Palestine. BMC Surg. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Satti, M.Z.; Hamza, M.; Sajid, Z.; Asif, O.; Ahmed, H.; Zaidi, M.J.; Irshad, U. Compliance Rate of Surgical Antimicrobial Prophylaxis and Its Association with Knowledge of Guidelines Among Surgical Residents in a Tertiary Care Public Hospital of a Developing Country Study Design. Cureus 2019, 11, 4776. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Surgical National Antimicrobial Prescribing Survey: Results of the 2016 Pilot. Available online: www.safetyandquality.gov.au (accessed on 25 January 2023).
- Shrestha, S.; Hann, K.; Kyaw KW, Y.; Koju, P.; Khogali, M. Surgical Antibiotic Prophylaxis Administration Practices. Public Health Action 2021, 11, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Population Government of Nepal National Antibiotic Treatment Guidelines-2014 (Technical Report); Government of Nepal: Kathmandu, Nepal, 2014.
- Kemp, S. Digital 2022: Nepal. Available online: https://datareportal.com/reports/digital-2022-nepal: (accessed on 25 January 2023).
- The GARP-Nepal National Working Group Situation Analysis and Recommendations Antibiotic Use and Resistance in Nepal. 2015. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj6iaKq0eWAAxWH6mEKHQd3AogQFnoECCIQAQ&url=https%3A%2F%2Fonehealthtrust.org%2Fwp-content%2Fuploads%2F2017%2F08%2Fgarp-nepal_sa.pdf&usg=AOvVaw0vbRUFv0N-kFyMCG5HMTvj&opi=89978449 (accessed on 25 January 2023).
- World Health Organization. SORT IT Operational Research and Training. Available online: https://tdr.who.int/activities/sort-it-operational-research-and-training (accessed on 6 June 2023).
- World Health Organization. Compliance with Surgical Antibiotic Prophylaxis, but More to Be Done, in a Referral Hospital in Nepal Key Messages. Available online: https://tdr.who.int/activities/sort-it-operational-research-and-training/communicating-research-findings (accessed on 6 June 2023).
- Bebell, L.M.; Muiru, A.N. Antibiotic Use and Emerging Resistance: How Can Resource-Limited Countries Turn the Tide? Glob. Heart 2014, 9, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Centre, C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 20–22. [Google Scholar]
- Shankar, P.R.; Subish, P.; Dubey, A.K.; Mishra, P.; Upadhyay, D.K. Surgical Antibiotic Prophylaxis in a Teaching Hospital in Western Nepal. J. Istitute Med. 2007, 29, 41–47. [Google Scholar]
- Schmitt, C.; Lacerda, R.A.; Turrini, R.N.T.; Padoveze, M.C. Improving Compliance with Surgical Antibiotic Prophylaxis Guidelines: A Multicenter Evaluation. Am. J. Infect. Control 2017, 45, 1111–1115. [Google Scholar] [CrossRef]
- Parulekar, L.; Soman, R.; Singhal, T.; Rodrigues, C.; Dastur, F.D.; Mehta, A. How Good Is Compliance with Surgical Antibiotic Prophylaxis Guidelines in a Tertiary Care Private Hospital in India? A Prospective Study. Indian J. Surg. 2009, 71, 15–18. [Google Scholar] [CrossRef][Green Version]
- So, J.P.; Aleem, I.S.; Tsang, D.S.; Matlow, A.G.; Wright, J.G. Increasing Compliance with an Antibiotic Prophylaxis Guideline to Prevent Pediatric Surgical Site Infection: Before and after Study. Ann. Surg. 2015, 262, 403–408. [Google Scholar] [CrossRef]
- Segala, F.V.; Murri, R.; Taddei, E.; Giovannenze, F.; Del Vecchio, P.; Birocchi, E.; Taccari, F.; Cauda, R.; Fantoni, M. Antibiotic Appropriateness and Adherence to Local Guidelines in Perioperative Prophylaxis: Results from an Antimicrobial Stewardship Intervention. Antimicrob. Resist. Infect. Control 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Ha, D.R.; Haste, N.M.; Gluckstein, D.P. The Role of Antibiotic Stewardship in Promoting Appropriate Antibiotic Use. Am. J. Lifestyle Med. 2019, 13, 376–383. [Google Scholar] [CrossRef] [PubMed]

| Recommendations | Action Status | Details of Action (When and What) |
|---|---|---|
| Establishment of a hospital committee for rational antibiotic use Establishment of an antibiotic stewardship program | Implemented Ongoing | July 2022 Hospital committee established. Lead persons assigned from internal medicine and pharmacology. One doctor is being trained in infection, prevention and control |
| Develop a dedicated SAP guideline | Implemented | May 2022 Seek funding for guidelines development and training. A proposal was accepted by the WHO country office in Nepal. 1500 US$ funding was provided by TDR. |
| December 2022 SAP guidelines developed and endorsed | ||
| Training of surgeons, anesthetists and nurses | Implemented | December 2022 Training done in batches and continued. |
| Wound Class | Definition | Indication for SAP | Timing for Initial Dose of SAP | Indication for Redosing |
|---|---|---|---|---|
| Clean | Primarily closed, elective procedures involving no inflammation, no break in technique, and no entry into the gastrointestinal, oropharyngeal, biliary, genitourinary tracts or tracheobronchial tracts (e.g., herniorrhaphy) | Not recommended Recommended when: (1) risk factors are present, for example patients with immunosuppressive states, diabetes mellitus, malignancies or (2) patient has prosthesis in-situ | IV bolus: should be administered no more than 60 min prior to skin incision. | A single pre-operative dose is enough for most of the procedures, however, redosing is recommended when: (1) there is prolonged surgery, more than four hours from the time of the initial dose or (2) if major blood loss occurs (1500 mL) |
| Clean-contaminated | Surgery during which colonized viscus (e.g., gastrointestinal, tracheobronchial or genitourinary tract) is entered; minor breaches in technique; procedures following blunt trauma; cholecystectomy; prostate surgery; upper and/or lower urinary tract surgery; or uncomplicated appendectomy | Recommended | ||
| Contaminated | Surgery in the presence of non-purulent inflammation or major spillage from a colonized viscus, major breach in aseptic technique, or traumatic wounds less than 4 h old | Recommended | ||
| Dirty | Surgery in the presence of established infection (e.g., perforated viscous, devitalized tissue) and traumatic wounds more than 4 h old | NA * |
| Characteristics | Baseline Study (July–December 2019) | Follow-Up Study (January–April 2023) | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | p Value | |
| Total | 874 | 751 | |||
| Sex | |||||
| Male | 497 | (57) | 428 | (57) | 0.959 |
| Female | 377 | (43) | 323 | (43) | |
| Age (years) | |||||
| Median [IQR] | 40 (26–53) | 43 (30–57) | 0.432 | ||
| Type of surgery | |||||
| Elective | 661 | (76) | 638 | (85) | <0.001 |
| Emergency | 213 | (24) | 113 | (15) | |
| Anatomical site of surgery | |||||
| Gastrointestinal | 476 | (54) | 339 | (45) | <0.001 |
| Inguinal Hernia | 128 | (15) | 68 | (9) | |
| Upper Urinary | 101 | (12) | 100 | (13) | |
| Lower Urinary | 54 | (6) | 48 | (7) | |
| Thoracic | 8 | (1) | 8 | (1) | |
| Vascular | 42 | (5) | 88 | (12) | |
| Others | 65 | (7) | 100 | (13) | |
| Surgical wound class | |||||
| Clean | 202 | (23) | 216 | (29) | 0.001 |
| Clean-contaminated | 587 | (67) | 434 | (58) | |
| Contaminated | 57 | (7) | 70 | (9) | |
| Dirty | 28 | (3) | 31 | (4) | |
| Comorbidity * | |||||
| None | 817 | (94) | 692 | (92) | 0.002 |
| Cancer | 16 | (2) | 5 | (0.7) | |
| HIV/AIDS | 0 | (0) | 1 | (0.1) | |
| TB | 8 | (1) | 2 | (0.3) | |
| Diabetes mellitus | 31 | (3) | 51 | (6.7) | |
| Presence/insertion of prosthesis | |||||
| No | 758 | (87) | 694 | (92) | <0.001 |
| Yes | 116 | (13) | 57 | (8) | |
| Characteristics | Baseline Study (July–December 2019) | Follow-Up Study (January–April 2023) | p Value * |
|---|---|---|---|
| n (%) | n (%) | ||
| Total patients | 846 | 720 | |
| Eligible for SAP | 717 | 569 | |
| Received initial dose | 708 (99) | 541 (95) | <0.001 |
| Not eligible for SAP (a) | 129 | 151 | |
| Received initial dose (b) | 65 (50) | 57 (38) | 0.045 |
| Eligible for redosing (c) | 164 | 27 | |
| Received redosing (d) | 23 (14) | 6 (22) | 0.272 |
| Not eligible for redosing (e) | 544 | 514 | |
| Received redosing (f) | 0 | 2 (0.4) | 0.145 |
| Overall SAP compliance * | 632 (75) | 612 (85) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 World Health Organization. Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the Creative Commons Attribution IGO License (http://creativecommons.org/licenses/by/3.0/igo), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any reproduction of this article there should not be any suggestion that WHO or this article endorse any specific organisation or products. The use of the WHO logo is not permitted. This notice should be preserved along with the article’s original URL.
Share and Cite
Shrestha, I.; Shrestha, S.; Vijayageetha, M.; Koju, P.; Shrestha, S.; Zachariah, R.; Khogali, M.A. Surgical Antibiotic Prophylaxis Administration Improved after introducing Dedicated Guidelines: A Before-and-After Study from Dhulikhel Hospital in Nepal (2019–2023). Trop. Med. Infect. Dis. 2023, 8, 420. https://doi.org/10.3390/tropicalmed8080420
Shrestha I, Shrestha S, Vijayageetha M, Koju P, Shrestha S, Zachariah R, Khogali MA. Surgical Antibiotic Prophylaxis Administration Improved after introducing Dedicated Guidelines: A Before-and-After Study from Dhulikhel Hospital in Nepal (2019–2023). Tropical Medicine and Infectious Disease. 2023; 8(8):420. https://doi.org/10.3390/tropicalmed8080420
Chicago/Turabian StyleShrestha, Indira, Sulekha Shrestha, Mathavaswami Vijayageetha, Pramesh Koju, Saugat Shrestha, Rony Zachariah, and Mohammed Ahmed Khogali. 2023. "Surgical Antibiotic Prophylaxis Administration Improved after introducing Dedicated Guidelines: A Before-and-After Study from Dhulikhel Hospital in Nepal (2019–2023)" Tropical Medicine and Infectious Disease 8, no. 8: 420. https://doi.org/10.3390/tropicalmed8080420
APA StyleShrestha, I., Shrestha, S., Vijayageetha, M., Koju, P., Shrestha, S., Zachariah, R., & Khogali, M. A. (2023). Surgical Antibiotic Prophylaxis Administration Improved after introducing Dedicated Guidelines: A Before-and-After Study from Dhulikhel Hospital in Nepal (2019–2023). Tropical Medicine and Infectious Disease, 8(8), 420. https://doi.org/10.3390/tropicalmed8080420







