Evaluation of an Innovative Point-of-Care Rapid Diagnostic Test for the Identification of Imported Malaria Parasites in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting, Participants and Design
2.2. Interpretation of the Results for RDTs
2.3. Data Analysis
3. Results
3.1. Diagnostic Performance of the Novel and Wondfo RDTs
3.2. Additive NRI and Absolute NRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Zhou, X.-N. China declared malaria-free: A milestone in the world malaria eradication and Chinese public health. Infect. Dis. Poverty 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Feng, X.-Y.; Zhou, S.-S.; Tang, L.-H.; Xia, Z.-G. Establishing and applying an adaptive strategy and approach to eliminating malaria: Practice and lessons learnt from China from 2011 to 2020. Emerg. Microbes Infect. 2022, 11, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, L.; Tu, H.; Zhou, S.S.; Xia, Z.G. From elimination to post-elimination: Characteristics, challenges and re-transmission preventing strategy of imported malaria in China. China Trop. Medicine. 2021, 21, 5–10. (In Chinese) [Google Scholar]
- Zhang, L.; Feng, J.; Zhang, S.S.; Xia, Z.-G.; Zhou, S.-S. Epidemiological characteristics of malaria and the progress towards its elimination in China in 2018. Chin. J. Parasitol. Parasit. Dis. 2019, 37, 241–247. (In Chinese) [Google Scholar]
- Li, Z.; Zhang, Q.; Zheng, C.; Zhou, S.; Sun, J.; Zhang, Z.; Geng, Q.; Zhang, H.; Wang, L.; Lai, S.; et al. Epidemiologic features of overseas imported malaria in the People’s Republic of China. Malar. J. 2016, 15, 141. [Google Scholar] [CrossRef]
- Mace, K.E.; Lucchi, N.W.; Tan, K.R. Malaria Surveillance—United States, 2018. MMWR Surveill. Summ. 2022, 71, 1–29. [Google Scholar] [CrossRef]
- Behrens, R.H.; Neave, P.E.; Jones, C.O. Imported malaria among people who travel to visit friends and relatives: Is current UK policy effective or does it need a strategic change? Malar. J. 2015, 14, 149. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.Q.; Wang, D.Q.; Auburn, S.; Lu, S.N.; Xu, X.; Lyu, X.; Yu, C.; Tian, C.; Li, S.; et al. Epidemiological profile of Plasmodium ovale spp. imported from Africa to Anhui Province, China, 2012–2019. Malar. J. 2021, 20, 15. [Google Scholar] [CrossRef]
- Feng, J.; Tu, H.; Zhang, L.; Xia, Z.; Zhou, S. Imported Malaria Cases—China, 2012–2018. China CDC Wkly. 2020, 2, 8. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Jiang, J.J.; Li, W.D. Course of malaria control and elimination in Anhui Province. J. Trop. Dis. Parasitol. 2020, 18, 65–69, 80. [Google Scholar]
- Li, J.; Wei, S.-j.; Zhang, W.-w.; Lin, K.-m.; Yan, H.; Feng, X.-y. Analysis of malaria epidemiological characteristics in Guangxi Province in 2010–2019. Chin. J. Parasitol. Parasit. Dis. 2020, 38, 589–594. [Google Scholar]
- Ju, L.; Kangmin, L.; Shujia, W.; Weiwe, Z.; Xiangyan, F.; Hui, Y. An overview of malaria control in Guangxi Zhuang autonomous region for 70 years. J. Trop. Med. 2020, 20, 997–1000, 1012. [Google Scholar]
- Wang, D.; Cotter, C.; Sun, X.; Bennett, A.; Gosling, R.D.; Xiao, N. Adapting the local response for malaria elimination through evaluation of the 1-3-7 system performance in the China–Myanmar border region. Malar. J. 2017, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-N.; Zhang, L.-L.; Ruan, W.; Chen, H.-L.; Lu, Q.-Y.; Yang, T.-T. Species identification in 5 imported cases previously diagnosed as Vivax malaria by parasitological and nested PCR techniques. Chin. J. Parasitol. Parasit. Dis. 2013, 31, 221–223, 234. [Google Scholar]
- Chavatte, J.-M.; Tan, S.B.H.; Snounou, G.; Lin, R.T.P.V. Molecular characterization of misidentified Plasmodium ovale imported cases in Singapore. Malar. J. 2015, 14, 454. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Jin, S.; Wu, Z.; Chin, D.P.; Koplan, J.P.; Wilson, M.E. Emergence and control of infectious diseases in China. Lancet 2008, 372, 1598–1605. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Cheng, Z.; Xiao, N.; Cotter, C.; Hwang, J.; Li, X.; Yin, S.; Wang, J.; Bai, L.; et al. Transmission riskfrom imported Plasmodium vivax malaria in the China–Myanmar borderregion. Emerg. Infect. Dis. 2015, 21, 1861–1864. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of China. Diagnosis of Malaria; National Health and Family Planning Commission of China: Beijing, China, 2015. (In Chinese)
- Perandin, F.; Manca, N.; Calderaro, A.; Piccolo, G.; Galati, L.; Ricci, L.; Medici, M.C.; Arcangeletti, M.C.; Snounou, G.; Dettori, G.; et al. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J. Clin. Microbiol. 2004, 42, 1214–1219. [Google Scholar] [CrossRef]
- Alba, A.C.; Agoritsas, T.; Walsh, M.; Hanna, S.; Iorio, A.; Devereaux, P.J.; McGinn, T.; Guyatt, G. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA 2017, 318, 1377–1384. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Tu, H.; Yin, J.H.; Xia, Z.G. Malaria epidemiology in China in 2020. Chin. J. Parasitol. Parasit. Dis. 2021, 39, 195–199. (In Chinese) [Google Scholar]
- Yin, J.; Yan, H.; Li, M. Prompt and precise identifcation of various sources of infection in response to the prevention of malaria re-establishment in China. Infect. Dis. Poverty. 2022, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhu, G.; Cao, C.; Miao, P.; Cao, Y.; Wang, W.; Gu, Y.; Xu, S.; Wang, S.; Zhou, H.; et al. The challenge of maintaining microscopist capacity at basic levels for malaria elimination in Jiangsu Province, China. BMC Public Health 2018, 18, 489. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Bell, D.; Wongsrichanalai, C.; Barnwell, J.W. Ensuring quality and access for malaria diagnosis: How can it be achieved? Nat. Rev. Microbiol. 2006, 4, S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Buchanan, I.; Choi, Y.H.; Makler, M.T. Opportunities for improving pLDH-based malaria diagnostic tests. Malar. J. 2011, 10, 213. [Google Scholar] [CrossRef]
- Azizi, H.; Majdzadeh, R.; Ahmadi, A.; Esmaeili, E.D.; Naghili, B.; Mansournia, M.A. Health workers readiness and practice in malaria case detection and appropriate treatment: A meta-analysis and meta-regression. Malar. J. 2021, 20, 420. [Google Scholar] [CrossRef]
- Tang, J.; Tang, F.; Zhu, H.; Lu, F.; Xu, S.; Cao, Y.; Gu, Y.; He, X.; Zhou, H.; Zhu, G.; et al. Assessment of false negative rates of lactate dehydrogenase-based malaria rapid diagnostic tests for Plasmodium ovale detection. PLoS Negl. Trop. Dis. 2019, 13, e0007254. [Google Scholar] [CrossRef]
- Mei, L.; Zhigui, X.; Ahuisen, Z. Analysis of inconsistence of plasmodium detection in some malaria cases. Chin. J. Paraist Dis. 2019, 37, 464–471. [Google Scholar]
- Mueller, I.; Zimmerman, P.A.; Reeder, J.C. Plasmodium malariae and Plasmodium ovale-the “bashful” malaria parasites. Trends Parasitol. 2007, 23, 278–283. [Google Scholar] [CrossRef]
- Oguike, M.C.; Betson, M.; Burke, M.; Nolder, D.; Stothard, R.; Kleinschmidt, I.; Proietti, C.; Bousema, T.; Ndounga, M.; Tanabe, K.; et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int. J. Parasitol. 2011, 41, 677–683. [Google Scholar] [CrossRef]
- Oriero, E.C.; Amenga-Etego, L.; Ishengoma, D.S.; Amambua-Ngwa, A. Plasmodium malariae, current knowledge and future re-search opportunities on a neglected malaria parasite species. Crit. Rev. Microbiol. 2021, 47, 44–56. [Google Scholar] [CrossRef]
- Kerlin, D.H.; Gatton, M.L. Preferential Invasion by Plasmodium Merozoites and the Self-Regulation of Parasite Burden. PLoS ONE 2013, 8, e57434. [Google Scholar] [CrossRef]
- Ota-Sullivan, K.; Blecker-Shelly, D.L. Use of the Rapid BinaxNOW Malaria Test in a 24-Hour Laboratory Associated with Accurate Detection and Decreased Malaria Testing Turnaround Times in a Pediatric Setting Where Malaria Is Not Endemic. J. Clin. Microbiol. 2013, 51, 1567–1569. [Google Scholar] [CrossRef]

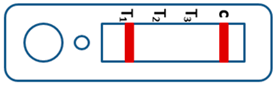
| Valid |  | Mixed infection of P. falciparum, P. vivax and P. ovale or/and P. malariae | Invalid |  |
 | Positive result for P. falciparum only |  | ||
 | Positive result for P. vivax only |  | ||
 | Positive result for P. ovale and/or P. malariae |  | ||
 | Mixed infection of P. falciparum and P. vivax | 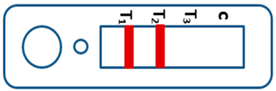 | ||
 | Mixed infection of P. falciparum and P. ovale and/or P. malariae | 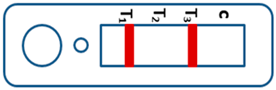 | ||
 | Mixed infection of P. vivax and P. ovale and/or P. malariae |  | ||
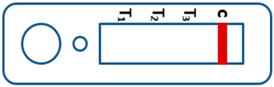 | Negative for any Plasmodium |  |
| Characteristics | The Novel RDTs | Wondfo RDTs | χ2 * | p-Value |
|---|---|---|---|---|
| Sensitivity [95% CI] | 78.37 [73.83–82.91] | 86.21 [82.40–90.01] | 11.294 * | 0.001 |
| Specificity [95% CI] | 95.05 [92.51–97.59] | 89.05 [85.39–92.71] | 13.474 * | <0.001 |
| PPV [95% CI] | 94.70 [91.98–97.42] | 89.87 [86.47–93.27] | 4.543 | 0.033 |
| NPV [95% CI] | 79.59 [75.27–83.90] | 85.14 [81.06–89.21] | 3.318 | 0.069 |
| Diagnostic accuracy rate [95% CI] | 86.21 [83.45–88.97] | 87.54 [84.90–90.19] | 0.466 | 0.495 |
| Kappa value [95% CI] | 0.726 [0.779–0.673] | 0.751 [0.698–0.804] | NA | NA |
| Species * | Type of RDTs | N | Results of RDTs (n) | Sensitivity (%) | χ2 # | p-Value | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| P. falciparum | The novel | 154 | 134 | 20 | 87.01 | 12.071 | <0.001 |
| Wondfo | 154 | 148 | 6 | 96.10 | |||
| P. ovale | The novel | 123 | 87 | 35 | 71.31 | 0.036 | 0.850 |
| Wondfo | 123 | 88 | 34 | 72.13 | |||
| P. vivax | The novel | 22 | 18 | 4 | 81.82 | 0.500 | 0.500 |
| Wondfo | 22 | 20 | 2 | 90.91 | |||
| P. malariae | The novel | 13 | 8 | 5 | 61.54 | 2.250 | 0.134 |
| Wondfo | 13 | 12 | 1 | 92.31 | |||
| Positive Samples (n = 309) | ||||
|---|---|---|---|---|
| The Novel RDTs | ||||
| Wondfo RDTs | Negative | Positive | Total | |
| Negative | 31 | 38 | 69 | |
| Positive | 13 | 237 | 250 | |
| Negative samples (n = 283) | ||||
| The novel RDTs | ||||
| Wondfo RDTs | Negative | Positive | Total | |
| Negative | 251 | 18 | 269 | |
| Positive | 1 | 13 | 14 | |
| Additive NRI | 1.83% | Absolute NRI | 1.33% | |
| Z | 0.673 | Z | 1.317 | |
| p | 0.501 | p | 0.188 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Wang, S.; Sui, Y.; Zhang, T.; Luo, F.; Shi, F.; Qian, Y.; Li, J.; Lu, S.; Cotter, C.; et al. Evaluation of an Innovative Point-of-Care Rapid Diagnostic Test for the Identification of Imported Malaria Parasites in China. Trop. Med. Infect. Dis. 2023, 8, 296. https://doi.org/10.3390/tropicalmed8060296
Lin K, Wang S, Sui Y, Zhang T, Luo F, Shi F, Qian Y, Li J, Lu S, Cotter C, et al. Evaluation of an Innovative Point-of-Care Rapid Diagnostic Test for the Identification of Imported Malaria Parasites in China. Tropical Medicine and Infectious Disease. 2023; 8(6):296. https://doi.org/10.3390/tropicalmed8060296
Chicago/Turabian StyleLin, Kangming, Shuqi Wang, Yuan Sui, Tao Zhang, Fei Luo, Feng Shi, Yingjun Qian, Jun Li, Shenning Lu, Chris Cotter, and et al. 2023. "Evaluation of an Innovative Point-of-Care Rapid Diagnostic Test for the Identification of Imported Malaria Parasites in China" Tropical Medicine and Infectious Disease 8, no. 6: 296. https://doi.org/10.3390/tropicalmed8060296
APA StyleLin, K., Wang, S., Sui, Y., Zhang, T., Luo, F., Shi, F., Qian, Y., Li, J., Lu, S., Cotter, C., Wang, D., & Li, S. (2023). Evaluation of an Innovative Point-of-Care Rapid Diagnostic Test for the Identification of Imported Malaria Parasites in China. Tropical Medicine and Infectious Disease, 8(6), 296. https://doi.org/10.3390/tropicalmed8060296








