Human Papillomavirus (HPV) Vaccination Knowledge, Beliefs, and Hesitancy Associated with Stages of Parental Readiness for Adolescent HPV Vaccination: Implications for HPV Vaccination Promotion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
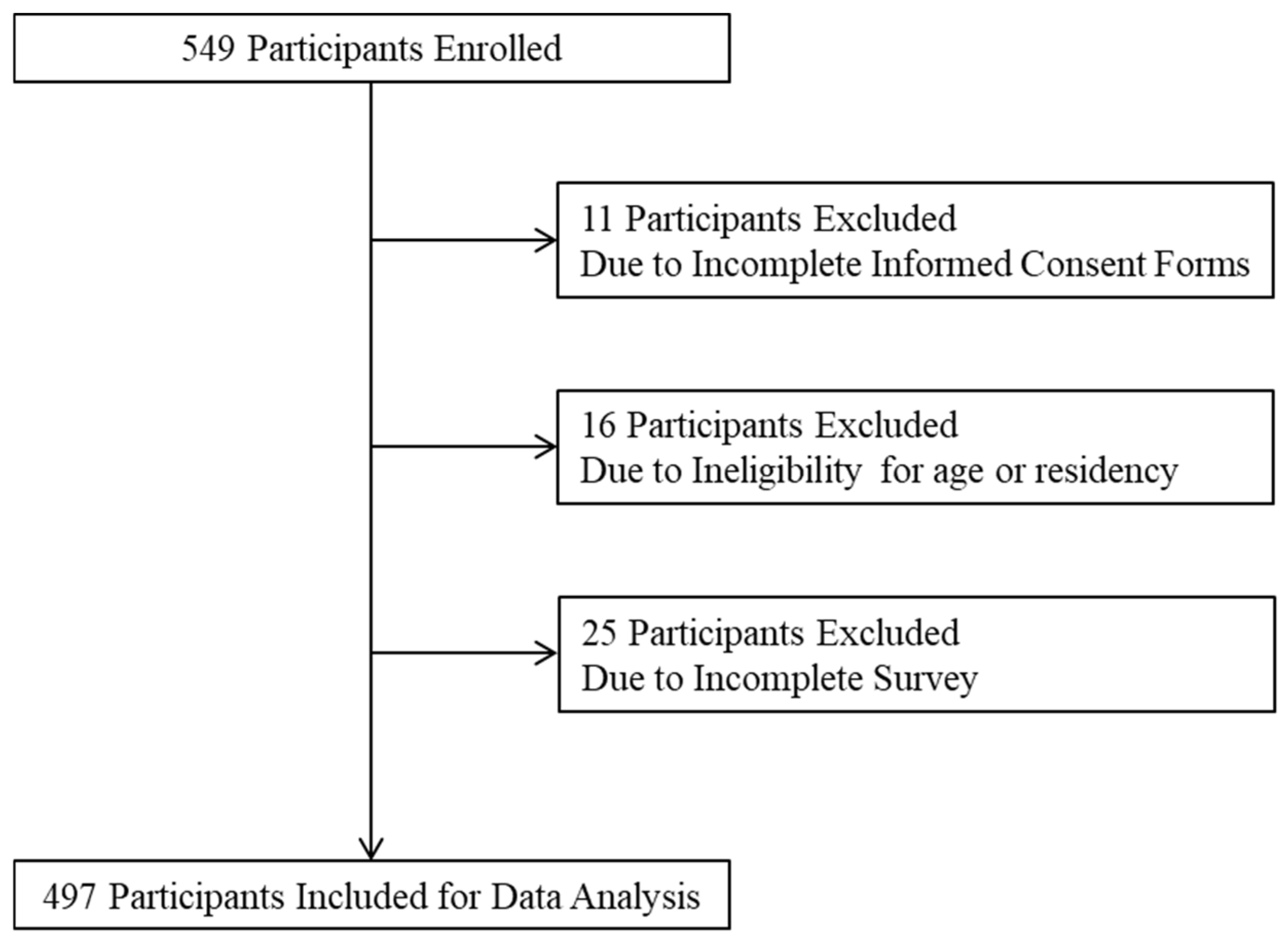
2.2. Sampling
2.3. Data Collection
2.4. Variables
2.4.1. Outcome Variable
2.4.2. Independent Variable
2.4.3. Control Variable
2.5. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Independent Variables by Stages of Readiness for Adolescent HPV Vaccination
3.3. Factors Associated with Stages of Readiness for Adolescent HPV Vaccination
3.3.1. Precontemplation Stage vs. Contemplation/Preparation/Action/Maintenance Stage
3.3.2. Precontemplation/Contemplation Stage vs. Preparation/Action/Maintenance Stage
3.3.3. Precontemplation/Contemplation/Preparation Stage vs. Action/Maintenance Stage
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meites, E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68. Available online: https://www.cdc.gov/mmwr/volumes/68/wr/mm6832a3.htm (accessed on 22 January 2022). [CrossRef] [PubMed]
- How Many Cancers Are Linked with HPV Each Year?|CDC. 2021. Available online: https://www.cdc.gov/cancer/hpv/statistics/cases.htm (accessed on 3 September 2022).
- HPV-Associated Cancer Statistics|CDC. Available online: https://www.cdc.gov/cancer/hpv/statistics/index.htm (accessed on 18 January 2022).
- HPV Vaccine Safety and Effectiveness|CDC. 2021. Available online: https://www.cdc.gov/vaccines/vpd/hpv/hcp/safety-effectiveness.html (accessed on 30 March 2022).
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D.; Penny, M.E.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of Quadrivalent HPV Vaccine against HPV Infection and Disease in Males. N. Engl. J. Med. 2011, 364, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Slade, B.A.; Leidel, L.; Vellozzi, C.; Woo, E.J.; Hua, W.; Sutherland, A.; Izurieta, H.S.; Ball, R.; Miller, N.; Braun, M.M.; et al. Postlicensure Safety Surveillance for Quadrivalent Human Papillomavirus Recombinant Vaccine. JAMA 2009, 302, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Arana, J.E.; Harrington, T.; Cano, M.; Lewis, P.; Mba-Jonas, A.; Rongxia, L.; Stewart, B.; Markowitz, L.E.; Shimabukuro, T.T. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009–2015. Vaccine 2018, 36, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Block, S.L.; Brown, D.R.; Chatterjee, A.; Gold, M.A.; Sings, H.L.; Meibohm, A.; Dana, A.; Haupt, R.M.; Barr, E.; Tamms, G.M.; et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr. Infect. Dis. J. 2010, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- HPV Vaccination Recommendations|CDC. 2022. Available online: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html (accessed on 2 September 2022).
- Policy (OIDP) O of ID and H. Featured Priority: HPV Vaccination. HHS.gov. 2018. Available online: https://www.hhs.gov/vaccines/featured-priorities/hpv-vaccination/index.html (accessed on 4 September 2022).
- Pingali, C. National Vaccination Coverage Among Adolescents Aged 13–17 Years—National Immunization Survey-Teen, United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7135a1.htm (accessed on 4 September 2022). [CrossRef] [PubMed]
- Increase the Proportion of Adolescents Who Get Recommended Doses of the HPV Vaccine—IID-08—Healthy People 2030|health.gov. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 (accessed on 4 September 2022).
- Supplementary Table 2. National Vaccination Coverage among Adolescents Aged 13–17 Years—National Immunization Survey-Teen, United States, 2021. Available online: https://stacks.cdc.gov/view/cdc/120476 (accessed on 5 September 2022).
- Dyne, E.A.V. Trends in Human Papillomavirus–Associated Cancers—United States, 1999–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67. Available online: https://www.cdc.gov/mmwr/volumes/67/wr/mm6733a2.htm (accessed on 23 October 2020).
- Vasudevan, L.; Ostermann, J.; Wang, Y.; Harrison, S.E.; Yelverton, V.; Fish, L.J.; Williams, C.; Walter, E.B. Association of caregiver attitudes with adolescent HPV vaccination in 13 southern US states. Vaccine X 2022, 11, 100181. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Santibanez, T.A.; Stokley, S.; Lindley, M.C.; Fisher, A.; Kim, D.; Greby, S.; Srivastav, A.; Singleton, J. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine 2021, 39, 2416–2423. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- WHO. WHO|SAGE Working Group Dealing with Vaccine Hesitancy (March 2012 to November 2014). Available online: https://www.who.int/immunization/sage/sage_wg_vaccine_hesitancy_apr12/en/ (accessed on 29 April 2021).
- Ten Health Issues WHO Will Tackle This Year. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 4 December 2022).
- Middleman, A.B.; Klein, J.; Quinn, J. Vaccine Hesitancy in the Time of COVID-19: Attitudes and Intentions of Teens and Parents Regarding the COVID-19 Vaccine. Vaccines 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Albertin, C.S.; Gurfinkel, D.; Saville, A.W.; Vangala, S.; Rice, J.D.; Helmkamp, L.; Zimet, G.D.; Valderrama, R.; Breck, A.; et al. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine 2020, 38, 6027–6037. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.; Martinez, B.; Shin, M.B.; Allee-Munoz, A.; Rodriguez, I.; Navarro, J.; Thomas-Barrios, K.R.; Kast, W.M.; Baezconde-Garbanati, L. Understanding medical mistrust and HPV vaccine hesitancy among multiethnic parents in Los Angeles. J. Behav. Med. 2022, 46, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Rositch, A.F.; Liu, T.; Chao, C.; Moran, M.; Beavis, A.L. Levels of Parental Human Papillomavirus Vaccine Hesitancy and Their Reasons for Not Intending to Vaccinate: Insights From the 2019 National Immunization Survey-Teen. J. Adolesc. Health 2022, 71, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Fleming, A.; Moore, A.C.; Sahm, L.J. Views of parents regarding human papillomavirus vaccination: A systematic review and meta-ethnographic synthesis of qualitative literature. Res. Soc. Adm. Pharm. 2019, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Mora, J.; Ferdous, T.; Shokar, N. HPV Vaccine Beliefs and Correlates of Uptake Among Hispanic Women and Their Children on the US-Mexico Border. Cancer Control 2020, 27, 1073274820968881. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, K.; Zhu, Y.; Lin, Y.-Y.; Damgacioglu, H.; Lin, Y.; Montealegre, J.R.; Deshmukh, A.A. HPV Vaccine Recommendations and Parental Intent. Pediatrics 2021, 147, e2020026286. [Google Scholar] [CrossRef] [PubMed]
- Hirth, J.M.; Fuchs, E.L.; Chang, M.; Fernandez, M.E.; Berenson, A.B. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008–2016). Vaccine 2019, 37, 595–601. [Google Scholar] [CrossRef]
- Li, L.; Wood, C.E.; Kostkova, P. Vaccine hesitancy and behavior change theory-based social media interventions: A systematic review. Transl. Behav. Med. 2022, 12, 243–272. [Google Scholar] [CrossRef]
- Health Behavior: Theory, Research, and Practice, 5th Edition|Wiley. Available online: https://www.wiley.com/en-us/Health+Behavior%3A+Theory%2C+Research%2C+and+Practice%2C+5th+Edition-p-9781118628980 (accessed on 27 May 2022).
- Urich, A. The Health Belief Model. 2017. Available online: https://psu.pb.unizin.org/kines082/chapter/the-health-belief-model/ (accessed on 24 September 2022).
- Snetselaar, L.G. CHAPTER 7—Nutrition Intervention: Lessons from Clinical Trials. In Nutrition in the Prevention and Treatment of Disease; Coulston, A.M., Rock, C.L., Monsen, E.R., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 95–104. ISBN 978-0-12-193155-1. Available online: https://www.sciencedirect.com/science/article/pii/B978012193155150009X (accessed on 25 September 2022).
- Limbu, Y.B.; Gautam, R.K.; Pham, L. The Health Belief Model Applied to COVID-19 Vaccine Hesitancy: A Systematic Review. Vaccines 2022, 10, 973. [Google Scholar] [CrossRef]
- Reiter, P.L.; Brewer, N.T.; Gottlieb, S.L.; McRee, A.-L.; Smith, J.S. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc. Sci. Med. 2009, 69, 475–480. [Google Scholar] [CrossRef] [PubMed]
- A Quality Improvement Initiative to Increase HPV Vaccine Rates Using an Educational and Reminder Strategy with Parents of Preteen Girls—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23522561/ (accessed on 27 May 2022).
- Gerend, M.A.; Shepherd, J.E. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann. Behav. 2012, 44, 171–180. [Google Scholar] [CrossRef]
- Harrison, S.E.; Yelverton, V.; Wang, Y.; Ostermann, J.; Fish, L.J.; Williams, C.L.; Vasudevan, L.; Walter, E.B. Examining Associations between Knowledge and Vaccine Uptake Using the Human Papillomavirus Knowledge Questionnaire (HPV-KQ). Am. J. Health Behav. 2021, 45, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Anuforo, B.; McGee-Avila, J.K.; Toler, L.; Xu, B.; Kohler, R.E.; Manne, S.; Tsui, J. Disparities in HPV vaccine knowledge and adolescent HPV vaccine uptake by parental nativity among diverse multiethnic parents in New Jersey. BMC Public Health 2022, 22, 195. [Google Scholar] [CrossRef]
- Victory, M.; Do, T.Q.N.; Kuo, Y.-F.; Rodriguez, A.M. Parental knowledge gaps and barriers for children receiving human papillomavirus vaccine in the Rio Grande Valley of Texas. Hum. Vaccines Immunother. 2019, 15, 1678–1687. [Google Scholar] [CrossRef]
- Naoum, P.; Athanasakis, K.; Zavras, D.; Kyriopoulos, J.; Pavi, E. Knowledge, Perceptions and Attitudes Toward HPV Vaccination: A Survey on Parents of Girls Aged 11–18 Years Old in Greece. Front. Glob. Womens Health 2022, 3, 871090. Available online: https://www.frontiersin.org/articles/10.3389/fgwh.2022.871090 (accessed on 4 April 2023). [CrossRef]
- Park, S.; Jang, I.; Lee, J.L.; Kim, Y. Factors Affecting Vaccination Status of Female Adolescents Subject to the Korean National HPV Immunization Program: Focusing on Mothers’ HPV Knowledge and Heath Beliefs of HPV Vaccines. J. Korean Soc. Sch. Health 2020, 33, 58–66. [Google Scholar] [CrossRef]
- Riley, M.A.; Holden, J.G. Dynamics of cognition. WIREs Cogn. Sci. 2012, 3, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. AJHP 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Spencer, L.; Pagell, F.; Adams, T. Applying the transtheoretical model to cancer screening behavior. Am. J. Health Behav. 2005, 29, 36–56. [Google Scholar] [CrossRef]
- Chu, H.; Liu, S. Integrating health behavior theories to predict American’s intention to receive a COVID-19 vaccine. Patient Educ. Couns. 2021, 104, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Amoyal, N.R.; Paiva, A.L.; Prochaska, J.O. Motivation for HPV Vaccination Among Young Adult Men: Validation of TTM Decisional Balance and Self-Efficacy Constructs. Am. J. Health Promot. 2016, 30, 163–171. [Google Scholar] [CrossRef]
- U.S. Census Bureau QuickFacts: Shelby County, Tennessee. Available online: https://www.census.gov/quickfacts/shelbycountytennessee (accessed on 3 January 2023).
- ACIP HPV Vaccine Recommendations|CDC. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html (accessed on 2 December 2022).
- Qualtrics XM: The Leading Experience Management Software. Available online: https://www.qualtrics.com/?utm_source=google&utm_medium=ppc&utm_campaign=US-Brand-Qualtrics-Brand&utm_keyword=qualtrics&MatchType=e&adid=522505679922&utm_content=522505679922&adgroupid=41339289338&campaignid=755409789&Target=&targetid=kwd-8232955280&Device=c&devicemodel=&loc_phsyical_ms=9013543&network=g&adposition=&gclid=Cj0KCQiA4aacBhCUARIsAI55maGCqGhim4Xhai5GQP7-hDI7BFCYn4M9CDlsldJqVzrsrTPP5jq_h6saAv3CEALw_wcB (accessed on 2 December 2022).
- Cunningham, J.; Wallston, K.A.; Wilkins, C.H.; Hull, P.C.; Miller, S.T. Development and Psychometric Evaluation of the HPV Clinical Trial Survey for Parents (CTSP-HPV) Using Traditional Survey Development Methods and Community Engagement Principles. Clin. Transl. Sci. 2015, 8, 702–709. [Google Scholar] [CrossRef]
- View HINTS Questions|HINTS. Available online: https://hints.cancer.gov/view-questions/all-hints-questions.aspx?PK_Article=228 (accessed on 4 April 2023).
- Patel, D.A.; Grunzweig, K.A.; Zochowski, M.K.; Dempsey, A.F.; Carlos, R.C.; Dalton, V.K. Human papillomavirus vaccine stages of change among male and female university students: Ready or not? J. Am. Coll. Health J. ACH 2013, 61, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Karki, I.; Dobbs, P.D.; Larson, D.; Maness, S.B. Human papillomavirus (HPV) knowledge, beliefs, and vaccine uptake among United States and international college students. J. Am. Coll. Health 2021, 70, 2483–2490. [Google Scholar] [CrossRef]
- McHugh, M.L. The Chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Census Profile: Memphis, TN-MS-AR Metro Area. Available online: http://censusreporter.org/profiles/31000US32820-memphis-tn-ms-ar-metro-area/ (accessed on 17 January 2023).
- Salmon, D.A.; Dudley, M.Z.; Glanz, J.M.; Omer, S.B. Vaccine Hesitancy: Causes, Consequences, and a Call to Action. Am. J. Prev. Med. 2015, 49, S391–S398. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar] [CrossRef]
- Schuster, M.; Eskola, J.; Duclos, P.; SAGE Working Group on Vaccine Hesitancy. Review of vaccine hesitancy: Rationale, remit and methods. Vaccine 2015, 33, 4157–4160. [Google Scholar] [CrossRef]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H. The Health Belief Model and Sick Role Behavior. Health Educ. Monogr. 1974, 2, 409–419. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Lee, R.C. Using the health belief model in qualitative focus groups to identify HPV vaccine acceptability in college men. Int. Q. Community Health Educ. 2012, 33, 175–187. [Google Scholar] [CrossRef]
- Hodge, F.S.; Itty, T.; Cardoza, B.; Samuel-Nakamura, C. HPV vaccine readiness among American Indian college students. Ethn. Dis. 2011, 21, 415–420. [Google Scholar]
- Aldossri, M.; Okoronkwo, C.; Dodd, V.; Manson, H.; Singhal, S. Determinants of dentists’ readiness to assess HPV risk and recommend immunization: A transtheoretical model of change-based cross-sectional study of Ontario dentists. PLoS ONE 2021, 16, e0247043. [Google Scholar] [CrossRef]
- Paiva, A.L.; Lipschitz, J.M.; Fernandez, A.C.; Redding, C.A.; Prochaska, J.O. Evaluation of the acceptability and feasibility of a computer-tailored intervention to increase human papillomavirus vaccination among young adult women. J. Am. Coll. Health 2014, 62, 32–38. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Lee, R.C. Designing and evaluating a health belief model-based intervention to increase intent of HPV vaccination among college males. Int. Q. Community Health Educ. 2013, 34, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, F.T.; Sanchez, J.; Rabionet, S.E.; Popovici, I.; Baraka, M.A. Predictor of HPV Vaccination Uptake among Foreign-Born College Students in the U.S.: An Exploration of the Role of Acculturation and the Health Belief Model. Vaccines 2023, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Fallucca, A.; Immordino, P.; Riggio, L.; Casuccio, A.; Vitale, F.; Restivo, V. Acceptability of HPV Vaccination in Young Students by Exploring Health Belief Model and Health Literacy. Vaccines 2022, 10, 998. [Google Scholar] [CrossRef]
- Fernandez, A.C.; Paiva, A.L.; Lipschitz, J.M.; Larson, H.E.; Amoyal, N.R.; Blaney, C.L.; Sillice, M.A.; Redding, C.A.; Prochaska, J.O. Disease prevention without relapse: Processes of change for HPV vaccination. Open J. Prev. Med. 2013, 3, 301–309. [Google Scholar] [CrossRef]
- Oh, N.L.; Biddell, C.B.; Rhodes, B.E.; Brewer, N.T. Provider communication and HPV vaccine uptake: A meta-analysis and systematic review. Prev. Med. 2021, 148, 106554. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, M.K.; Bednarczyk, R.A.; O’Leary, S.T.; Schwartz, J.L.; Shapiro, E.D.; Niccolai, L.M. Understanding the Factors Influencing Health Care Provider Recommendations about Adolescent Vaccines: A Proposed Framework. J. Behav. Med. 2022, 46, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.C.; Madden, C.A.; Thompson, D.M.; Garbe, M.C.; Roberts, J.R.; Jacobson, R.M.; Darden, P.M. The impact of provider recommendation on human papillomavirus vaccine and other adolescent vaccines. Hum. Vaccines Immunother. 2021, 17, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Hull, P.C.; Williams, E.A.; Khabele, D.; Dean, C.; Bond, B.; Sanderson, M. HPV vaccine use among African American girls: Qualitative formative research using a participatory social marketing approach. Gynecol. Oncol. 2014, 132 (Suppl. S1), S13–S20. [Google Scholar] [CrossRef]
- Williams, W.W.; Lu, P.-J.; Saraiya, M.; Yankey, D.; Dorell, C.; Rodriguez, J.L.; Kepka, D.; Markowitz, L.E. Factors associated with human papillomavirus vaccination among young adult women in the United States. Vaccine 2013, 31, 2937–2946. [Google Scholar] [CrossRef]
- D’Errico, M.P.; Tung, W.-C.; Lu, M.; D’Errico, R. Barriers and Recommendations Associated With Human Papillomavirus Vaccination Among College Students. J. Nurse Pract. 2020, 16, 533–537. [Google Scholar] [CrossRef]
- Barnard, M.; Cole, A.C.; Ward, L.; Gravlee, E.; Cole, M.L.; Compretta, C. Interventions to increase uptake of the human papillomavirus vaccine in unvaccinated college students: A systematic literature review. Prev. Med. Rep. 2019, 14, 100884. [Google Scholar] [CrossRef] [PubMed]
- Costanza, M.E.; Luckmann, R.; Stoddard, A.M.; Avrunin, J.S.; White, M.J.; Stark, J.R.; Clemow, L.; Rosal, M.C. Applying a stage model of behavior change to colon cancer screening. Prev. Med. 2005, 41, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Vickberg, S.M. The transtheoretical model of behavior change: Measuring our success one stage at a time. Educ. Update 1999, 4, 1–2. [Google Scholar] [PubMed]
| Variables | Total n = 495 | Precontemplation n = 21 | Contemplation n = 115 | Preparation n = 286 | Action/Maintenance n = 73 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Age (mean = 39.6, SD = 6.02) | ||||||||||
| ≤29 | 20 | 4.31 | 5 | 29.41 | 5 | 4.55 | 8 | 3.00 | 2 | 2.86 |
| 30–39 | 186 | 40.09 | 11 | 64.71 | 44 | 40.00 | 96 | 35.96 | 35 | 50.00 |
| 40–49 | 249 | 53.66 | 1 | 5.88 | 58 | 52.73 | 158 | 59.18 | 32 | 45.71 |
| ≥50 | 9 | 1.94 | 0 | 0.00 | 3 | 2.73 | 5 | 1.87 | 1 | 1.43 |
| Gender | ||||||||||
| Female | 264 | 54.66 | 13 | 68.42 | 43 | 39.45 | 166 | 58.66 | 42 | 58.33 |
| Male | 219 | 45.34 | 6 | 31.58 | 66 | 60.55 | 117 | 41.34 | 30 | 41.67 |
| Race | ||||||||||
| Black | 57 | 11.66 | 8 | 42.11 | 14 | 12.17 | 22 | 7.80 | 13 | 17.81 |
| White | 387 | 79.14 | 9 | 47.37 | 96 | 83.48 | 225 | 79.79 | 57 | 78.08 |
| Others | 45 | 9.20 | 2 | 10.53 | 5 | 4.35 | 35 | 12.41 | 3 | 4.11 |
| Employment | ||||||||||
| Employed | 463 | 95.46 | 19 | 100.00 | 100 | 89.29 | 272 | 96.80 | 72 | 98.63 |
| Unemployed | 22 | 4.54 | 0 | 0.00 | 12 | 10.71 | 9 | 3.20 | 1 | 1.37 |
| Annual household income | ||||||||||
| <20,000 | 16 | 3.30 | 6 | 31.58 | 1 | 0.88 | 7 | 2.49 | 2 | 2.78 |
| $20,001–$40,000 | 45 | 9.28 | 1 | 5.26 | 14 | 12.39 | 30 | 10.68 | 0 | 0.00 |
| $40,001–$60,000 | 197 | 40.62 | 6 | 31.58 | 46 | 40.71 | 123 | 43.77 | 22 | 30.56 |
| $60,001–$80,000 | 110 | 22.68 | 5 | 26.32 | 16 | 14.16 | 63 | 22.42 | 26 | 36.11 |
| $80,001–$100,000 | 85 | 17.53 | 1 | 5.26 | 29 | 25.66 | 42 | 14.95 | 13 | 18.06 |
| >$100,000 | 32 | 6.60 | 0 | 0.00 | 7 | 6.19 | 16 | 5.69 | 9 | 12.50 |
| Insurance | ||||||||||
| Yes | 454 | 93.42 | 18 | 94.74 | 105 | 92.92 | 262 | 93.24 | 69 | 94.52 |
| No | 32 | 6.58 | 1 | 5.26 | 8 | 7.08 | 19 | 6.76 | 4 | 5.48 |
| Primary healthcare provider | ||||||||||
| Yes | 386 | 79.42 | 17 | 89.47 | 87 | 76.99 | 231 | 82.21 | 51 | 69.86 |
| No | 100 | 20.58 | 2 | 10.53 | 26 | 23.01 | 50 | 17.79 | 22 | 30.14 |
| Health status | ||||||||||
| Very poor/poor/fair | 157 | 32.3 | 6 | 31.58 | 41 | 36.28 | 94 | 33.46 | 16 | 21.92 |
| Good or excellent | 329 | 67.7 | 13 | 68.42 | 72 | 63.72 | 187 | 66.55 | 57 | 78.08 |
| Number of chronic conditions | ||||||||||
| None | 313 | 64.54 | 12 | 66.67 | 63 | 55.75 | 189 | 67.26 | 49 | 67.12 |
| One | 100 | 20.62 | 1 | 5.56 | 21 | 18.58 | 65 | 23.13 | 13 | 17.81 |
| Two or more | 72 | 14.85 | 5 | 27.78 | 29 | 25.66 | 27 | 9.61 | 11 | 15.07 |
| Religiosity | ||||||||||
| Important | 451 | 93.96 | 15 | 78.95 | 106 | 96.36 | 262 | 93.91 | 68 | 94.44 |
| Not important | 29 | 6.04 | 4 | 21.05 | 4 | 3.64 | 17 | 6.09 | 4 | 5.56 |
| Variables | Total n = 495 | Precontemplation (a) n = 21 | Contemplation (b) n = 115 | Preparation (c) n = 286 | Action/Maintenance (d) n = 73 | F (p-Value) | Scheffe | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| HPV vaccine knowledge (Range: 0–8) | 4.50 | 2.50 | 3.71 | 3.05 | 3.44 | 2.30 | 4.99 | 2.45 | 4.45 | 2.33 | 11.95 (0.000) | b < c |
| Health Belief Model | ||||||||||||
| Susceptibility (Range: 2–8) | 5.82 | 1.27 | 5.71 | 1.59 | 5.49 | 1.33 | 6.02 | 1.20 | 5.59 | 1.22 | 6.10 (0.000) | b < c |
| Severity (Range: 3–12) | 8.98 | 1.81 | 8.81 | 2.02 | 8.55 | 1.92 | 9.17 | 1.76 | 8.93 | 1.55 | 3.47 (0.016) | b < c |
| Benefit (Range: 3–12) | 8.94 | 1.69 | 8.29 | 2.15 | 8.39 | 1.81 | 9.15 | 1.55 | 9.16 | 1.63 | 7.29 (0.000) | b < c,d |
| Barrier (Range: 4–16) | 10.84 | 2.36 | 10.81 | 2.48 | 10.91 | 2.36 | 10.98 | 2.26 | 10.22 | 2.62 | 2.09 (0.101) | - |
| HPV vaccine hesitancy (Range: 8–32) | 17.47 | 3.05 | 18.90 | 4.30 | 18.28 | 2.70 | 17.28 | 2.92 | 16.55 | 3.33 | 7.06 (0.000) | a > d b > c,d |
| Variables | Precontemplation vs. Contemplation/Preparation/Action/Maintenance | Precontemplation/Contemplation vs. Preparation/Action/Maintenance | Precontemplation/Contemplation/Preparation vs. Action/Maintenance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | |
| HPV vaccine knowledge | 0.94 | [0.72, 1.23] | 0.636 | 1.28 | [1.15, 1.43] | 0.000 | 0.98 | [0.86, 1.11] | 0.735 |
| Health Belief Model | |||||||||
| Susceptibility | 1.03 | [0.59, 1.79] | 0.916 | 1.36 | [1.10, 1.69] | 0.004 | 0.87 | [0.68, 1.10] | 0.237 |
| Severity | 0.84 | [0.50, 1.40] | 0.500 | 0.94 | [0.81, 1.11] | 0.473 | 0.95 | [0.79, 1.14] | 0.569 |
| Benefit | 1.20 | [0.74, 1.95] | 0.451 | 1.19 | [0.99, 1.43] | 062 | 1.09 | [0.87, 1.35] | 0.470 |
| Barrier | 1.03 | [0.75, 1.42] | 0.839 | 1.00 | [0.89, 1.13] | 0.950 | 0.98 | [0.86, 1.12] | 0.777 |
| HPV vaccine hesitancy | 0.95 | [0.73, 1.24] | 0.718 | 0.97 | [0.87, 1.08] | 0.555 | 0.86 | [0.77, 0.98] | 0.018 |
| Age | 1.26 | [1.12, 1.42] | 0.000 | 1.00 | [0.96, 1.05] | 0.835 | 0.98 | [0.93, 1.03] | 0.380 |
| Gender (ref. = female) | |||||||||
| Male | 1.57 | [0.43, 5.72] | 0.496 | 0.61 | [0.38, 0.98] | 0.039 | 1.01 | [0.57, 1.79] | 0.978 |
| Race (ref. = black) | |||||||||
| White | 1.65 | [0.35, 7.87] | 0.528 | 0.73 | [0.33, 1.61] | 0.437 | 0.32 | [0.13, 0.79] | 0.013 |
| Others | 0.57 | [0.07, 5.02] | 0.616 | 1.53 | [0.48, 4.83] | 0.473 | 0.05 | [0.01, 0.25] | 0.000 |
| Employment (ref. = unemployed) | |||||||||
| Employed | 1.00 | [-,-] | - | 2.28 | [0.86, 6.04] | 0.096 | 2.87 | [0.35, 23.59] | 0.327 |
| Annual household income | 1.79 | [1.04, 3.07] | 0.036 | 1.16 | [0.92, 1.45] | 0.201 | 1.54 | [1.17, 2.01] | 0.002 |
| Insurance (ref. = no) | |||||||||
| Yes | 3.31 | [0.15, 72.22] | 0.447 | 0.75 | [0.27, 2.07] | 0.578 | 1.60 | [0.47, 5.50] | 0.452 |
| Primary healthcare provider (ref. = no) | |||||||||
| Yes | 0.25 | [0.03, 2.43] | 0.235 | 0.87 | [0.46, 1.62] | 0.650 | 0.35 | [0.18, 0.71] | 0.004 |
| Health status (ref. = very poor/poor/fair) | |||||||||
| Good or excellent | 0.47 | [0.11, 1.97] | 0.300 | 0.70 | [0.41, 1.18] | 0.178 | 1.39 | [0.71, 2.72] | 0.331 |
| Number of chronic conditions (ref. = none) | |||||||||
| One | 4.91 | [0.49, 49.17] | 0.176 | 1.31 | [0.69, 2.48] | 0.408 | 0.83 | [0.39, 1.79] | 0.636 |
| Two or more | 0.99 | [0.20, 4.97] | 0.992 | 0.41 | [0.21, 0.80] | 0.009 | 0.83 | [0.34, 2.03] | 0.688 |
| Religiosity (ref. = Not important) | |||||||||
| Important | 3.90 | [0.60, 25.41] | 0.154 | 0.79 | [0.27, 2.30] | 0.669 | 1.37 | [0.37, 5.11] | 0.639 |
| Number of observations | 427 | 448 | 448 | ||||||
| (p) | 41.91 (0.001) | 72.46 (0.000) | 45.40 (0.000) | ||||||
| 0.307 | 0.141 | 0.119 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.W.; Lee, Y.; Brandt, H.M. Human Papillomavirus (HPV) Vaccination Knowledge, Beliefs, and Hesitancy Associated with Stages of Parental Readiness for Adolescent HPV Vaccination: Implications for HPV Vaccination Promotion. Trop. Med. Infect. Dis. 2023, 8, 251. https://doi.org/10.3390/tropicalmed8050251
Jin SW, Lee Y, Brandt HM. Human Papillomavirus (HPV) Vaccination Knowledge, Beliefs, and Hesitancy Associated with Stages of Parental Readiness for Adolescent HPV Vaccination: Implications for HPV Vaccination Promotion. Tropical Medicine and Infectious Disease. 2023; 8(5):251. https://doi.org/10.3390/tropicalmed8050251
Chicago/Turabian StyleJin, Seok Won, Yeonggeul Lee, and Heather M. Brandt. 2023. "Human Papillomavirus (HPV) Vaccination Knowledge, Beliefs, and Hesitancy Associated with Stages of Parental Readiness for Adolescent HPV Vaccination: Implications for HPV Vaccination Promotion" Tropical Medicine and Infectious Disease 8, no. 5: 251. https://doi.org/10.3390/tropicalmed8050251
APA StyleJin, S. W., Lee, Y., & Brandt, H. M. (2023). Human Papillomavirus (HPV) Vaccination Knowledge, Beliefs, and Hesitancy Associated with Stages of Parental Readiness for Adolescent HPV Vaccination: Implications for HPV Vaccination Promotion. Tropical Medicine and Infectious Disease, 8(5), 251. https://doi.org/10.3390/tropicalmed8050251







