Abstract
Human intestinal spirochetosis (HIS) can cause gastrointestinal symptoms, although asymptomatic infections have been described. Individuals from low-income countries, people living with HIV, and men who have sex with men (MSM) show increased risk. A retrospective review of all patients diagnosed with HIS (n = 165) between January 2013 and October 2020 at a tertiary hospital in Madrid, Spain, was performed to assess risk factors for symptomatic HIS, symptoms, and response to treatment. Most patients were male (n = 156; 94.5%), 86.7% were MSM, and 23.5% practiced chemsex, of whom most were symptomatic (p = 0.039). Most patients (78.4%) reported unprotected oral-anal intercourse. A total of 124 (81.1%) were symptomatic; diarrhea was the most common complaint (68.3%). Multivariable regression showed increased odds of symptoms associated with age under 41 (odds ratio 5.44, 95% CI 1.87–15.88; p = 0.002). Colonoscopy was normal in 153 (92.7%). Furthermore, 66.7% presented previous or concomitant sexually transmitted diseases (STDs). Among the patients, 102 underwent testing for other gastrointestinal pathogens, with positive results in 20 (19.6%). All symptomatic patients without concomitant gastrointestinal infection presenting improvement on follow-up (42 of 53) had received either metronidazole or doxycycline (p = 0.049). HIS should be considered as a cause of chronic diarrhea in MSM with high-risk sexual behavior after other causes have been ruled out; treatment with metronidazole is recommended. Coinfection with other STDs is common.
Keywords:
intestinal spirochetosis; diarrhea; Brachyspira; MSM; chemsex; HIV infection; histopathology; metronidazole 1. Introduction
Human intestinal spirochetosis (HIS) is defined as the colonization of the luminal surface of colonic and appendiceal epithelial cells by anaerobic spirochetes from the genus Brachyspira [1,2,3]. Of the nine identified Brachyspira species, only B. aalborgi and B. pilosicoli are known to colonize humans [4,5,6,7].
Intestinal spirochetes were first described by Van Leeuwenhoek in 1719, who noticed moving spiral particles in his own stools, which he denominated “animalcules” [8]. In the late 19th century, during a devastating cholera epidemic in Naples, Escherich observed spirochetes in the stools of both symptomatic and asymptomatic individuals [9]. The term HIS was coined in 1967 by Harland and Lee to describe the intestinal colonization of a 64-year-old male patient presenting with a 3-year history of chronic diarrhea [1]. Since then, numerous cases of HIS from low, middle, and high-income countries have been reported.
Spirochetes are a well-known cause of diarrhea in animals [10,11,12,13,14] and have been isolated from the feces of birds, pigs, dogs, and primates. In developing countries, contaminated water and infected animals are primary sources of HIS, whereas, in developed countries, homosexual intercourse is a common risk factor [15,16,17,18,19].
The pathogenic ability of spirochetes is still under debate, with some authors considering HIS harmless [20]. However, a recent systematic review and meta-analysis found that HIS was significantly associated with diarrhea and abdominal pain [7]. Van Mook et al. suggest that, occasionally, the microorganism may gain pathogenicity and become invasive [17,20,21]. The clinical spectrum of HIS is broad, ranging from asymptomatic colonization and incidental diagnosis to symptoms such as abdominal pain, meteorism, diarrhea, constipation, or bleeding. When symptomatic, HIS usually presents as chronic, watery diarrhea [22].
HIS has traditionally been associated with immunosuppression [23,24], especially in patients with HIV infection, though it may also occur in patients with a normal immune status [7,25]. Previous reports suggest that HIS prevalence is significantly higher amongst men who have sex with men (MSM), leading to the question of whether HIS should be considered a sexually transmitted disease (STD) [26]. Individuals at high risk for STDs include those having unprotected sexual intercourse. Those participating in sexualized drug use or chemsex, the use of substances (typically crystal methamphetamine, mephedrone, GHB/GBL, and ketamine) to facilitate, disinhibit, prolong and/or intensify sexual experience [27] (mainly MSM) are at an increased risk for STDs, due to a lower perception of risk and a subsequent increase in unprotected sexual intercourse.
A diagnosis of HIS is made by routine hematoxylin and eosin staining of colonic mucosal biopsies, revealing a dense, bluish haze on the luminal surface of enterocytes caused by a palisade-like arrangement of bacteria that gives the impression of a tight ‘false brush border’ [21]. Subsequent confirmation with Warthin-Starry or Dieterle silver impregnation is highly recommended, as is immunochemistry.
Treatment is not recommended in asymptomatic patients, and a wait-and-see attitude may be followed [21]. However, metronidazole has been described as an effective treatment of anaerobic spirochetes in patients presenting with chronic diarrhea [7,28,29].
The aim of this study was to analyze the clinical significance of HIS in patients from a tertiary hospital in Spain by investigating possible modes of transmission, risk factors for symptomatic disease, and describing histological features and response to treatment.
2. Materials and Methods
We performed a retrospective study of all cases of HIS diagnosed between 1 January 2013 and 31 October 2020 at the Fundación Jiménez Díaz University Hospital in Madrid (Spain). This study included 165 consecutive colorectal biopsies.
2.1. Study Population and Sample Collection
All consecutive cases in which spirochetes were visualized in colonic biopsies obtained from colonoscopies performed from January 2013 until October 2020 were included. A colonoscopy was performed to study symptomatic patients presenting with abdominal pain, chronic diarrhea, or bleeding. Colonoscopies were performed in asymptomatic individuals as the screening procedure in patients with a family history of colon cancer; furthermore, biopsies were also obtained in macroscopically normal colonoscopies in order to rule out microscopic colitis such as lymphocytic and collagenous colitis. Biopsy specimens were obtained from the colon and rectum and evaluated histologically at the Department of Pathology at the Fundación Jiménez Díaz University Hospital in Madrid (Spain). HIS was diagnosed using light microscopy of colorectal mucosa biopsies. The identification of a 3 µm hematoxylinophilic fringe formation adjacent to the normal brush border of the luminal side of the enterocytes was considered a positive result. This finding is proof of spirochete colonization of the colon. The stepped biopsy specimens were fixed in 10% formalin, dehydrated in an increasing series of alcohols and xylol, and then embedded in paraffin. After being deparaffinized, the 4 mm-thick stepped sections (at least 8 sections per block) were routinely stained with hematoxylin and eosin. Diagnoses were confirmed with Warthin-Starry silver stain, and in selected cases, immunohistochemistry with anti-Treponema pallidum antibodies was also performed (rabbit polyclonal antibody Ref: AP10661; AP10661C. Gennova Scientific, S.L. Seville, Spain).
Informed consent was obtained from all subjects involved in the study. Clinical and epidemiological data were retrospectively collected from the medical records of patients to whom a standardized questionnaire had previously been administered. However, some data are missing either because they were not collected at admission or because the patient did not answer or consent to the recording of some personal information; therefore, the denominators for the different characteristics vary. Information about endoscopic findings, treatment, and post-treatment symptoms was also obtained from clinical records. This included: (i) demographic characteristics, e.g., age, sex, and country of birth, (ii) behavioral habits, e.g., contact with animals, travel to developing countries, hand and fruit/vegetable washing, and whether there has been any occurrence of diarrhea in the participant or their family members (iii) drinking and recreational water use, e.g., type of drinking water, whether they had swum in pools, and (iv) sexual attitudes, e.g., sex orientation, sex-role preference, sex-risk behavior, and sexualized drug use (chemsex).
2.2. Scanning Electron Microscopy
In one case, an additional electron microscopic examination was carried out (JEOL JSM6400 scanning microscope, Tokyo, Japan). Scanning Electron Microscopy (SEM) was retrospectively performed on a formalin-fixed large bowel sample from a colonoscopic biopsy. Tissue was processed according to the conventional SEM protocol of fixation with 2% glutaraldehyde and 4% paraformaldehyde in Millonig’s buffer solution at room temperature for one hour, followed by waxing in distilled water and subsequent dehydration with a series of increasing ethanol concentrations. Millonig buffer consists of 2.26% sodium phosphate dibasic solution, and it was laboratory-made with 2.26 g NaH2PO4 + 100 mL of double-distilled water; 2.52% sodium hydroxide solution (2.52 g NaOH + 100 mL of double-distilled water) and 5.4% glucose solution (5.4 g C6H12O6 + 100 mL of double-distilled water). The solution is 0.1 M, and its pH 7.3. Fixation with glutaraldehyde (25% solution EM Sciences, Hatfield, PA, USA) and paraformaldehyde (16% solution EM Sciences, Hatfield, PA, USA) result in Karnovsky fixer. This fixer is preferred above others since bacterial coating and culture features are better preserved. The dehydration process was completed using a critical point dryer. Finally, the sample was mounted in a holder for observation with a JEOL JSM6400 scanning microscope at 20 kV.
2.3. Microbiological Studies
Patients diagnosed with HIS were screened for concomitant STDs and gastrointestinal infections.
2.3.1. Sexually Transmitted Diseases
STDs included sexually acquired HIV, hepatitis C, hepatitis B, and syphilis. Additionally, urethritis and proctitis were studied and defined as the presence of one or more signs or symptoms as depicted in specific guidelines: dysuria, urethral pruritis, mucoid, mucopurulent, and purulent discharge for urethritis and rectal bleeding, pain, tenesmus, constipation, and anal discharge for proctitis [30,31].
Chlamydia trachomatis was detected by polymerase chain reaction on urethral or rectal swab specimens. The swabs used for sample collections were CE medical devices class IIa, DELTALAB (Barcelona, Spain). We used multiplex polymerase chain reaction (C. trachomatis/Neisseria gonorrhoeae/Mycoplasma genitalium Real-TM; Sacace Biotechnologies Srl, Como, Italy) to detect C. trachomatis infection by targeting an 89-bp region of the cryptic plasmid and a 162- to 165-bp region in the genome. The identification of serovar and serogroup was made by specific probes (RHA kit Ct Genotyping, Labo Biomedical Products BV, Rijswijk, The Netherlands); since performing this test takes up to 5 h and because of the large number of samples received in our laboratory, the result could be delayed a few days. When performing all tests, the manufacturer’s instructions were strictly followed.
Urethral or rectal swabs for N. gonorrhoeae culture on selective media, other selective media for facultative bacteria, and polymerase chain reaction to detect herpes simplex virus (artusR Herpes simplex virus 1/2 QS-RGQ; Qiagen, Hilden, Germany) were also performed. Likewise, we also carried out blood tests to detect other STDs, such as HIV, syphilis, hepatitis B, and hepatitis C serologic tests.
2.3.2. Gastrointestinal Infections
From 2013 to 2020, fecal samples were processed using standard microbiological culture methods, namely bacterial culture, microscopy examination for parasites, and immunochromatography for adenovirus, rotavirus, or norovirus. Furthermore, molecular detection of protist intestinal parasites was performed in samples with a positive result on direct microscopy, and the BioFire®FilmArray® gastrointestinal (GI) (Biomerieux, Marcy l’Étoile, France) panel was only carried out when requested by the clinician.
- Bacterial culture
Fecal samples were cultured on CCDA agar plates (Oxoid, Basingstoke, UK) for Campylobacter isolation, SS agar plates (Becton Dickinson, Heidelberg, Germany) for Shigella and Salmonella spp., and CIN agar plates (Oxoid) for Yersinia. In order to detect oxidase-positive Gram-negative bacilli, such as Vibrio, Plesiomonas, and Aeromonas, an oxidase test was performed on the bacterial colonies grown on blood agar (Oxoid, Basingstoke, UK) and MacConkey agar (Becton Dickinson, Heidelberg, Germany) [32]. A Rappaport–Vassiliadis Salmonella Enrichment Broth (VWK Chemicals, MerckKGaA, Darmstadt, Germany) was used as an enrichment step for the recovery of Salmonella, followed by plating on SS agar. The final identification was achieved using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) (Bruker, Bremen, Germany) following the manufacturer’s instructions [32].
- Direct microscopy
Wet mounts for direct microscopic observation of fresh stools were performed at the Department of Microbiology of Fundación Jiménez Díaz University Hospital. When Entamoeba histolytica was suspected, stool samples were periodically sent to the Reference and Parasitological Research Laboratory of the National Microbiology Centre (Instituto de Salud Carlos III, Madrid, Spain) for PCR techniques to differentiate it from Entamoeba dispar, a morphologically identical non-pathogenic species on direct microscopy [33].
- Molecular detection
Genomic DNA was isolated from about 200 mg of each fecal specimen of wild ungulate origin by using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, except for samples mixed with InhibitEX buffer that were incubated for 10 min at 95 °C. Extracted and purified DNA samples were eluted in 200 µL of PCR-grade water and kept at 4 °C until further molecular analysis.
Detection and differential diagnosis of E. histolytica and E. dispar were carried out by a qPCR method targeting a 172-bp fragment of the ssu rRNA gene of the E. histolytica/E. dispar complex using the generic primer set Ehd-239F/Ehd-88R [34]. All qPCR reactions were run on a Corbett Rotor-Gene 6000 qPCR cycler (QIAGEN). All direct and nested PCR reactions were run on a 2720 thermocycler (Applied Biosystems, Foster City, CA, USA). Reaction mixtures included 2.5 units of MyTAQ™ DNA polymerase (Bioline GmbH, Luckenwalde, Germany), a 5xMyTAQ Reaction Buffer containing 5 mM dNTPs, and 15 mM MgCl2. PCR amplicons were visualized on 2% D5 agarose gels (Conda, Madrid, Spain) and stained with Pronasafe nucleic acid staining solution (Conda).
When requested by the clinician, assays on the feces samples were performed using the Biofire®FilmArray® gastrointestinal panel for the detection of 22 pathogens, which are causative agents of infectious diarrhea in humans, including bacteria, Campylobacter (jejuni, coli, upsaliensis), Clostridioides difficile toxin A/B, Plesiomonas shigelloides, Salmonella, Yersinia enterocolitica, Vibrio (parahaemolyticus, vulnificus, cholerae), Escherichia coli O157, enteroaggregative E.coli (EAEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC) Shiga toxin/verotoxin-producing or enterohaemorrhagic E. coli (STEC) stx1/stx2, and Shigella/Enteroinvasive E. coli (EIEC); parasites, Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, and Giardia lamblia; and viruses including Adenovirus F 40/41, Astrovirus, Norovirus GI/GII, Rotavirus A, and Sapovirus(I, II, IV, and V). For each sample, 200 µL was added to each panel, following the manufacturer’s instructions, and was then analyzed in a Biofire®Filmarray® integrated system (Biomerieux, Marcy l’Étoile, France).
2.4. Statistical Analysis
Continuous variables were presented as median and interquartile ranges (IQR), and categorical variables as proportions unless otherwise specified. We used the Mann-Whitney U-test, χ2 test, or Fisher’s exact test to compare differences between groups, as appropriate. For these comparisons, a p-value of 0.05 or lower was considered of statistical significance. Statistical analysis was performed using SPSS® v27.0 (IBM Corp., Armonk, NY, USA).
Univariable and multivariable logistic regression models served to analyze risk factors associated with symptoms. Values are expressed as odds ratio (OR) and 95% confidence intervals (95% CI). The multivariable logistic regression model was adjusted by the variables that had p-value ≤ 0.05, which were further selected by a stepwise forward selection method (pin < 0.05 and pout < 0.10). Significant differences are shown in bold.
3. Results
3.1. Age and Sex
A total of 165 patients were diagnosed with HIS, with a median age of 41.0 years (IQR 34.0–50.0). Most patients were male (156/165; 94.5%), and the majority were Caucasian (148/165; 89.7%). The baseline characteristics of the patients are shown in Table 1.

Table 1.
Sociodemographic variables and association with symptomatic HIS.
3.2. Lifestyle and Risk Factors
A total of 99 patients were smokers (99/165; 60%), 36 patients acknowledged using one or more recreational drugs (36/119; 30.2%), and 27 (27/115; 23.5%) engaged in chemsex. The most common recreational drugs were GHB, mephedrone, popper, crystal methamphetamine, and, to a lesser extent, ketamine.
Regarding other potential risk factors for HIS, 67 patients (67/106; 63.2%) had traveled to low and middle-income countries in the past, and 61 patients (61/101; 60.4%) reported previous or current animal ownership.
3.3. Sexual Orientation, HIV Status, and Sexually Transmitted Diseases
Data regarding sexual orientation and drug use were collected from patients who agreed to share this information; the sample size of each variable is specified in Table 1. HIV was diagnosed after HIS was detected. In the male group, 124 patients reported having sex with other men (124/143; 86.7%); 85 patients did not have a stable sexual partner (85/125; 68.0%), and 98 patients reported habitual, unprotected oral-anal contacts (98/125; 78.4%).
Information on HIV testing was only available for 153 patients, of whom 65 (42.5%) were HIV-positive. In one case, HIV was diagnosed after HIS was detected. During the period of study, 98 patients (98/147; 66.7%) presented a concomitant STD. A total of 12 patients (12/147; 8.2%) had a previous diagnosis of sexually acquired hepatitis C, 4 tested positive for hepatitis B surface antigen, and 47 (47/141; 33.3%) for a positive core antigen (HBcAg). Additionally, 44 patients (44/127; 34.6%) had a positive treponemal test. Moreover, 43 patients (43/103; 41.7%) had presented a previous episode of urethritis, most commonly caused by Neisseria gonorrhoeae, and 33 patients (33/99; 33.3%) proctitis, mainly caused by Chlamydia trachomatis (Table 2).

Table 2.
Sexually transmitted diseases in MSM patients with HIS.
3.4. Symptomatology
Gastrointestinal symptoms were recorded in 133 patients (81.1%); 112 patients (68.3%) presented with diarrhea, 79 patients complained of changes in their bowel habits (48.2%), 51 had experienced rectal bleeding (31.1%), and 49 abdominal pain (29.9%).
Risk factors associated with the presence of symptomatic disease are shown in Table 3. Multivariable regression showed an increased odds of symptoms associated with age under 41 (odds ratio 5.44, 95% CI 1.87–15.88; p = 0.002).

Table 3.
Risk factors associated with symptomatic HIS.
3.5. Localization and Histologic Findings
Macroscopic colonoscopy findings were normal in 152 patients (92.1%). Unspecific inflammatory changes were observed in the remaining 7.9%. HIS was mainly detected in the cecum and ascending colon in 128 patients (77.6%), followed by the transverse colon (50.9%), descending colon (44.2%), and rectum (26.1%). Biopsies showed spirochetes in a variety of localizations: on the intact surface epithelium (n = 152; 92.1%); in the upper part of crypts (n = 9; 5.5%); on the surface of microabscesses (n = 2, 1.2%); and in both the upper part of crypts and microabscesses (n = 1, 0.6%). Spirochetes were found coating adenomas in 25 samples (15.15%). Under light microscopy, spirochetes were observed attached to the surface of the colonic epithelium with hematoxylin-eosin staining. Warthin-Starry silver staining was performed in all biopsies, confirming the presence of HIS (Figure 1).
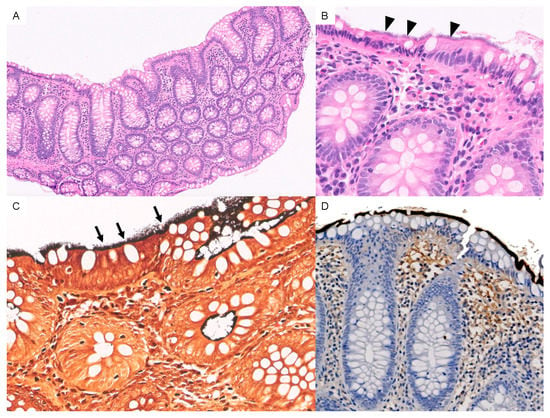
Figure 1.
(A) Panoramic view of HIS with hematoxylin and eosin staining [10×]. (B) A 3 μm-thick basophilic fringe on the luminal surface of the enterocytes is visible (arrowheads) [40×]. (C) Spirochetes with Warthin-starry silver stain are observed (arrows). [40×] (D) Immunohistochemistry for anti-Treponema pallidum antibodies highlights spirochetes in dark brown-black color.
Additionally, immunochemistry for anti-Treponema pallidum antibodies was carried out in some samples (Figure 1D).
Scanning Electron Microscopy (SEM)
One specimen was obtained for study under SEM, where the colonic epithelium was found to be covered with a forest of spirochetes. B. aalborgi was identified using polymerase chain reaction (PCR). Figure 2 shows how the microorganism disrupts the normal architecture of the human colon, invading the epithelium and creating a biofilm.

Figure 2.
(A) Spirochetes attached to the colonic epithelium. (B) Detail of spirochetes disrupting the normal architecture of the human colon, invading the epithelium. (C) Biofilm formation of Brachyspira aalborgi. (D) Detail of spirochetes with their distinctive sinusoidal shape.
3.6. Additional Microbiological Studies
In total, 102 fecal samples from symptomatic patients were sent for microbiological testing. Among them, 20 (19.6%) were positive for an infective microorganism (Table 4), and seven patients were coinfected with more than one pathogen. The most common microorganism was Giardia duodenalis (n = 8), followed by EAEC (n = 5), Shigella/EIEC (n = 3), EPEC (n = 3), STEC (n = 2), Blastocystis hominis (n = 2), Entamoeba hystolitica (n = 1), Norovirus (n = 1), Sapovirus (n = 1) and Yersinia enterocolitica (n = 1). It was necessary to perform molecular differentiation between E. hystolitica and E. dispar in 14 cases. In only one patient, E. hystolitica was detected, while Entamoeba dispar DNA was detected in all of the others (92.8%). The results of pathogenic microorganisms are shown in Table 4.

Table 4.
Results of microbiological studies in stool samples from 102 patients.
3.7. Blood Tests
Blood test results showed no specific findings and were otherwise unremarkable (Table 5).

Table 5.
Results of blood tests.
No statistically significant difference was found when comparing these values between symptomatic and asymptomatic patients.
3.8. Treatment
Out of 165 patients, 107 (69.0%) were treated. Additionally, 91 (58.7%) received metronidazole, 12 were treated with doxycycline (7.3%), and the four remaining patients received different antimicrobial agents (macrolides or combined treatment).
Of the 82 symptomatic patients without concomitant gastrointestinal infection, 53 followed up after treatment; all patients reporting symptom improvement (n = 42, 79.2%) had received metronidazole or doxycycline (p = 0.049).
4. Discussion
HIS is caused by the slow-growing anaerobic gram-negative spirochetes B. aalborgi and B. pilosicoli. Previous descriptions of HIS report an association with HIV infection and MSM, but various studies have confirmed that it also affects a wider range of patients, including those living in relatively crowded conditions, lacking proper hygienic facilities, and living in close contact with animals [1,15,17,19].
Every patient in whom HIS was incidentally found during a colonoscopy was selected. Colonoscopies were performed irrespective of the patient’s sexual orientation, finding the vast majority of HIS in MSM as in other studies [4,7,15,16,17,18,19,35,36]. The age profile of our cohort is similar to that of prior publications [6,37]. HIS has traditionally been associated with HIV infection, and patients with a new diagnosis of HIS should be screened for HIV infection. In our series, one new HIV diagnosis was made after screening in the context of HIS.
We observed a high N. gonorrhoeae and C. trachomatis coinfection rate, a finding reported in other studies [19,26]. Giardia duodenalis, Entamoeba histolytica, and other intestinal protozoa cause symptoms resembling those of symptomatic HIS; metronidazole is the treatment of choice for these pathogens, but it is also first-line therapy for HIS. Screening for intestinal protozoa and appropriate treatment may be considered as a first step when evaluating patients with possible HIS to minimize confounding factors and avoid unnecessary invasive procedures.
In our series, coinfection with another gastrointestinal pathogen was detected in 20 symptomatic patients (19.6%), with HIS being the sole infectious agent in 80.4%. All symptomatic patients without a concomitant gastrointestinal infection showing improvement at the follow-up had received treatment with metronidazole or doxycycline.
To date, and to the best of our knowledge, no previous studies have assessed the role of sexualized drug use in HIS. A vast majority of HIS was detected in MSM (86.7%), among whom 23.5% practiced chemsex. Although a higher number of chemsex users was found in symptomatic compared to asymptomatic HIS, chemsex was not confirmed in the multivariate analysis as an independent risk factor for the development of symptoms, being rather a co-factor that increases rates of unprotected sex, leading to an increased risk of STDs [38,39].
The absence of macroscopical inflammation in our series contrasts with the findings of Calderaro et al., who describe hyperemia, mucosal erosion, and inflammatory changes in the 17 patients included in their study [36]. However, our results are similar to those described by Anthony et al., who found no macroscopical inflammation or, at most, minimal inflammatory changes [6].
Therapeutic recommendations for HIS have changed over the last decades, and response to treatment varies. Some patients may experience complete remission of diarrhea and gastrointestinal symptoms, whereas others fail to improve despite confirmation of HIS eradication in subsequent histologic exams [22]. Some authors suggest that the level of histological invasion could be associated with symptom severity, with patients who present spirochaetal invasion beyond the superficial epithelium showing better response to treatment [40]. In symptomatic patients, a trial of antibiotic treatment with metronidazole is recommended [7,28,29,41], although clinical responses may vary. Metronidazole is one of the mainstay drugs for the treatment of anaerobic infections; it exerts rapid bactericidal effects against anaerobic bacteria with a killing rate proportional to the drug concentration. The most common adverse effects are gastrointestinal, and patients may present with symptoms such as nausea, anorexia, vomiting, and diarrhea. It can also cause dizziness, confusion and peripheral neuropathy, and a disulfiram-like reaction may be observed in patients drinking ethanol [42].
Doxycycline, in turn, inhibits protein synthesis by binding with the 30S ribosomal subunit of susceptible bacteria. It may cause skin photosensitivity reactions, and also esophagitis and esophageal ulcers may occur due to local caustic injury produced by direct local contact and because of its low pH. In our study, we found that all symptomatic patients without concomitant gastrointestinal infection improved after being treated with metronidazole or doxycycline. No adverse reactions were reported in either case.
Our histopathological findings of HIS using light microscopy are similar to those described in the literature. We only recruited one sample for scanning electron microscopy, which showed a disruption of the normal architecture of the human colon and the presence of biofilm. Recent studies have demonstrated the capability of some spirochetes to form biofilms, as in the case of Borrelia burgdorferi, Leptospira interrogans, and Treponema denticola [43,44]. This is the first report of biofilm formation in Brachyspira spp, although the finding should be confirmed in future studies.
The main limitation of this study is its retrospective nature, so the statistical analysis was most likely influenced by the limited availability of some data. As reported in the methods, clinical and epidemiological data were retrospectively collected from the medical records of patients to whom a standardized questionnaire had previously been administered. PCR in fecal samples was performed irrespective of symptomatology, but data were not available for all samples. Moreover, molecular studies to identify the species of Brachyspira were only performed in one case.
5. Conclusions
HIS should be considered as a cause of chronic diarrhea in MSM with high-risk sexual behavior after other causes have been ruled out. Treatment with metronidazole is recommended in these cases. Coinfection with other sexually transmitted diseases is common.
Author Contributions
Conceptualization, L.P.-P.; methodology, L.P.-P. and R.P.-T.; software, R.P.-T. and A.M.V.M.; validation, L.P.-P. and R.P.-T.; formal analysis, R.P.-T. and M.d.P.T.; investigation, M.d.P.T.; resources, A.M.V.M., D.C. and P.K.; data curation, M.d.P.T.; writing—original draft preparation, L.P.-P., R.P.-T. and M.d.P.T.; writing—review and editing, L.P.-P., R.P.-T., M.d.P.T., A.M.V.M., D.C., P.K., M.G., J.R.F.A. and A.C.-Ú.; visualization, M.d.P.T., R.P.-T. and L.P.-P.; supervision, L.P.-P. and R.P.-T.; project administration, L.P.-P. and R.P.-T.; funding acquisition, L.P.-P., M.G. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of IIS-Fundación Jiménez Díaz Ethical Review Board (Ref. PIC061-18_FJD; 12 July 2018). Standard clinical practice was followed for all procedures, and participating patients’ information was anonymized and deidentified prior to analysis.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Standard clinical practice was followed for all procedures, and participating patients’ information was anonymized and reidentified prior to analysis.
Data Availability Statement
Data cannot be shared publicly because they are confidential. Data are available from the Department of Infectious Diseases of IIS-Fundación Jiménez Díaz for researchers who meet the criteria for access to confidential data.
Acknowledgments
We would like to thank Félix Manzarbeitia, for selecting HIS case records from the Pathology Department and Bernadette Pfang, for her help in reviewing and editing the English version of the manuscript. We acknowledge the ICTS-CNME for the electron microscope support. We also appreciate the help to Rafael Rojas for his technical help.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Harland, W.A.; Lee, F.D. Intestinal Spirochaetosis. Br. Med. J. 1967, 3, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.D.; Kraszewski, A.; Gordon, J.; Howie, J.G.R.; Mcseveney, D.; Harland, W.A. Intestinal Spirochaetosis. Gut 1971, 12, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.A.; Torbenson, M.S. Intestinal Spirochetosis. Mayo Clin. Proc. 2020, 95, 427–428. [Google Scholar] [CrossRef]
- Weisheit, B.; Bethke, B.; Stolte, M. Human Intestinal Spirochetosis: Analysis of the Symptoms of 209 Patients. Scand. J. Gastroenterol. 2007, 42, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.J. Hiding in Plain Sight: Colonic Spirochetosis in Humans. J. Bacteriol. 2019, 201, e00465-19. [Google Scholar] [CrossRef]
- Anthony, N.E.; Blackwell, J.; Ahrens, W.; Lovell, R.; Scobey, M.W. Intestinal Spirochetosis: An Enigmatic Disease. Dig. Dis. Sci. 2013, 58, 202–208. [Google Scholar] [CrossRef]
- Fan, K.; Eslick, G.D.; Nair, P.M.; Burns, G.L.; Walker, M.M.; Hoedt, E.C.; Keely, S.; Talley, N.J. Human Intestinal Spirochetosis, Irritable Bowel Syndrome, and Colonic Polyps: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2022, 37, 1222–1234. [Google Scholar] [CrossRef]
- Escherich, T. Beiträge Zur Kenntniss Der Darmbakterien. München Med. Wochenschr. 1886, 33, 815–817. [Google Scholar]
- Escherich, T. Klinisch-therapeutische Beobachtungen aus der Cholera-Epidemiein Neapel. Aerztliches Intell. 1984, 31, 561–564. [Google Scholar]
- Stephens, C.P.; Hampson, D.J. Intestinal Spirochete Infections of Chickens: A Review of Disease Associations, Epidemiology and Control. Anim. Health Res. Rev. 2001, 2, 83–91. [Google Scholar] [CrossRef]
- Mikosza, A.S.J.; Hampson, D.J. Human Intestinal Spirochetosis: Brachyspira Aalborgi and/or Brachyspira Pilosicoli? Anim. Health Res. Rev. 2001, 2, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.J.; Huxtable, C.R.; Hampson, D.J. Experimental Infection of Newly Weaned Pigs with Human and Porcine Strains of Serpulina Pilosicoli. Infect. Immun. 1996, 64, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.J.; Mclaren, A.J.; Hampson, D.J. Pathogenicity of Human and Porcine Intestinal Spirochetes in One-Day-Old Specific-Pathogen-Free Chicks: An animal model of intestinal spirochetosis. Infect. Immun. 1995, 63, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Munshi, M.A.; Traub, R.J.; Robertson, I.D.; Mikosza, A.S.J.; Hampson, D.J. Colonization and Risk Factors for Brachyspiraa Alborgi and Brachyspira Pilosicoli in Humans and Dogs on Tea Estates in Assam, India. Epidemiol. Infect. 2004, 132, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.J.; Miller, J.N.; Lance Georgel’, A.W. Microbiological and Biochemical Characterization of Spirochetes Isolated from the Feces of Homosexual Males. J. Clin. Microbiol. 1986, 24, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.S.; Waugh, M.A.; Cooke, E.M. Isolation of Intestinal Spirochaetes from Homosexuals. J. Clin. Pathol. 1981, 34, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Law, C.L.H.; Grierson, J.M.; Stevens, S.M.B.; Law, C. Rectal Spirochaetosis in Homosexual Men: The Association with Sexual Practices, HIV Infection and Enteric Flora. Genitourin. Med. 1994, 70, 26–29. [Google Scholar] [CrossRef]
- Cooper, C.; Cotton, D.W.K.; Hudson, M.J.; Kirkham, N.; Wilmott, F.E.W. Rectal Spirochaetosis in Homosexual Men: Characterisation of the Organism and Pathophysiology. Genitourin. Med. 1986, 62, 47–52. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Roberts, P.L.; Ann Rompalo, M.; Quinn, T.C.; Holmes, K.K.; Stamm, W.E. Intestinal Spirochetosis in Homosexual Men. Am. J. Med. 1987, 82, 587–592. [Google Scholar] [CrossRef]
- Nielsen, R.H.; Orholm, M.; Pedersen, J.O.; Hovind-Hougen, K.; StubbeTeglbjærg, P.; Hess Thaysen, E. Colorectal Spirochetosis: Clinical Significance of the Infestation. Gastroenterology 1983, 85, 62–67. [Google Scholar] [CrossRef]
- van Mook, W.N.K.A.; Koek, G.H.; van der Ven, A.J.A.M.; Ceelen, T.L.; Bos, R.P. Human Intestinal Spirochaetosis: Any Clinical Significance? Eur. J. Gastroenterol. Hepatol. 2004, 16, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Tsinganou, E.; Gebbers, J.-O. Human Intestinal Spirochetosis—A Review. GER Med. Sci. 2010, 8, Doc01. [Google Scholar]
- Kanavaki, S.; Mantadakis, E.; Thomakos, N.; Pefanis, A.; Matsiota-Bernard, P.; Karabela, S.; Samonis, G. Brachyspira (Serpulina) Pilosicoli Spirochetemia in an Immunocompromised Patient. Infection 2002, 30, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, T.; Hayashi, S.; Adachi, Y.; Sunada, K.; Hayashi, Y.; Nishimura, N.; Yano, T.; Miyata, T.; Yamamoto, H.; Hirai, Y.; et al. Human Intestinal Spirochetosis in an Immunocompromised Host: Evaluation of Eradication Therapy by Endoscopy, Histopathology and Bacteriology. Clin. J. Gastroenterol. 2012, 5, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Takahashi, M.; Horiguchi, S.I.; Funata, N.; Koizumi, K.; Okudela, K.; Hishima, T.; Ohashi, K. Clinicopathologic Study of Intestinal Spirochetosis in Japan with Special Reference to Human Immunodeficiency Virus Infection Status and Species Types: Analysis of 5265 Consecutive Colorectal Biopsies. BMC Infect. Dis. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, D.; Vall-Mayans, M.; Coll-Estrada, S.; Naranjo-Hans, L.; Armengol, P.; Iglesias, M.A.; Barberá, M.J.; Arando, M. Human Intestinal Spirochetosis, a Sexually Transmissible Infection? Review of Six Cases from Two Sexually Transmitted Infection Centres in Barcelona. Int. J. STD AIDS 2021, 32, 52–58. [Google Scholar] [CrossRef]
- Edmundson, C.; Heinsbroek, E.; Glass, R.; Hope, V.; Mohammed, H.; White, M.; Desai, M. Sexualised Drug Use in the United Kingdom (UK): A Review of the Literature. Int. J. Drug Policy 2018, 55, 131–148. [Google Scholar] [CrossRef]
- Peghini, P.L.; Guccion, J.G.; Sharma, A. Improvement of Chronic Diarrhea After Treatment for Intestinal Spirochetosis. Dig. Dis. Sci. 2000, 45, 1006–1010. [Google Scholar] [CrossRef]
- Esteve, M.; Salas, A.; Fernández-Bañares, F.; Lloreta, J.; Mariné, M.; Gonzalez, C.I.; Forné, M.; Casalots, J.; Santaolalla, R.; Espinós, J.C.; et al. Intestinal Spirochetosis and Chronic Watery Diarrhea: Clinical and Histological Response to Treatment and Long-Term Follow Up. J. Gastroenterol. Hepatol. 2006, 21, 1326–1333. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Nori, A.v.; Kiellberg Larsen, H.; Kreuter, A.; Padovese, V.; Pallawela, S.; Vall-Mayans, M.; Ross, J. 2021 European Guideline on the Management of Proctitis, Proctocolitis and Enteritis Caused by Sexually Transmissible Pathogens. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1434–1443. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; de Barbeyrac, B.; de Vrieze, N.H.N.; Viset, J.D.; White, J.A.; Vall-Mayans, M.; Unemo, M. 2019 European Guideline on the Management of Lymphogranuloma Venereum. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Zboromyrska, Y.; Hurtado, J.C.; Salvador, P.; Alvarez-Martínez, M.J.; Valls, M.E.; Mas, J.; Marcos, M.A.; Gascón, J.; Vila, J. Aetiology of traveller’s diarrhoea: Evaluation of a multiplex PCR tool to detect different enteropathogens. Clin. Microbiol. Infect. 2014, 20, O753–O759. [Google Scholar] [CrossRef]
- Stanley, S.L., Jr. Amoebiasis. Lancet 2003, 361, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cisneros, M.J.; Cogollos, R.; López-Vélez, R.; Martín-Rabadá, P.; Martínez-Ruiz, R.; Subirats, M.; Merino, F.J.; Fuentes, I. Application of Real-Time PCR for the Differentiation of Entamoeba Histolytica and E. Dispar in Cyst-Positive Faecal Samples from 130 Immigrants Living in Spain. Ann. Trop. Med. Parasitol. 2010, 104, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J.; Mahajan, H.; Tan, K.L.; Sharma, R. The Histological Features of Intestinal Spirochetosis in a Series of 113 Patients. Int. J. Surg. Pathol. 2010, 18, 144–148. [Google Scholar] [CrossRef]
- Mcmillan, A.; Lee, F.D. Sigmoidoscopic and Microscopic Appearance of the Rectal Mucosa in Homosexual Men. Gut 1981, 22, 1035–1041. [Google Scholar] [CrossRef]
- Calderaro, A.; Bommezzadri, S.; Gorrini, C.; Piccolo, G.; Peruzzi, S.; Villanacci, V.; Zambelli, C.; Dettori, G.; Chezzi, C. Infective Colitis Associated with Human Intestinal Spirochetosis. J. Gastroenterol. Hepatol. 2007, 22, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Heiligenberg, M.; Wermeling, P.R.; van Rooijen, M.S.; Urbanus, A.T.; Speksnijder, A.G.C.L.; Heijman, T.; Prins, M.; Coutinho, R.A.; van der Loeff, M.F.S. Recreational Drug Use during Sex and Sexually Transmitted Infections among Clients of a City Sexually Transmitted Infections Clinic in Amsterdam, the Netherlands. Sex. Transm. Dis. 2012, 39, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, P.; Hickson, F.; Reid, D.; Torres-Rueda, S.; Bourne, A. Motivations and Values Associated with Combining Sex and Illicit Drugs (Chemsex) among Gay Men in South London: Findings from a Qualitative Study. Sex. Transm. Infect. 2017, 93, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Gebbers, J.O. Clinical Significance of Human Intestinal Spirochetosis—A Morphologic Approach. Infection 2003, 31, 341–349. [Google Scholar] [CrossRef]
- Tong, Y.T.; Younes, M. Intestinal Spirochetosis: Case Series and Review of Literature. Ann. Clin. Lab. Sci. 2020, 50, 386–390. [Google Scholar] [PubMed]
- Edwards, D.L.; Fink, P.C.; Van-Dyke, P.O. Disulfiram-like reaction associated with intravenous trimethoprim-sulfamethoxazole and metronidazole. Clin. Pharm. 1986, 5, 999–1000. [Google Scholar] [PubMed]
- Ristow, P.; Bourhy, P.; Kerneis, S.; Schmitt, C.; Prevost, M.C.; Lilenbaum, W.; Picardeau, M. Biofilm Formation by Saprophytic and Pathogenic Leptospires. Microbiology 2008, 154, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.N.; dos Santos Ribeiro, P.; da França, G.V.; Souza, F.N.; Ramos, E.A.G.; Figueira, C.P.; Reis, M.G.; Costa, F.; Ristow, P. Leptospira Interrogans Biofilm Formation in Rattus Norvegicus (Norway Rats) Natural Reservoirs. PLoS Negl. Trop. Dis. 2021, 15, e0009736. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).