Virus Identification for Monkeypox in Human Seminal Fluid Samples: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Eligibility Criteria
2.3. Sources of Information and Search Techniques
2.4. Study Selection
2.5. Outcomes
2.6. Data Collection Process and Data Items
2.7. Tables
3. Results
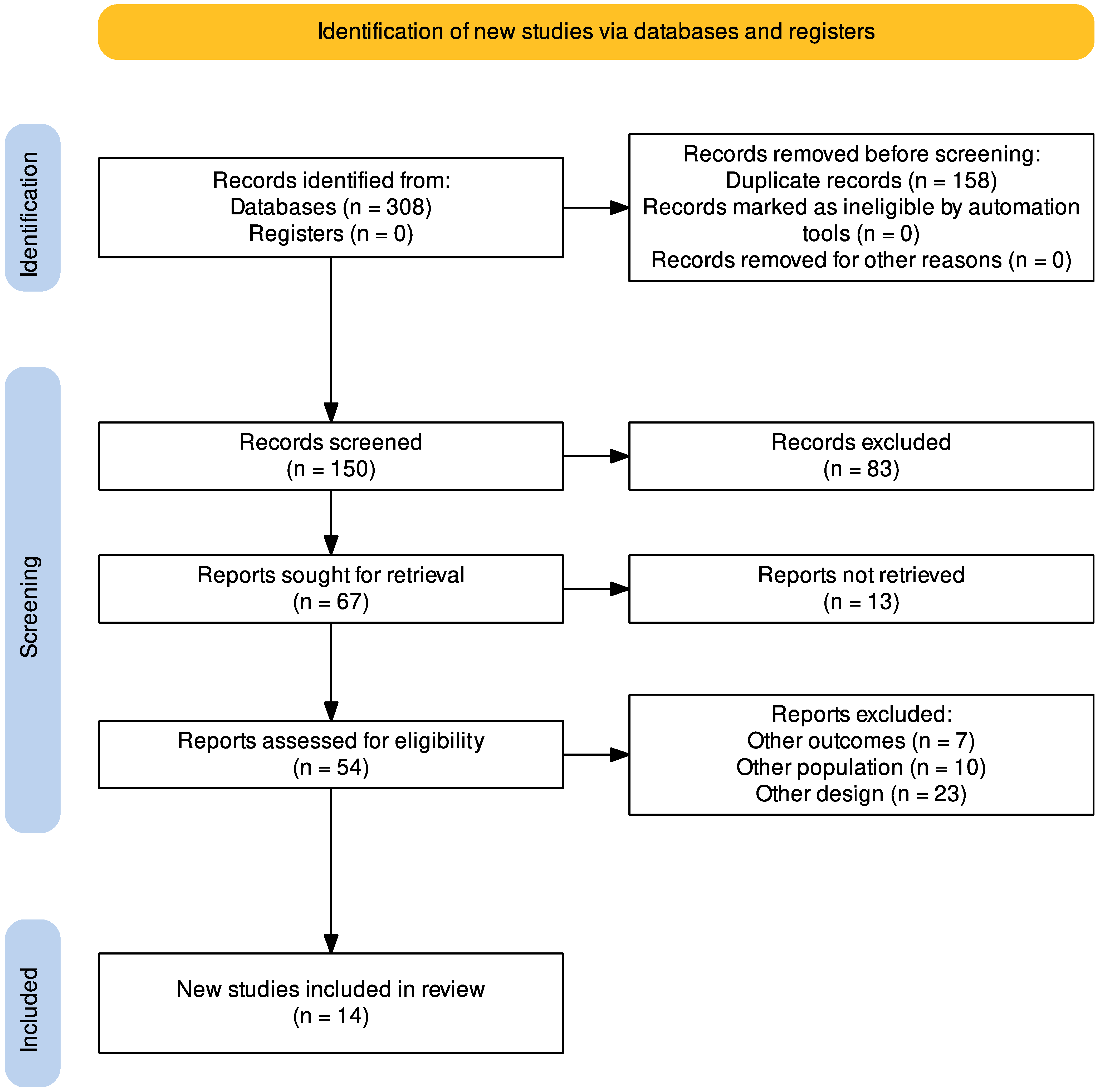
3.1. Study Selection
3.2. Study Characteristics
3.3. Demographic Characteristics and MPXV-Positive Samples
3.4. Clinical Manifestations, Localization of Skin Lesions, and Outcome
4. Discussion
4.1. Main Findings and Current Epidemiological Data
4.2. Detection of MPX in Seminal Fluid Samples from Confirmed Cases
4.3. Clinical Characteristics of Patients with MPX
4.4. Future Perspectives on the MPX
4.5. Limitations and Strengths
4.6. Relevance of Findings in Public Health
4.7. Prevention Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox–After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Elsayed, S.; Bondy, L.; Hanage, W.P. Monkeypox Virus Infections in Humans. Clin. Microbiol. Rev. 2022, 35, e00092-22. [Google Scholar] [CrossRef]
- De Baetselier, I.; Van Dijck, C.; Kenyon, C.; Coppens, J.; Michiels, J.; de Block, T.; Smet, H.; Coppens, S.; Vanroye, F.; Bugert, J.J.; et al. Retrospective Detection of Asymptomatic Monkeypox Virus Infections among Male Sexual Health Clinic Attendees in Belgium. Nat. Med. 2022, 28, 2288–2292. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The Never-Ending Global Emergence of Viral Zoonoses after COVID-19? The Rising Concern of Monkeypox in Europe, North America and Beyond. Travel Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Bonilla-Aldana, D.K.; et al. Human Monkeypox Disease (MPX). Infez. Med. 2022, 30, 372–391. [Google Scholar] [CrossRef]
- Rezza, G. Emergence of Human Monkeypox in West Africa. Lancet Infect. Dis. 2019, 19, 797–799. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, L.; Wang, B.; Sun, Y.; Wu, X.; Peng, X.; Li, Y.; Lin, Y.-F.; Fitzpatrick, T.; Vermund, S.H.; et al. Clinical Characteristics of Human Mpox (Monkeypox) in 2022: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 146. [Google Scholar] [CrossRef]
- Fink, D.L.; Callaby, H.; Luintel, A.; Beynon, W.; Bond, H.; Lim, E.Y.; Gkrania-Klotsas, E.; Heskin, J.; Bracchi, M.; Rathish, B.; et al. Clinical Features and Management of Individuals Admitted to Hospital with Monkeypox and Associated Complications across the UK: A Retrospective Cohort Study. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Tomasoni, L.R.; Castelli, F.; Quiros-Roldan, E. Monkeypox, a Literature Review: What Is New and Where Does This Concerning Virus Come From? Viruses 2022, 14, 1894. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Barboza, J.J.; Garcia-Vasquez, E.A.; Bonilla-Aldana, D.K.; Diaz-Torres, M.; Saldaña-Cumpa, H.M.; Diaz-Murillo, M.T.; Cruz, O.C.-S.; Rodriguez-Morales, A.J. Epidemiological Situation of Monkeypox Transmission by Possible Sexual Contact: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- León-Figueroa, D.A.; Barboza, J.J.; Saldaña-Cumpa, H.M.; Moreno-Ramos, E.; Bonilla-Aldana, D.K.; Valladares-Garrido, M.J.; Sah, R.; Rodriguez-Morales, A.J. Detection of Monkeypox Virus According to The Collection Site of Samples from Confirmed Cases: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 4. [Google Scholar] [CrossRef]
- Gul, I.; Liu, C.; Yuan, X.; Du, Z.; Zhai, S.; Lei, Z.; Chen, Q.; Raheem, M.A.; He, Q.; Hu, Q.; et al. Current and Perspective Sensing Methods for Monkeypox Virus. Bioengineering 2022, 9, 571. [Google Scholar] [CrossRef]
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of Monkeypox Virus-An Overview. Travel Med. Infect. Dis. 2022, 50, 102459. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Abdelaal, A.; Brakat, A.M.; Lashin, B.I.; Abouelkheir, M.; Abdelazeem, B.; Rodriguez-Morales, A.J.; Sah, R. Monkeypox Viral Detection In Semen Specimens of Confirmed Cases: A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 95, e28250. [Google Scholar] [CrossRef] [PubMed]
- Brundu, M.; Marinello, S.; Scaglione, V.; Ferrari, A.; Franchin, E.; Mazzitelli, M.; Cattelan, A.M. The First Case of Monkeypox Virus and Acute HIV Infection: Should We Consider Monkeypox a New Possible Sexually Transmitted Infection? J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Hornuss, D.; Daehne, T.; Goetz, V.; Mueller, M.; Usadel, S.; Lorz, A.; Mockenhaupt, M.; Huzly, D.; Bierbaum, S.; Fuchs, J.; et al. Transmission Characteristics, Replication Patterns and Clinical Manifestations of Human Monkeypox Virus-an in-Depth Analysis of Four Cases from Germany. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 29, 112-e5. [Google Scholar] [CrossRef]
- Pettke, A.; Filén, F.; Widgren, K.; Jacks, A.; Glans, H.; Andreasson, S.; Muradrasoli, S.; Helgesson, S.; Hauzenberger, E.; Karlberg, M.L.; et al. Ten-Week Follow-Up of Monkeypox Case-Patient, Sweden, 2022. Emerg. Infect. Dis. 2022, 28, 2074–2077. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Jaeranny, S.; Li, M.; Sukhdeo, S.S.; Monge, J.C.; Callejas, M.F.; Hasso, M.; Fattouh, R.; Lalonde, S.D.; Lam, J.; et al. Atypical Clinical Presentation of Monkeypox Complicated by Myopericarditis. Open Forum Infect. Dis. 2022, 9, ofac394. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- Noe, S.; Zange, S.; Seilmaier, M.; Antwerpen, M.H.; Fenzl, T.; Schneider, J.; Spinner, C.D.; Bugert, J.J.; Wendtner, C.-M.; Wölfel, R. Clinical and Virological Features of First Human Monkeypox Cases in Germany. Infection 2022, 51, 265–270. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J.; et al. Frequent Detection of Monkeypox Virus DNA in Saliva, Semen, and Other Clinical Samples from 12 Patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Candela, C.; Mileto, D.; Canetti, D.; Bruzzesi, E.; Rizzo, A.; Castagna, A.; Nozza, S. Monkeypox Infection among Men Who Have Sex with Men: PCR Testing on Seminal Fluids. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef]
- Friedel, N.; Gallo, E.S.; Horovitz, T.; Ben Ami, R.; Sprecher, E. Sexually Transmitted Monkeypox with Pseudo-Koebnerization within a Tattoo. JAAD Case Rep. 2022, 31, 112–114. [Google Scholar] [CrossRef]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral Loads in Clinical Samples of Men with Monkeypox Virus Infection: A French Case Series. Lancet Infect. Dis. 2022, 23, 74–80. [Google Scholar] [CrossRef]
- Duarte, A.; Iannantuono, M.V.; Perez, M.; Masciocchi, M.; Payaslian, S.; Cuesta, M.C. Monkeypox and exudative pharyngitis in Argentina. Medicina 2022, 82, 770–773. [Google Scholar]
- Taylor, L. Monkeypox: WHO Declares a Public Health Emergency of International Concern. BMJ 2022, 378, o1874. [Google Scholar] [CrossRef]
- CDC Mpox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 8 January 2023).
- Multi-Country Outbreak of Mpox (Monkeypox)-External Situation Report 13, Published 5 January 2023-World|ReliefWeb. Available online: https://reliefweb.int/report/world/multi-country-outbreak-mpox-monkeypox-external-situation-report-13-published-5-january-2023 (accessed on 7 January 2023).
- Science Brief: Detection and Transmission of Mpox (Formerly Monkeypox) Virus during the 2022 Clade IIb Outbreak. Available online: https://stacks.cdc.gov/view/cdc/124367 (accessed on 8 February 2023).
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika Virus Infection Damages the Testes in Mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef]
- Perry, D.L.; Huzella, L.M.; Bernbaum, J.G.; Holbrook, M.R.; Jahrling, P.B.; Hagen, K.R.; Schnell, M.J.; Johnson, R.F. Ebola Virus Localization in the Macaque Reproductive Tract during Acute Ebola Virus Disease. Am. J. Pathol. 2018, 188, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Coffin, K.M.; Liu, J.; Warren, T.K.; Blancett, C.D.; Kuehl, K.A.; Nichols, D.K.; Bearss, J.J.; Schellhase, C.W.; Retterer, C.J.; Weidner, J.M.; et al. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe 2018, 24, 405–416.e3. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Shoemaker, C.J.; Zeng, X.; Garrison, A.R.; Golden, J.W.; Schellhase, C.W.; Pratt, W.; Rossi, F.; Fitzpatrick, C.J.; Shamblin, J.; et al. Persistent Crimean-Congo Hemorrhagic Fever Virus Infection in the Testes and within Granulomas of Non-Human Primates with Latent Tuberculosis. PLoS Pathog. 2019, 15, e1008050. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mucker, E.M.; Chapman, J.L.; Babka, A.M.; Gordon, J.M.; Bryan, A.V.; Raymond, J.L.W.; Bell, T.M.; Facemire, P.R.; Goff, A.J.; et al. Retrospective Detection of Monkeypox Virus in the Testes of Nonhuman Primate Survivors. Nat. Microbiol. 2022, 7, 1980–1986. [Google Scholar] [CrossRef]
- Feldmann, H. Virus in Semen and the Risk of Sexual Transmission. N. Engl. J. Med. 2018, 378, 1440–1441. [Google Scholar] [CrossRef]
- Gimenes, F.; Medina, F.S.; de Abreu, A.L.P.; Irie, M.M.T.; Esquiçati, I.B.; Malagutti, N.; Vasconcellos, V.R.B.; Discacciati, M.G.; Bonini, M.G.; Maria-Engler, S.S.; et al. Sensitive Simultaneous Detection of Seven Sexually Transmitted Agents in Semen by Multiplex-PCR and of HPV by Single PCR. PLoS ONE 2014, 9, e98862. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Monkeypox and Sexually Transmitted Diseases. Adv. Exp. Med. Biol. 2022. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, B.; Montes-Madariaga, E.S.; Cabanillas-Ramirez, C.; Alva, N.; Ricardo-Martínez, A.; León-Figueroa, D.A.; Barboza, J.J.; Mohanty, A.; Padhi, B.K.; Sah, R. Epidemiologic Situation of HIV and Monkeypox Coinfection: A Systematic Review. Vaccines 2023, 11, 246. [Google Scholar] [CrossRef]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Mosquera-Rojas, M.D.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Clinical Features, Hospitalisation and Deaths Associated with Monkeypox: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 36. [Google Scholar] [CrossRef]
- Ghazanfar, A. Epidemiology, Clinical Features, Diagnosis and Management of Monkeypox Virus: A Clinical Review Article. Cureus 2022, 14, e28598. [Google Scholar] [CrossRef]
- Cassir, N.; Cardona, F.; Tissot-Dupont, H.; Bruel, C.; Doudier, B.; Lahouel, S.; Bendamardji, K.; Boschi, C.; Aherfi, S.; Edouard, S.; et al. Observational Cohort Study of Evolving Epidemiologic, Clinical, and Virologic Features of Monkeypox in Southern France. Emerg. Infect. Dis. 2022, 28, 2409–2415. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, B.; León-Figueroa, D.A.; Montes-Madariaga, E.S.; Ricardo-Martínez, A.; Alva, N.; Cabanillas-Ramirez, C.; Barboza, J.J.; Siddiq, A.; Coaguila Cusicanqui, L.A.; Bonilla-Aldana, D.K.; et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop. Med. Infect. Dis. 2022, 7, 369. [Google Scholar] [CrossRef]
- Desai, A.N.; Thompson, G.R., III; Neumeister, S.M.; Arutyunova, A.M.; Trigg, K.; Cohen, S.H. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA 2022, 328, 1348–1350. [Google Scholar] [CrossRef]
- Fabrizio, C.; Bruno, G.; Cristiano, L.; Buccoliero, G.B. Cidofovir for Treating Complicated Monkeypox in a Man with Acquired Immune Deficiency Syndrome. Infection 2022. [Google Scholar] [CrossRef]
- Sudarmaji, N.; Kifli, N.; Hermansyah, A.; Yeoh, S.F.; Goh, B.-H.; Ming, L.C. Prevention and Treatment of Monkeypox: A Systematic Review of Preclinical Studies. Viruses 2022, 14, 2496. [Google Scholar] [CrossRef]
- Poland, G.A.; Kennedy, R.B.; Tosh, P.K. Prevention of Monkeypox with Vaccines: A Rapid Review. Lancet Infect. Dis. 2022, 22, e349–e358. [Google Scholar] [CrossRef]
- Kuroda, N.; Shimizu, T.; Hirano, D.; Ishikane, M.; Kataoka, Y. Lack of Clinical Evidence of Antiviral Therapy for Human Monkeypox: A Scoping Review. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2023, 29, 228–231. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef]
- Raj, S.M. Efficacy of Three Key Antiviral Drugs Used to Treat Orthopoxvirus Infections: A Systematic Review. Glob. Biosecurity 2019, 1. [Google Scholar] [CrossRef]
- Webb, E.; Rigby, I.; Michelen, M.; Dagens, A.; Cheng, V.; Rojek, A.M.; Dahmash, D.; Khader, S.; Gedela, K.; Norton, A.; et al. Availability, Scope and Quality of Monkeypox Clinical Management Guidelines Globally: A Systematic Review. BMJ Glob. Health 2022, 7, e009838. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.; Reda, A.; Lashin, B.I.; Katamesh, B.E.; Brakat, A.M.; AL-Manaseer, B.M.; Kaur, S.; Asija, A.; Patel, N.K.; Basnyat, S.; et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and MRNA Vaccines? Vaccines 2022, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Mishra, S.; Rabaan, A.A.; Mohanty, A.; Padhi, B.K.; Sah, R. Monkeypox Breakthrough Infections and Side-Effects: Clarion Call for Nex-Gen Novel Vaccine. New Microbes New Infect. 2023, 52, 101084. [Google Scholar] [CrossRef] [PubMed]
- Berens-Riha, N.; De Block, T.; Rutgers, J.; Michiels, J.; Van Gestel, L.; Hens, M.; ITM Monkeypox Study Group; Kenyon, C.; Bottieau, E.; Soentjens, P.; et al. Severe Mpox (Formerly Monkeypox) Disease in Five Patients after Recent Vaccination with MVA-BN Vaccine, Belgium, July to October 2022. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 2200894. [Google Scholar] [CrossRef] [PubMed]
- Dukers-Muijrers, N.H.T.M.; Evers, Y.; Widdershoven, V.; Davidovich, U.; Adam, P.C.G.; Op de Coul, E.L.M.; Zantkuijl, P.; Matser, A.; Prins, M.; de Vries, H.J.C.; et al. Mpox Vaccination Willingness, Determinants, and Communication Needs in Gay, Bisexual, and Other Men Who Have Sex with Men, in the Context of Limited Vaccine Availability in the Netherlands (Dutch Mpox-Survey). Front. Public Health 2022, 10, 1058807. [Google Scholar] [CrossRef]
- Tehranchinia, Z.; Robati, R.M.; Moravvej, H.; Memariani, M.; Memariani, H. Monkeypox Disease with a Focus on the 2022 Outbreak; a Narrative Review. Arch. Acad. Emerg. Med. 2023, 11, e19. [Google Scholar] [CrossRef]
- Maldonado, M.G.S.; Rodríguez, A.J.L.; Pacheco, R.A.P.; Cevallos, L.C.M.; Saavedra, E.U.Z.; Zapata, L.R.P.; Huayta, F.A.L.; Prado, E.D.M. Epidemiological Characteristics and Clinical Features of Patients with Mpox Virus Infection from a Hospital in Peru between July and September 2022. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, in press. [Google Scholar] [CrossRef]
- Ayorinde, T.A.; Olufadewa, I.I.; Adesina, M.A.; Oladele, R.I.; Oladoye, M.J.; Adene, T.; Asaolu, O. The Reemergence of the Human Monkeypox: Strengthening Africa’s Epidemic Preparedness and Response System. Ann. Med. Surg. 2023, 85, 24–27. [Google Scholar] [CrossRef]
- Vera, M.N.; Sanca, K.; Leon, S.R. Comment on Sah et al. Monkeypox and Its Possible Sexual Transmission: Where Are We Now with Its Evidence? Pathogens 2022, 11, 924. Pathogens 2022, 11, 1417. [Google Scholar] [CrossRef]
- Reda, A.; Sah, R.; Rodríguez-Morales, A.J. More Evidence about Monkeypox Sexual Transmission in the Current 2022 Multi-Country Outbreak. Reply to Vera et al. Comment on “Sah et al. Monkeypox and Its Possible Sexual Transmission: Where Are We Now with Its Evidence? Pathogens 2022, 11, 924”. Pathogens 2022, 11, 1418. [Google Scholar] [CrossRef]
- Eid, R.E.; Allaw, F.; Haddad, S.F.; Kanj, S.S. Human Monkeypox: A Review of the Literature. PLoS Pathog. 2022, 18, e1010768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barboza, J.J.; León-Figueroa, D.A.; Saldaña-Cumpa, H.M.; Valladares-Garrido, M.J.; Moreno-Ramos, E.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Virus Identification for Monkeypox in Human Seminal Fluid Samples: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 173. https://doi.org/10.3390/tropicalmed8030173
Barboza JJ, León-Figueroa DA, Saldaña-Cumpa HM, Valladares-Garrido MJ, Moreno-Ramos E, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ. Virus Identification for Monkeypox in Human Seminal Fluid Samples: A Systematic Review. Tropical Medicine and Infectious Disease. 2023; 8(3):173. https://doi.org/10.3390/tropicalmed8030173
Chicago/Turabian StyleBarboza, Joshuan J., Darwin A. León-Figueroa, Hortencia M. Saldaña-Cumpa, Mario J. Valladares-Garrido, Emilly Moreno-Ramos, Ranjit Sah, D. Katterine Bonilla-Aldana, and Alfonso J. Rodriguez-Morales. 2023. "Virus Identification for Monkeypox in Human Seminal Fluid Samples: A Systematic Review" Tropical Medicine and Infectious Disease 8, no. 3: 173. https://doi.org/10.3390/tropicalmed8030173
APA StyleBarboza, J. J., León-Figueroa, D. A., Saldaña-Cumpa, H. M., Valladares-Garrido, M. J., Moreno-Ramos, E., Sah, R., Bonilla-Aldana, D. K., & Rodriguez-Morales, A. J. (2023). Virus Identification for Monkeypox in Human Seminal Fluid Samples: A Systematic Review. Tropical Medicine and Infectious Disease, 8(3), 173. https://doi.org/10.3390/tropicalmed8030173









