Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum
Abstract
1. Introduction
2. Materials and Methods
2.1. Guanidine Compound Preparation
2.2. Reference Anti-L. infantum Drug
2.3. L. infantum Culture Conditions
2.4. Anti-L. infantum Activity
2.5. Red Blood Cell Lysis Assay
2.6. In Vitro Cytotoxicity in Peripheral Blood Mononuclear Cells
2.7. Apoptosis/Necrosis Profiling with Annexin V/PI Staining in Promastigote and Amastigote-Like Forms
2.8. Nitrite Assay
2.9. Data Analysis and Statistics
2.10. Ethics Statement
3. Results
3.1. In Vitro Antileishmanial Activity and Selectivity Index Calculation
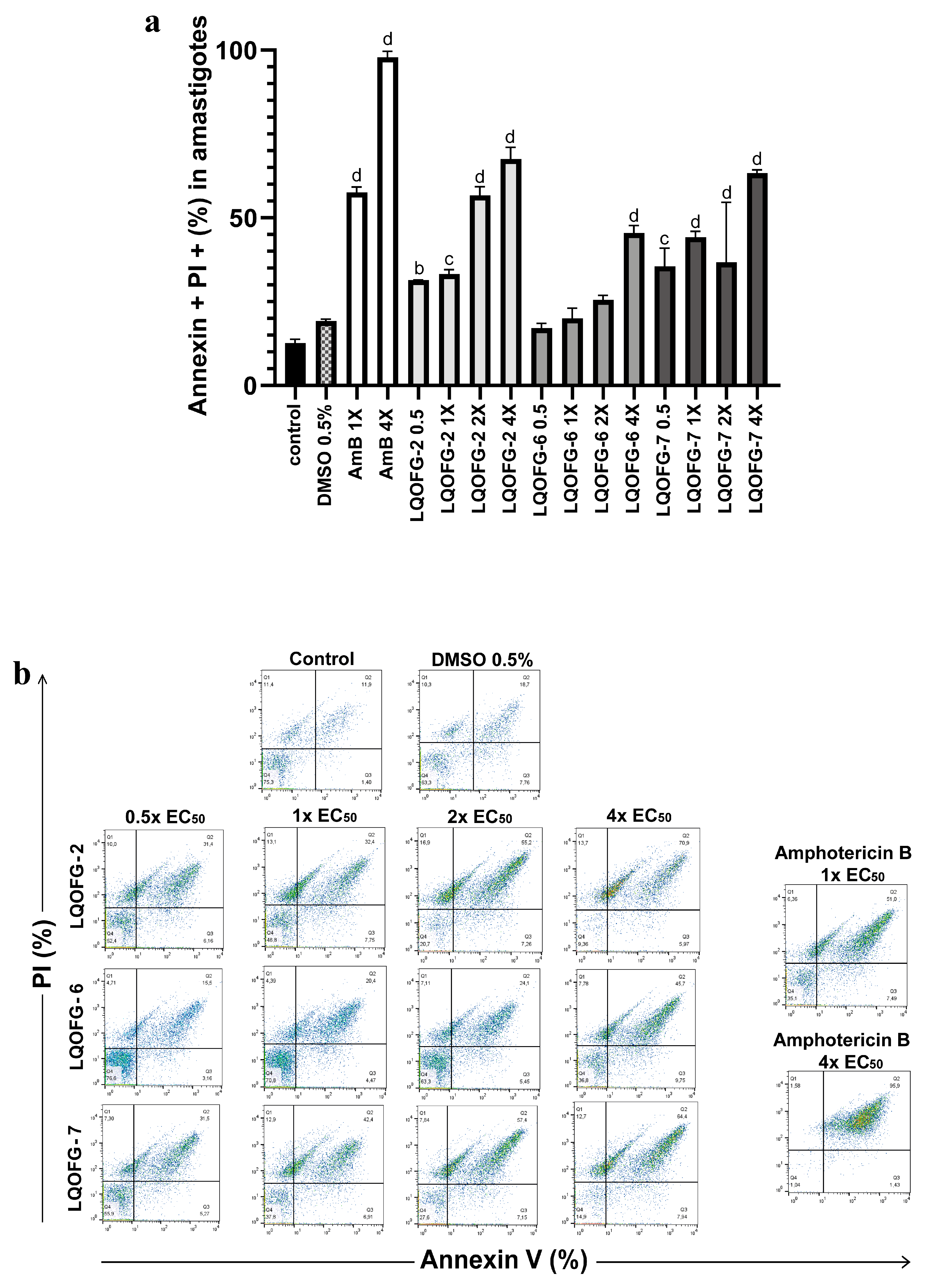
3.2. Apoptosis/Necrosis Profiling in Promastigote and Amastigote-Like Forms
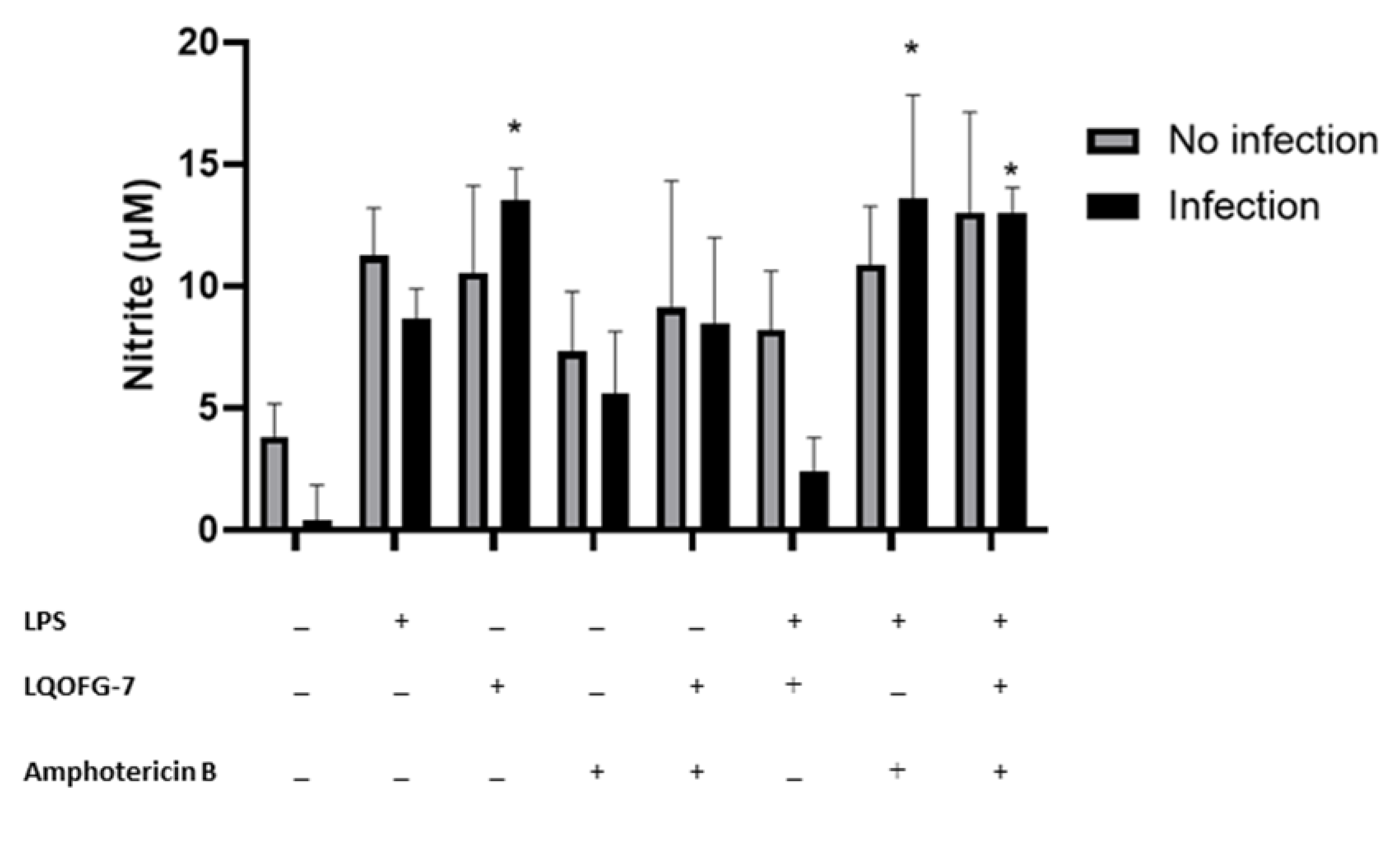
3.3. Nitrite Levels from PBMC after L. infantum Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pace, D. Leishmaniasis. J. Infect. 2014, 69, S10–S18. [Google Scholar] [CrossRef]
- Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 15 April 2022).
- van Griensven, J.; Diro, E. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- van Griensven, J.; Diro, E. Visceral Leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan Persister-like Cells and Drug Treatment Failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, K. Molecular Targets and Pathways for the Treatment of Visceral Leishmaniasis. Drug Discov. Today 2018, 23, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Semenya, D.; Castagnolo, D. Antimicrobial Drugs Bearing Guanidine Moieties: A Review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Baugh, S.D.P.; Chaly, A.; Weaver, D.G.; Pelletier, J.C.; Thanna, S.; Freeman, K.B.; Reitz, A.B.; Scott, R.W. Highly Potent, Broadly Active Antifungal Agents for the Treatment of Invasive Fungal Infections. Bioorg. Med. Chem. Lett. 2021, 33, 127727. [Google Scholar] [CrossRef] [PubMed]
- Ngernna, S.; Chim-Ong, A.; Roobsoong, W.; Sattabongkot, J.; Cui, L.; Nguitragool, W. Efficient Synchronization of Plasmodium Knowlesi in Vitro Cultures Using Guanidine Hydrochloride. Malar. J. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- do Espírito Santo, R.D.; Velásquez, Á.M.A.; Passianoto, L.V.G.; Sepulveda, A.A.L.; da Costa Clementino, L.; Assis, R.P.; Baviera, A.M.; Kalaba, P.; dos Santos, F.N.; Éberlin, M.N.; et al. N, N′ N″-Trisubstituted Guanidines: Synthesis, Characterization and Evaluation of Their Leishmanicidal Activity. Eur. J. Med. Chem. 2019, 171, 116–128. [Google Scholar] [CrossRef]
- Reid, C.M.; Ebikeme, C.; Barrett, M.P.; Patzewitz, E.M.; Müller, S.; Robins, D.J.; Sutherland, A. Synthesis of Novel Benzamidine- and Guanidine-Derived Polyazamacrocycles: Selective Anti-Protozoal Activity for Human African Trypanosomiasis. Bioorg. Med. Chem. Lett. 2008, 18, 5399–5401. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Bertonha, A.F.; Takaki, M.; Rodriguez, J.P.G. The Chemistry and Biology of Guanidine Natural Products. Nat. Prod. Rep. 2017, 34, 1264–1301. [Google Scholar] [CrossRef]
- Pasero, C.; D’Agostino, I.; de Luca, F.; Zamperini, C.; Deodato, D.; Truglio, G.I.; Sannio, F.; del Prete, R.; Ferraro, T.; Visaggio, D.; et al. Alkyl-Guanidine Compounds as Potent Broad-Spectrum Antibacterial Agents: Chemical Library Extension and Biological Characterization. J. Med. Chem. 2018, 61, 9162–9176. [Google Scholar] [CrossRef]
- Dantas, N.; de Aquino, T.M.; de Araújo-Júnior, J.X.; da Silva-Júnior, E.; Gomes, E.A.; Gomes, A.A.S.; Siqueira-Júnior, J.P.; Mendonça Junior, F.J.B. Aminoguanidine Hydrazones (AGH’s) as Modulators of Norfloxacin Resistance in Staphylococcus Aureus That Overexpress NorA Efflux Pump. Chem. Biol. Interact. 2018, 280, 8–14. [Google Scholar] [CrossRef]
- Manetti, F.; Castagnolo, D.; Raffi, F.; Zizzari, A.T.; Rajamaki, S.; D’Arezzo, S.; Visca, P.; Cona, A.; Fracasso, M.E.; Doria, D.; et al. Synthesis of New Linear Guanidines and Macrocyclic Amidinourea Derivatives Endowed with High Antifungal Activity against Candida Spp. and Aspergillus Spp. J. Med. Chem. 2009, 52, 7376–7379. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Li, Q.; Kozar, M.P.; Melendez, V.; O Neil, M.T.; Lin, A.J. Synthesis and Antimalarial Activity of 2-Guanidino-4-Oxoimidazoline Derivatives. J. Med. Chem. 2011, 54, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Mesquita, J.T.; Pinto, E.G.; Costa-Silva, T.A.; Borborema, S.E.T.; Galisteo Junior, A.J.; Neves, B.J.; Andrade, C.H.; Shuhaib, Z.; Bennett, E.L.; et al. Analogues of Marine Guanidine Alkaloids Are in Vitro Effective against Trypanosoma Cruzi and Selectively Eliminate Leishmania (L.) Infantum Intracellular Amastigotes. J. Nat. Prod. 2016, 79, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, S.; Späth, G.F.; Rachidi, N.; Prina, E. The Enemy within: Targeting Host–Parasite Interaction for Antileishmanial Drug Discovery. PLoS Negl. Trop. Dis. 2017, 11, e0005480. [Google Scholar] [CrossRef] [PubMed]
- Debrabant, A.; Joshi, M.B.; Pimenta, P.F.P.; Dwyer, D.M. Generation of Leishmania Donovani Axenic Amastigotes: Their Growth and Biological Characteristics. Int. J. Parasitol. 2004, 34, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.A.D.F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; Carvalho, F.A.D.A. Syzygium cumini (L.) Skeels Essential Oil and Its Major Constituent α-Pinene Exhibit Anti-Leishmania Activity through Immunomodulation in Vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Verma, A.K.; Mishra, P.R.; Jain, N.K. Surface-Engineered Dendrimeric Nanoconjugates for Macrophage-Targeted Delivery of Amphotericin B: Formulation Development and in Vitro and in Vivo Evaluation. Antimicrob. Agents Chemother. 2015, 59, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Vieira, É.L.M.; Keesen, T.S.L.; Machado, P.R.; Guimarães, L.H.; Carvalho, E.M.; Dutra, W.O.; Gollob, K.J. Immunoregulatory Profile of Monocytes from Cutaneous Leishmaniasis Patients and Association with Lesion Size. Parasite Immunol. 2013, 35, 65. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.G.; Magalhães, L.M.D.; Giunchetti, R.C.; Dutra, W.O.; Gollob, K.J. Infection of Human Monocytes with Leishmania Infantum Strains Induces a Downmodulated Response When Compared with Infection with Leishmania braziliensis. Front. Immunol. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.D.; Viana, A.; Chiari, E.; Galvão, L.M.C.; Gollob, K.J.; Dutra, W.O. Differential Activation of Human Monocytes and Lymphocytes by Distinct Strains of Trypanosoma Cruzi. PLoS Negl. Trop. Dis. 2015, 9, e0003816. [Google Scholar] [CrossRef]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, Peripheral Blood Mononuclear Cells, and THP-1 Cells Exhibit Different Cytokine Expression Patterns Following Stimulation with Lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef]
- Lapara, N.J.; Kelly, B.L. Suppression of LPS-Induced Inflammatory Responses in Macrophages Infected with Leishmania. J. Inflamm. 2010, 7, 8. [Google Scholar] [CrossRef]
- Bartosh, T.; Ylostalo, J. Macrophage Inflammatory Assay. Bio-Protoc. 2014, 4, e1108. [Google Scholar] [CrossRef]
- Reza, S.; Hasan, N.A.; Maryam, N.F.; Fahimeh, B.J.; Ghahremani, A.; GholamReza, H.; Amin, G.M. Cytokine Profile and Nitric Oxide Levels in Macrophages Exposed to Leishmania Infantum FML. Exp. Parasitol. 2019, 203, 1–7. [Google Scholar] [CrossRef]
- Brajtburg, J.; Elberg, S.; Schwartz, D.R.; Vertut-Croquin, A.; Schlessinger, D.; Kobayashi, G.S.; Medoff, G. Involvement of Oxidative Damage in Erythrocyte Lysis Induced by Amphotericin B. Antimicrob. Agents Chemother. 1985, 27, 172. [Google Scholar] [CrossRef]
- da Câmara Rocha, J.; da Franca Rodrigues, K.A.; do Nascimento Néris, P.L.; da Silva, L.V.; Almeida, F.S.; Lima, V.S.; Peixoto, R.F.; da Câmara Rocha, J.; de Azevedo, F.d.L.A.A.; Veras, R.C.; et al. Biological Activity of Morita-Baylis-Hillman Adduct Homodimers in L. Infantum and L. Amazonensis: Anti-Leishmania Activity and Cytotoxicity. Parasitol. Res. 2019, 118, 3067–3076. [Google Scholar] [CrossRef]
- Karampetsou, K.; Koutsoni, O.S.; Gogou, G.; Angelis, A.; Skaltsounis, L.A.; Dotsika, E. Total Phenolic Fraction (TPF) from Extra Virgin Olive Oil: Induction of Apoptotic-like Cell Death in Leishmania Spp. Promastigotes and in Vivo Potential of Therapeutic Immunomodulation. PLoS Negl. Trop. Dis. 2021, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.N. Interdependence between Physical Parameters and Selection of Substituent Groups for Correlation Studies. J. Med. Chem. 1971, 14, 680–684. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Rodrigues, V.; Pemberton, S.; Laforge, M.; Fortier, Y.; Cordeiro-da-Silva, A.; MacDougall, J.; Estaquier, J. Antileishmanial Drugs Modulate IL-12 Expression and Inflammasome Activation in Primary Human Cells. J. Immunol. 2020, 204, 1869–1880. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.S. Cell Death Pathways in Pathogenic Trypanosomatids: Lessons of (over)Kill. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, A.; Alzate, J.F.; MacLeod, E.T.; Lüder, C.G.K.; Fasel, N.; Hurd, H. Apoptotic Markers in Protozoan Parasites. Parasit Vectors 2010, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, J.L.M.; Moreira, M.E.C.; Benjamin, A.; Bonomo, A.C.; Barcinski, M.A. Mimicry of Apoptotic Cells by Exposing Phosphatidylserine Participates in the Establishment of Amastigotes of Leishmania (L) amazonensis in Mammalian Hosts. J. Immunol. 2006, 176, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- El-Hani, C.N.; Borges, V.M.; Wanderley, J.L.M.; Barcinski, M.A. Apoptosis and Apoptotic Mimicry in Leishmania: An Evolutionary Perspective. Front. Cell Infect. Microbiol. 2012, 2, 96. [Google Scholar] [CrossRef]
- Scariot, D.B.; Volpato, H.; de Souza Fernandes, N.; Soares, E.F.P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Din, Z.U.; Rodrigues-Filho, E.; Rubira, A.F.; Borges, O.; et al. Activity and Cell-Death Pathway in Leishmania Infantum Induced by Sugiol: Vectorization Using Yeast Cell Wall Particles Obtained from Saccharomyces Cerevisiae. Front. Cell. Infect. Microbiol. 2019, 9, 208. [Google Scholar] [CrossRef]
- Shadab, M.; Jha, B.; Asad, M.; Deepthi, M.; Kamran, M.; Ali, N. Apoptosis-like Cell Death in Leishmania Donovani Treated with KalsomeTM10, a New Liposomal Amphotericin B. PLoS ONE 2017, 12, e0171306. [Google Scholar] [CrossRef]
- Mendonça, D.B.D.; Silva, R.E.C.; Palace-Berl, F.; Takakura, C.F.H.; Soares, S.R.C.; Braz, L.M.A.; Tavares, L.C.; Lindoso, J.A.L. Nitro-Heterocyclic Compounds Induce Apoptosis-like Effects in Leishmania (L). Amazonensis Promastigotes. J Venom Anim Toxins Incl. Trop. Dis. 2019, 25, e144418. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Casanova, M. Cell Death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef]
- Brajtburg, J.; Bolard, J. Carrier Effects on Biological Activity of Amphotericin B. Clin. Microbiol. Rev. 1996, 9, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Ibiza, S.; Serrador, J.M. The Role of Nitric Oxide in the Regulation of Adaptive Immune Responses. Inmunología 2008, 27, 103–117. [Google Scholar] [CrossRef]
- Thwe, P.M.; Amiel, E. The Role of Nitric Oxide in Metabolic Regulation of Dendritic Cell Immune Function. Cancer Lett. 2018, 412, 236–242. [Google Scholar] [CrossRef]
- Kupani, M.; Sharma, S.; Pandey, R.K.; Kumar, R.; Sundar, S.; Mehrotra, S. IL-10 and TGF-β Induced Arginase Expression Contributes to Deficient Nitric Oxide Response in Human Visceral Leishmaniasis. Front. Cell Infect. Microbiol. 2020, 10, 614165. [Google Scholar] [CrossRef]
- Akbari, M.; Oryan, A.; Hatam, G. Immunotherapy in Treatment of Leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Formaglio, P.; Alabdullah, M.; Siokis, A.; Handschuh, J.; Sauerland, I.; Fu, Y.; Krone, A.; Gintschel, P.; Stettin, J.; Heyde, S.; et al. Nitric Oxide Controls Proliferation of Leishmania Major by Inhibiting the Recruitment of Permissive Host Cells. Immunity 2021, 54, 2724–2739.e10. [Google Scholar] [CrossRef]
- Mansuy, D.; Boucher, J.L. Alternative Nitric Oxide-Producing Substrates for No Synthases. Free Radic. Biol. Med. 2004, 37, 1105–1121. [Google Scholar] [CrossRef]
- Pandya, S.; Verma, R.K.; Khare, P.; Tiwari, B.; Srinivasarao, D.A.; Dube, A.; Goyal, N.; Misra, A. Supplementation of Host Response by Targeting Nitric Oxide to the Macrophage Cytosol Is Efficacious in the Hamster Model of Visceral Leishmaniasis and Adds to Efficacy of Amphotericin B. Int. J. Parasitol. Drugs Drug. Resist. 2016, 6, 125–132. [Google Scholar] [CrossRef]
- Gatto, M.; de Abreu, M.M.; Tasca, K.I.; de Golim, M.A.; da Silva, L.D.M.; Simão, J.C.; Fortaleza, C.M.C.B.; de Soares, É.M.V.C.; Calvi, S.A. The Involvement of TLR2 and TLR4 in Cytokine and Nitric Oxide Production in Visceral Leishmaniasis Patients before and after Treatment with Anti-Leishmanial Drugs. PLoS ONE 2015, 10, e0117977. [Google Scholar] [CrossRef]
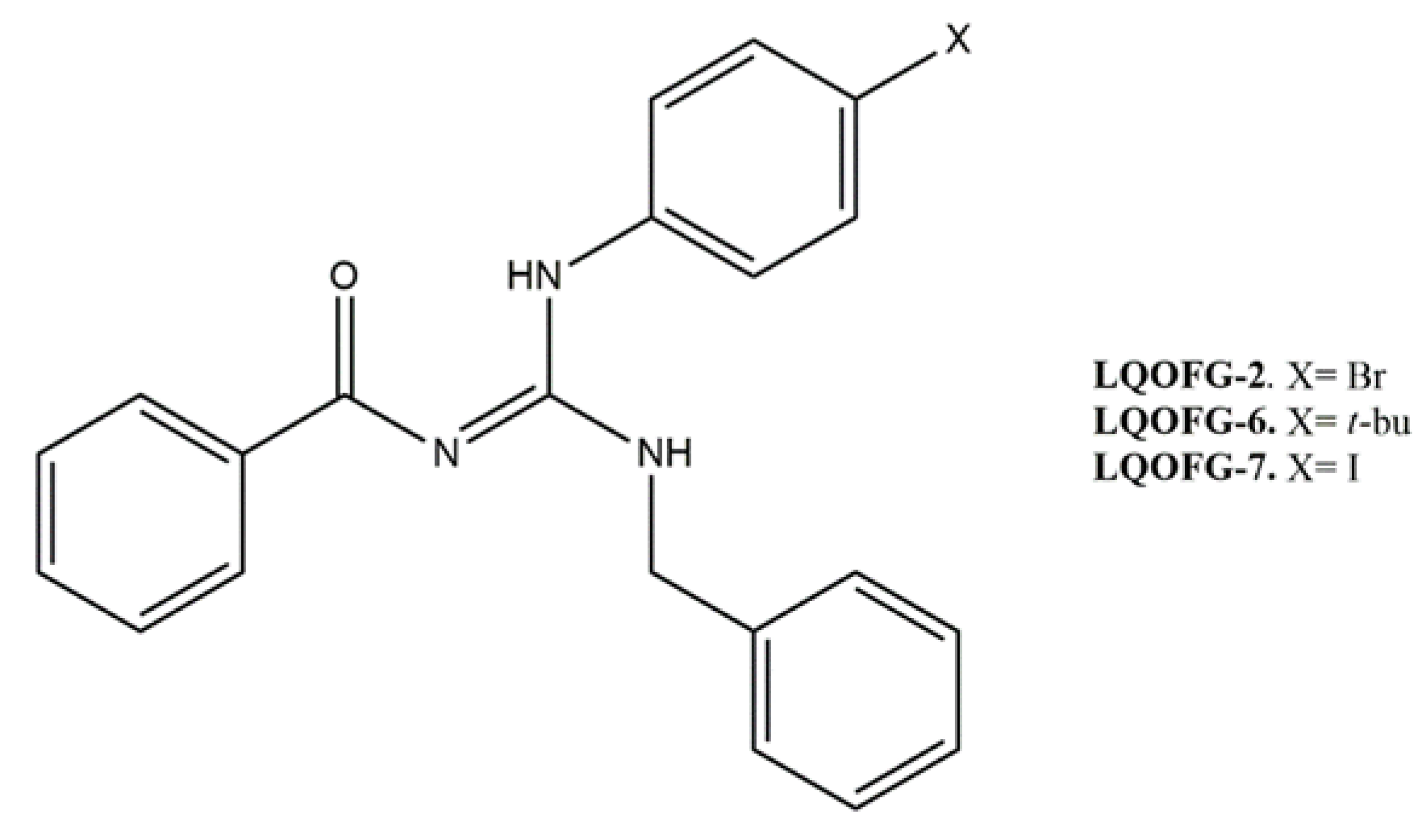
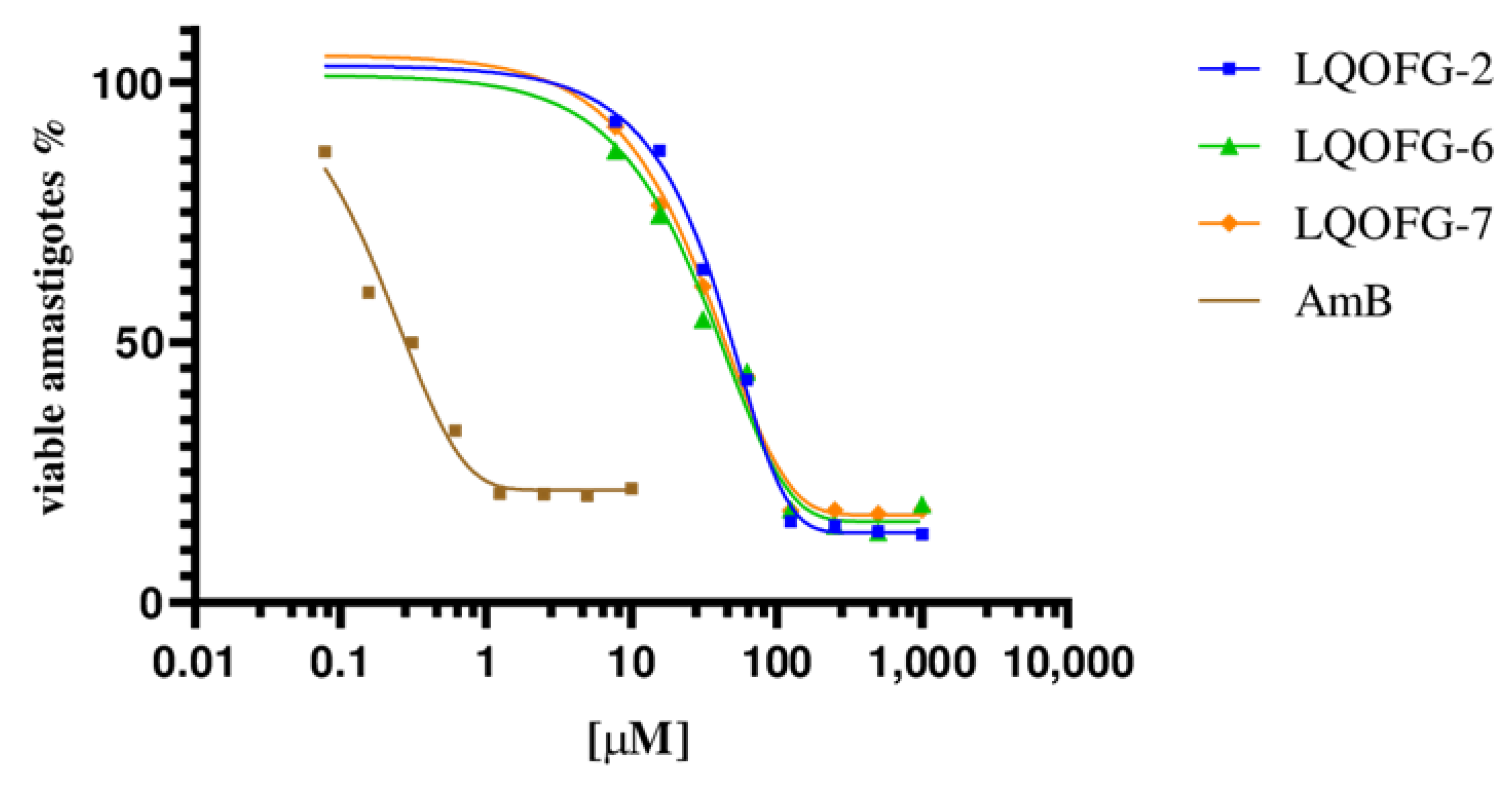
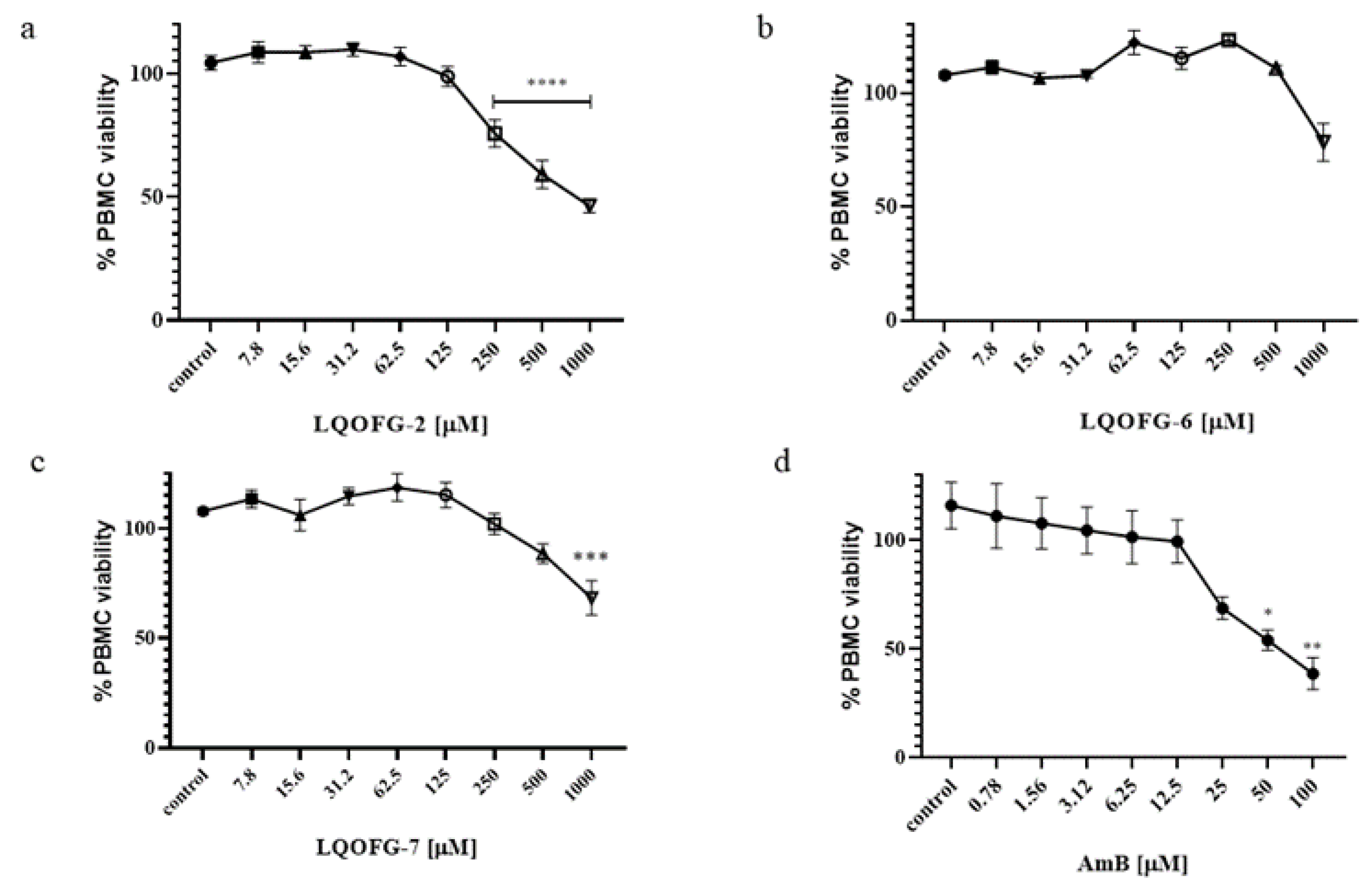
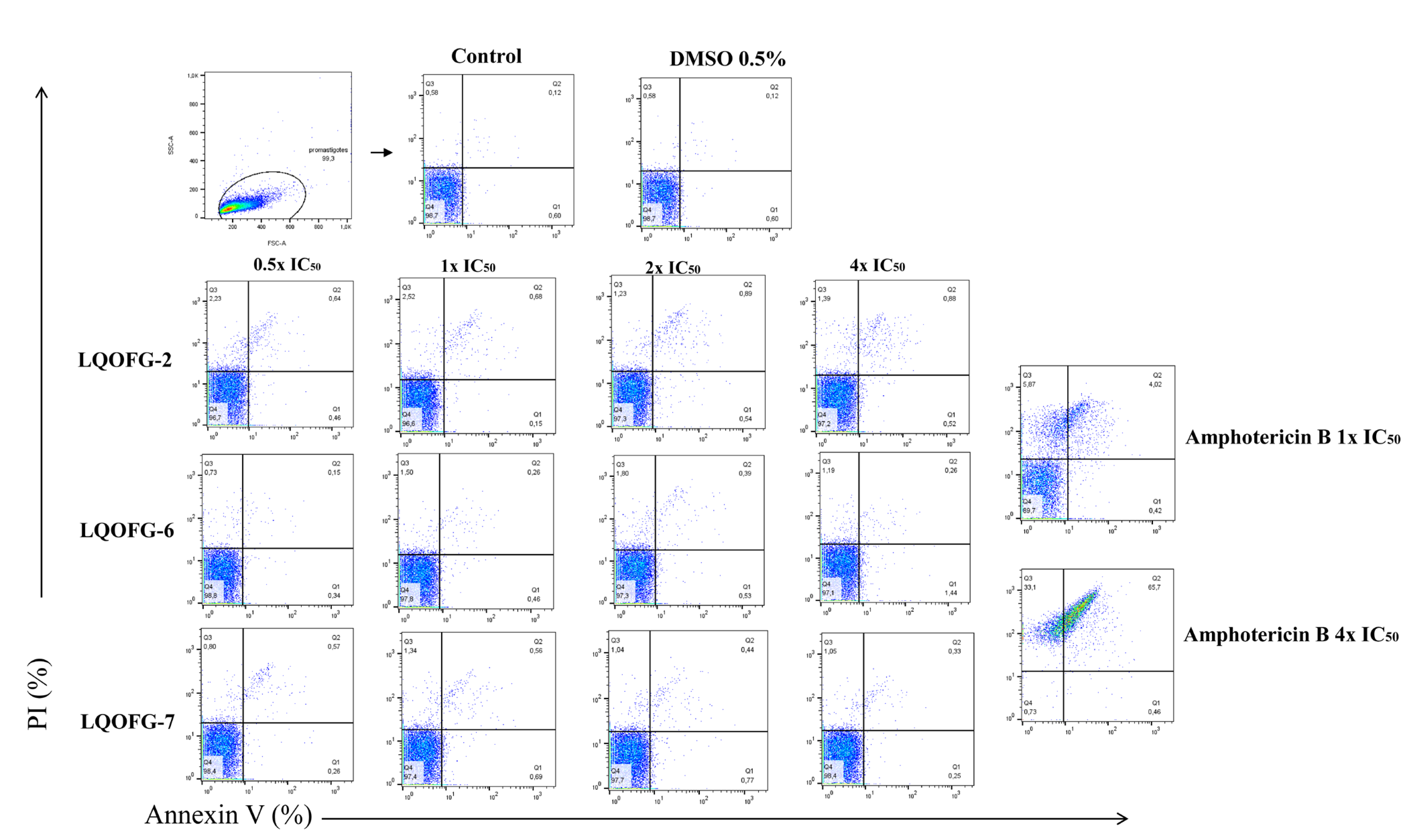
| Compounds | IC50 (µM) | HC50 (µM) | SI |
|---|---|---|---|
| LQOFG-2 | 12.7 ± 0.25 | >1000 | >78.2 |
| LQOFG-6 | 24.4 ± 0.76 | >1000 | >40.98 |
| LQOFG-7 | 23.6 ± 0.64 | >1000 | >42.37 |
| Amphotericin B | 1.5 ± 0.23 | 7.10 ± 2.09 | 4.73 |
| Compounds | EC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|
| LQOFG-2 | 26.1± 1.22 | 313.1 ± 3.23 | 12.00 |
| LQOFG-6 | 21.1 ± 1.13 | >1000 | >47.4 |
| LQOFG-7 | 18.6 ± 1.23 | 745.5 ± 3.58 | 39.54 |
| Amphotericin B | 0.30 ± 1.29 | 53.8 ± 3.59 | 177.6 |
| % Cells (Amastigote) | |||
|---|---|---|---|
| Early Apoptosis (AV+, PI−) | Late Apoptosis (AV+, PI+) | Necrosis (AV−, PI+) | |
| Cells | 1.99 ± 0.29 | 12.63 ± 0.68 | 15.57 ± 0.33 |
| DMSO 0.5% | 3.99 ± 0.48 | 19.20 ± 0.55 | 13.00 ± 2.26 |
| LQOFG-2 0.5 × EC50 | 5.68 ± 0.29 a | 31.43 ± 0.03 b | 13.20 ± 1.62 |
| LQOFG-2 1 × EC50 | 8.13 ± 0.84 d | 33.23 ± 0.73 c | 11.97 ± 0.66 |
| LQOFG-2 2 × EC50 | 7.68 ± 0.39 d | 56.27 ± 1.51 d | 15.93 ± 1.02 |
| LQOFG-2 4 × EC50 | 7.55 ± 1.18 d | 67.50 ± 2.05 d | 13.80 ± 1.47 |
| LQOFG-6 0.5 × EC50 | 3.62 ± 0.39 | 17.10 ± 0.80 | 13.20 ± 1.62 |
| LQOFG-6 1 × EC50 | 4.05 ± 0.22 | 20.00 ± 1.74 | 6.57 ± 1.20 |
| LQOFG-6 2 × EC50 | 5.72 ± 0.20 a | 25.50 ± 0.80 | 7.15 ± 0.21 |
| LQOFG-6 4 × EC50 | 9.06 ± 0.70 d | 45.47 ± 1.30 d | 8.14 ± 0.78 |
| LQOFG-7 0.5 × EC50 | 5.56 ± 0.46 a | 35.53 ± 3.13 c | 9.83 ± 1.27 |
| LQOFG-7 1 × EC50 | 5.84 ± 0.57 b | 44.20 ± 1.01 d | 13.53 ± 0.32 |
| LQOFG-7 2 × EC50 | 10.02 ± 1.53 d | 36.70 ± 10.35 d | 5.63 ± 1.24 |
| LQOFG-7 4 × EC50 | 8.01 ± 0.14 d | 63.27 ± 0.59 d | 12.27 ± 0.48 |
| Amphotericin B 1 × EC50 | 4.73 ± 0.97 | 57.50 ± 1.72 d | 11.52 ± 1.89 |
| Amphotericin B 4 × EC50 | 0.60 ± 0.42 | 97.87 ± 1.01 d | 0.96 ± 0.33 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, F.S.; Moreira, V.P.; Silva, E.d.S.; Cardoso, L.L.; de Sousa Palmeira, P.H.; Cavalcante-Silva, L.H.A.; Araújo, D.A.M.d.; Amaral, I.P.G.d.; González, E.R.P.; Keesen, T.S.L. Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Trop. Med. Infect. Dis. 2023, 8, 141. https://doi.org/10.3390/tropicalmed8030141
Almeida FS, Moreira VP, Silva EdS, Cardoso LL, de Sousa Palmeira PH, Cavalcante-Silva LHA, Araújo DAMd, Amaral IPGd, González ERP, Keesen TSL. Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Tropical Medicine and Infectious Disease. 2023; 8(3):141. https://doi.org/10.3390/tropicalmed8030141
Chicago/Turabian StyleAlmeida, Fernanda Silva, Vitor Partite Moreira, Edson dos Santos Silva, Leonardo Lima Cardoso, Pedro Henrique de Sousa Palmeira, Luiz Henrique Agra Cavalcante-Silva, Demétrius A. M. de Araújo, Ian P. G. do Amaral, Eduardo René Pérez González, and Tatjana S. L. Keesen. 2023. "Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum" Tropical Medicine and Infectious Disease 8, no. 3: 141. https://doi.org/10.3390/tropicalmed8030141
APA StyleAlmeida, F. S., Moreira, V. P., Silva, E. d. S., Cardoso, L. L., de Sousa Palmeira, P. H., Cavalcante-Silva, L. H. A., Araújo, D. A. M. d., Amaral, I. P. G. d., González, E. R. P., & Keesen, T. S. L. (2023). Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Tropical Medicine and Infectious Disease, 8(3), 141. https://doi.org/10.3390/tropicalmed8030141







