Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths
Abstract
1. Introduction
2. Materials and Methods
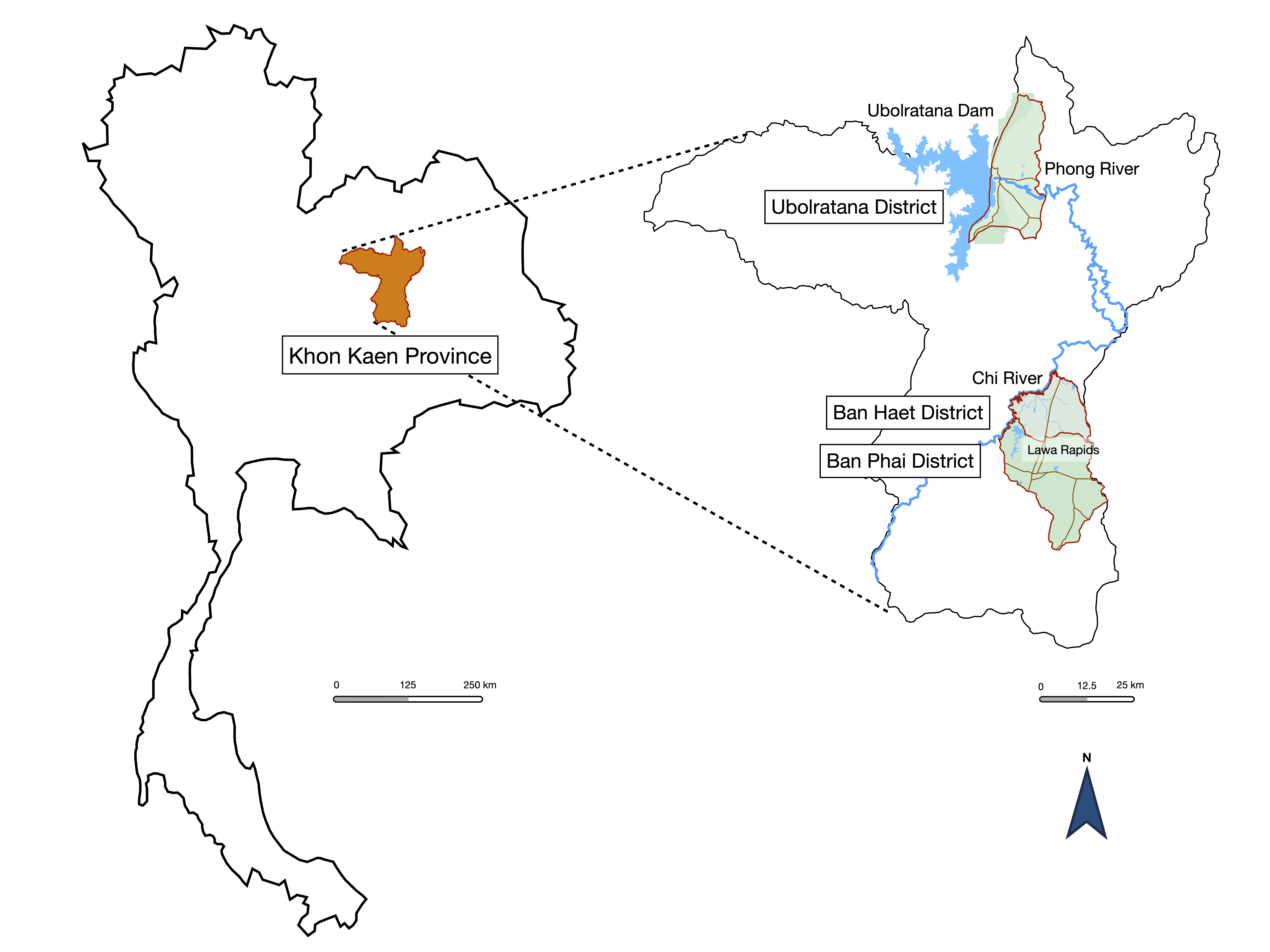
2.1. Study Design and Setting
2.2. Study Population
2.3. Sample Size Estimation
2.4. Survey of Parasitic Infection and Data Collection
2.5. The Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
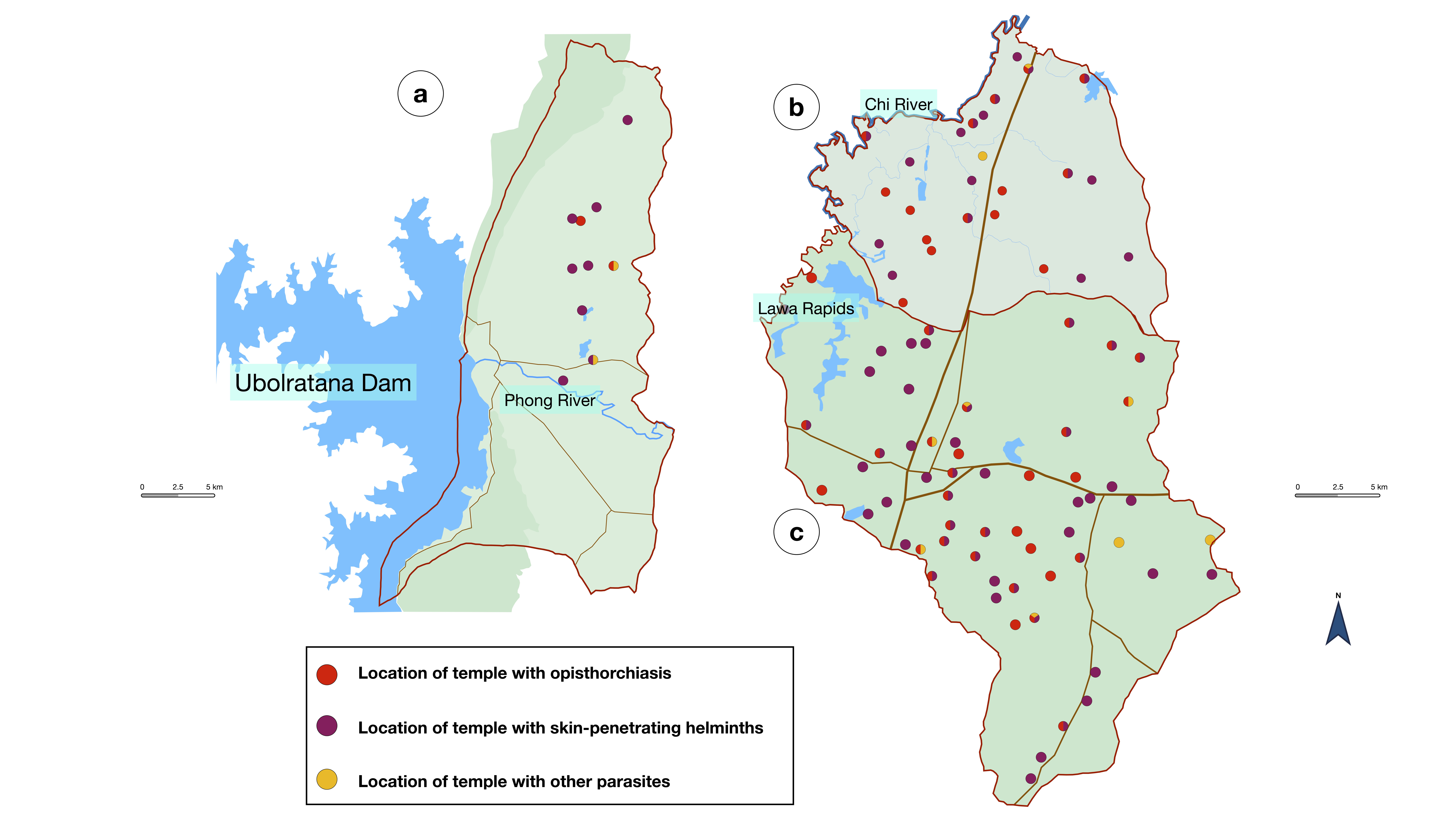
3.2. Prevalence of Parasitic Infection in Monks
3.3. Pre-Monkhood Raw Fish Ingestion and Offering Raw Fish Dishes to Monks Associated with Liver Fluke Infection
3.4. The Forbidden Footwear Rule Assigns a Risk for Skin-Penetrating Helminth Infection to Monks
3.5. Defecating on Ground Soil While Performing Off-Site Work Spread Liver Fluke and Skin-Penetrating Helminths
3.6. Univariate Regression for Opisthorchiasis and Skin-Penetrating Helminth
3.6.1. Opisthorchiasis
3.6.2. Skin-Penetrating Helminth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahamakut Buddhist University. The Three Basket and Commentary Translation-Basket of the Discipline, Volume 5; Mahamakut buddhist University Printing House: Bangkok, Thailand, 1991; pp. 13–22, 65, 97–101, 130–135, 167. [Google Scholar]
- Mahamakut Buddhist University. The Three Basket and Commentary Translation-Basket of the Discipline, Volume 2; Mahamakut buddhist University Printing House: Bangkok, Thailand, 1991; pp. 630–636, 685–687. [Google Scholar]
- Mahamakut Buddhist University. The Three Basket and Commentary Translation-Basket of the Discipline, Volume 7; Mahamakut buddhist University Printing house: Bangkok, Thailand, 1991; pp. 27–28, 451, 517. [Google Scholar]
- Amoah, I.D.; Adegoke, A.A.; Stenström, T.A. Soil-transmitted helminth infections associated with wastewater and sludge reuse: A review of current evidence. Trop. Med. Int. Health 2018, 23, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Echazú, A.; Bonanno, D.; Juarez, M.; Cajal, S.P.; Heredia, V.; Caropresi, S.; Cimino, R.O.; Caro, N.; Vargas, P.A.; Paredes, G.; et al. Effect of Poor Access to Water and Sanitation as Risk Factors for Soil-Transmitted Helminth Infection: Selectiveness by the Infective Route. PLoS Negl. Trop. Dis. 2015, 9, e0004111. [Google Scholar] [CrossRef] [PubMed]
- Makata, K.; Ensink, J.; Ayieko, P.; Hansen, C.; Sichalwe, S.; Mngara, J.; Mcharo, O.; Mazigo, H.; Seni, J.; Dreibelbis, R.; et al. Hand hygiene intervention to optimise soil-transmitted helminth infection control among primary school children: The Mikono Safi cluster randomised controlled trial in northwestern Tanzania. BMC Med. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-155011-6.
- Wongrach, K. The virtue of offering shoes to monks. AJPU 2018, 9, 251–262. [Google Scholar]
- Aksorn, N.; Subrungruang, I.; Charoensuk, L.; Suwannahitatorn, P.; Sinsophonphap, T.; Poonyakariyakorn, S.; Karndee, T.; Arunotong, P.; Juicharoen, P.; Prasitnok, W. The Prevalence of parasitic infection and Health risk behaviors of Thai Buddhist monks in Wachira Phayaban Region, Dusit District, Bangkok Metropolitan. Vajira Med. J. 2020, 64, 145–158. [Google Scholar]
- Jariya, P.; Tesjaroen, S.; Lertlaituan, P.; Bedavanij, A. Intestinal Parasitosis in the Monks and Novices at Kanmphaeng Saen District, Nakhon Pathom Province. 1989. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/sea-138270?lang=en (accessed on 10 January 2023).
- Inpankaew, T.; Traub, R.; Thompson, R.C.A.; Sukthana, Y. Canine parasitic zoonoses in Bangkok temples. Southeast Asian J. Trop. Med. Public Health 2007, 38, 247–255. [Google Scholar]
- Sriamporn, S.; Pisani, P.; Pipitgool, V.; Suwanrungruang, K.; Kamsa-Ard, S.; Parkin, D.M. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop. Med. Int. Health 2004, 9, 588–594. [Google Scholar] [CrossRef]
- Cholangiocarcinoma Foundation of Thailand. Isan Cohort. Available online: https://cloud.cascap.in.th/rp/ov/sum?s=t&year=&p=40&a=&sort=-amphur (accessed on 10 January 2023).
- Pumhirunroj, B.; Aukkanimart, R.; Boonmars, T.; Sriraj, P.; Boueroy, P.; Artchayasawat, A.; Songsri, J.; Sripan, P.; Chomphumee, K.; Ratanasuwan, P. Liver fluke-infected cyprinoid fish in northeastern thailand (2016–2017). Southeast Asian J. Trop. Med. Public Health 2020, 51, 1–7. [Google Scholar]
- Wayne, W.D. Biostatistics: A Foundation for Analysis in The Health Science, 6th ed.; John Wiley & Sons Inc.: New York, NY, USA, 1995; pp. 180–181. [Google Scholar]
- Boonjaraspinyo, S.; Boonmars, T.; Ekobol, N.; Artchayasawat, A.; Sriraj, P.; Aukkanimart, R.; Pumhirunroj, B.; Sripan, P.; Songsri, J.; Juasook, A.; et al. Prevalence and Associated Risk Factors of Intestinal Parasitic Infections: A Population-Based Study in Phra Lap Sub-District, Mueang Khon Kaen District, Khon Kaen Province, Northeastern Thailand. Trop. Med. Infect. Dis. 2023, 8, 22. [Google Scholar] [CrossRef]
- Sengthong, C.; Yingklang, M.; Intuyod, K.; Hongsrichan, N.; Pinlaor, S. An optimized agar plate culture improves diagnostic efficiency for Strongyloides stercoralis infection in an endemic community. Parasitol. Res. 2020, 119, 1409–1413. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Grundy-Warr, C.; Namsanor, J.; Kenney-Lazar, M.; Tang, C.J.Y.; Goh, L.Y.W.; Chong, Y.C.; Sithithaworn, P.; Ngonkum, S.; Khuntikeo, N. Masculinity and misinformation: Social dynamics of liver fluke infection risk in Thailand. Parasitol. Int. 2021, 84, 102382. [Google Scholar] [CrossRef]
- Thinkhamrop, K.; Khuntikeo, N.; Laohasiriwong, W.; Chupanit, P.; Kelly, M.; Suwannatrai, A.T. Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0009741. [Google Scholar] [CrossRef]
- Khuntikeo, N.; on behalf of the CASCAP Investigators; Chamadol, N.; Yongvanit, P.; Loilome, W.; Namwat, N.; Sithithaworn, P.; Andrews, R.H.; Petney, T.N.; Promthet, S.; et al. Cohort profile: Cholangiocarcinoma screening and care program (CASCAP). BMC Cancer 2015, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Diemert, D.J. 119—Cestode and Trematode Infections. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1032–1037.e1. [Google Scholar]
- Bunmee, T.; Chaiwang, N.; Kaewkot, C.; Jaturasitha, S. Current situation and future prospects for beef production in Thailand—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, R.M.; Thomas, L.; Gabriël, S.; Bobić, B.; Devleesschauwer, B.; Robertson, L.J.; Saratsis, A.; Torgerson, P.R.; Braae, U.C.; Dermauw, V.; et al. Epidemiology of Taenia saginata taeniosis/cysticercosis: A systematic review of the distribution in East, Southeast and South Asia. Parasites Vectors 2020, 13, 234. [Google Scholar] [CrossRef]
- Saenna, P.; Hurst, C.; Echaubard, P.; Wilcox, B.A.; Sripa, B. Fish sharing as a risk factor for Opisthorchis viverrini infection: Evidence from two villages in north-eastern Thailand. Infect. Dis. Poverty 2017, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Kaewma, J.; Tongterm, T.; Thitiset, T. Behavior of Food Offering to Buddhist Monks of the Elderly in Sisaket Province. AMBJ 2020, 5, 119–133. [Google Scholar]
- Zhou, M.; Zhang, N.; Zhang, M.; Ma, G. Culture, eating behavior, and infectious disease control and prevention. J. Ethn. Foods 2020, 7, 40. [Google Scholar] [CrossRef]
- Yingklang, M.; Chaidee, A.; Dangtakot, R.; Jantawong, C.; Haonon, O.; Sitthirach, C.; Hai, N.T.; Cha’On, U.; Anutrakulchai, S.; Kamsa-Ard, S.; et al. Association of Strongyloides stercoralis infection and type 2 diabetes mellitus in northeastern Thailand: Impact on diabetic complication-related renal biochemical parameters. PLoS ONE 2022, 17, e0269080. [Google Scholar] [CrossRef]
- Tomczyk, S.; Deribe, K.; Brooker, S.J.; Clark, H.; Rafique, K.; Knopp, S.; Utzinger, J.; Davey, G. Association between Footwear Use and Neglected Tropical Diseases: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2014, 8, e3285. [Google Scholar] [CrossRef]
- Haldeman, M.S.; Nolan, M.S.; Ng’Habi, K.R. Human hookworm infection: Is effective control possible? A review of hookworm control efforts and future directions. Acta Trop. 2020, 201, 105214. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.B.; Friant, S.; Clech, L.; Malavé, C.; Kemigabo, C.; Obeti, R.; Goldberg, T.L. Combining Footwear with Public Health Iconography to Prevent Soil-Transmitted Helminth Infections. Am. J. Trop. Med. Hyg. 2017, 96, 205–213. [Google Scholar] [CrossRef]
- Dhammavajiro, P.V. Vinaya: Principles of problem prevention and solution for Buddhist monks. DSR 2022, 5, 162–175. [Google Scholar]
- Kache, R.; Phasuk, N.; Viriyavejakul, P.; Punsawad, C. Prevalence of soil-transmitted helminth infections and associated risk factors among elderly individuals living in rural areas of southern Thailand. BMC Public Health 2020, 20, 1882. [Google Scholar] [CrossRef] [PubMed]
- Babayan, S.A.; Sinclair, A.; Duprez, J.S.; Selman, C. Chronic helminth infection burden differentially affects haematopoietic cell development while ageing selectively impairs adaptive responses to infection. Sci. Rep. 2018, 8, 3802. [Google Scholar] [CrossRef]
- Abdoli, A.; Ardakani, H.M. Helminth infections and immunosenescence: The friend of my enemy. Exp. Gerontol. 2020, 133, 110852. [Google Scholar] [CrossRef]
- Hai, N.T.; Hongsrichan, N.; Intuyod, K.; Pinlaor, P.; Yingklang, M.; Chaidee, A.; Pongking, T.; Anutrakulchai, S.; Cha’On, U.; Pinlaor, S. Strongyloides stercoralis infection induces gut dysbiosis in chronic kidney disease patients. PLoS Negl. Trop. Dis. 2022, 16, e0010302. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Agustí, A.; Melén, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene–environment interactions across the lifespan. Lancet Respir. Med. 2022, 10, 512–524. [Google Scholar] [CrossRef]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- Roulette, C.J.; Mann, H.; Kemp, B.M.; Remiker, M.; Roulette, J.W.; Hewlett, B.S.; Kazanji, M.; Breurec, S.; Monchy, D.; Sullivan, R.J. Tobacco use vs. helminths in Congo basin hunter-gatherers: Self-medication in humans? Evol. Hum. Behav. 2014, 35, 397–407. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Gazzinelli-Guimaraes, P.; Knight, J.M.; Fujiwara, R.; Hotez, P.J.; Bottazzi, M.E.; Corry, D.B. Host Immunity and Inflammation to Pulmonary Helminth Infections. Front. Immunol. 2020, 11, 594520. [Google Scholar] [CrossRef]
- Smith, M.T.; Umenai, T. Smoking among Buddhist monks in Phnom Penh, Cambodia. Tob. Control 2000, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Perski, O.; Theodoraki, M.; Cox, S.; Kock, L.; Shahab, L.; Brown, J. Associations between smoking to relieve stress, motivation to stop and quit attempts across the social spectrum: A population survey in England. PLoS ONE 2022, 17, e0268447. [Google Scholar] [CrossRef] [PubMed]
- Erawan, P. Healthy Schools Promotion: An Experience in Thailand. Procedia Soc. Behav. Sci. 2015, 186, 513–521. [Google Scholar] [CrossRef]
- Laoraksawong, P.; Sanpool, O.; Rodpai, R.; Thanchomnang, T.; Kanarkard, W.; Maleewong, W.; Kraiklang, R.; Intapan, P.M. Impact of the health education and preventive equipment package (HEPEP) on prevention of Strongyloides stercoralis infection among rural communities in Northeast Thailand: A cluster randomized controlled trial. BMC Public Health 2018, 18, 1184. [Google Scholar] [CrossRef]


| Variables | No. of Participants |
|---|---|
| n (%) | |
| Age (n = 511) | |
| ≤20 | 26 (5.1) |
| 21–40 | 98 (19.2) |
| 41–60 | 204 (39.9) |
| ≥61 | 183 (35.8) |
| Ordinate (n = 450) | |
| Monkhood ≤ 1 year | 51 (11.3) |
| Monkhood > 1 year | 399 (88.7) |
| Secular education (n = 476) | |
| ≤Primary education | 226 (47.5) |
| >Primary education | 250 (52.5) |
| Dharma education (n = 438) | |
| <Dharma scholar advanced level | 206 (47.0) |
| Dharma scholar advanced level | 232 (53.0) |
| Study area (n = 512) | |
| Ubolratana | 58 (11.3) |
| Ban Haet | 132 (25.8) |
| Ban Phai | 322 (62.9) |
| Underlying disease (n = 454) | |
| No | 247 (54.4) |
| Yes | 207 (45.6) |
| Illegal combined drugs (n = 433) | |
| No | 394 (91.0) |
| Yes | 39 (9.0) |
| Smoking (n = 480) | |
| No | 196 (40.8) |
| Ex- or current smoking | 284 (59.2) |
| Alcohol before monkhood (n = 411) | |
| No | 196 (47.7) |
| Yes | 215 (52.3) |
| Education of parasitic infection (n = 404) | |
| Never | 216 (53.5) |
| Received | 188 (46.5) |
| Examination of parasitic infection (n = 467) | |
| No | 320 (68.5) |
| Yes | 147 (31.5) |
| History of parasitic infection (n = 408) | |
| No | 307 (75.2) |
| Yes | 101 (24.8) |
| Anthelmintic drugs per year ≥ 1 time/year (n = 480) | |
| No | 134 (27.9) |
| Yes | 346 (72.1) |
| Offer raw meats to monk (n = 485) | |
| No | 98 (20.2) |
| Yes | 387 (79.8) |
| Raw beef dishes (n = 475) | 327 (68.8) |
| Raw fish dishes (n = 475) | 320 (67.4) |
| Raw shrimp dishes (n = 447) | 257 (57.5) |
| Raw pork dishes (n = 462) | 256 (55.4) |
| Parasitic Infection (n = 514) | n | (%) |
|---|---|---|
| Single infection | 120 | (23.3) |
| Multi-infection | 28 | (5.5) |
| Total infections | 148 | (28.8) |
| Helminth | ||
| Opisthorchis viverrini | 57 | (11.1) |
| Strongyloides stercoralis | 80 | (15.6) |
| Hookworm | 36 | (7.0) |
| Taenia spp. | 3 | (0.6) |
| Echinostome sp. | 1 | (0.2) |
| Minute intestinal flukes | 1 | (0.2) |
| Ascaris lumbricoides | 1 | (0.2) |
| Protozoa | ||
| Giardia lamblia | 4 | (0.8) |
| Entamoeba histolytica | 1 | (0.2) |
| Isospora belli | 1 | (0.2) |
| Entamoeba coli | 3 | (0.6) |
| Variables | O. viverrini Negative | O. viverrini Positive | ORcrude (95% CI) | p-Value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age (n = 511) | ||||
| <40 | 109 (90.1) | 12 (9.9) | 1 | |
| ≥40 | 345 (88.5) | 45 (11.5) | 1.18 (0.60–2.32) | 0.621 |
| Ordinate (n = 450) | ||||
| Monkhood ≤ 1 year | 46 (90.2) | 5 (9.8) | 1 | |
| Monkhood > 1 year | 354 (88.7) | 45 (11.3) | 1.17 (0.44–3.10) | 0.753 |
| Secular education (n = 476) | ||||
| ≤Primary education | 195 (86.3) | 31 (13.7) | 1 | |
| >Primary education | 226 (90.4) | 24 (9.6) | 0.67 (0.38–1.18) | 0.163 |
| Underlying disease (n = 454) | ||||
| No underlying disease | 219 (88.7) | 28 (11.3) | 1 | |
| Other underlying disease | 180 (89.1) | 22 (10.9) | 0.96 (0.53–1.73) | 0.881 |
| Chronic kidney disease with other underlying disease | 5 (100) | 0 (0) | – | – |
| Alcohol drinking before monkhood (n = 411) | ||||
| No | 176 (89.8) | 20 (10.2) | 1 | |
| Yes | 188 (87.4) | 27 (12.6) | 1.26 (0.68–2.33) | 0.455 |
| Smoking (n = 480) | ||||
| No smoking | 175 (89.3) | 21 (10.7) | 1 | |
| Ex- or current smoking | 251 (88.4) | 33 (11.6) | 1.1 (0.61–1.96) | 0.758 |
| Education about parasitic infection (n = 404) | ||||
| Never | 189 (87.5) | 27 (12.5) | 1 | |
| Received | 171 (91.0) | 17 (9.0) | 0.67 (0.37–1.32) | 0.268 |
| Anthelmintic drugs used ≥ 1 time/year (n = 331) | ||||
| No | 67 (88.2) | 9 (11.8) | 1 | |
| Yes | 230 (90.2) | 25 (9.8) | 0.81 (0.36–1.81) | 0.608 |
| Offered raw fish dish ≥ 1 type (n = 475) | ||||
| No | 147 (94.8) | 8 (5.2) | 1 | |
| Yes | 271 (84.7) | 49 (15.3) | 3.32 (1.53–7.20) | 0.002 * |
| Defecation on ground soil (n = 376) | ||||
| No | 199 (87.7) | 28 (12.3) | 1 | |
| Yes | 136 (91.3) | 13 (8.7) | 0.68 (0.34–1.36) | 0.274 |
| Variables | Skin-Penetrating Helminth Negative | Skin-Penetrating Helminth Positive | ORcrude (95% CI) | p-Value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age (n = 511) | ||||
| <40 | 114 (94.2) | 7 (5.8) | 1 | |
| ≥40 | 298 (76.4) | 92 (23.6) | 5.02 (2.26–11.17) | <0.001 * |
| Ordinate (n = 450) | ||||
| Monkhood ≤ 1 year | 47 (92.2) | 4 (7.8) | 1 | |
| Monkhood > 1 year | 312 (78.2) | 87 (21.8) | 3.28 (1.15–9.34) | 0.026 * |
| Secular education (n = 476) | ||||
| ≤Primary education | 166 (73.5) | 60 (26.5) | 1 | |
| >Primary education | 218 (872) | 32 (12.8) | 0.41 (0.25–0.65) | <0.001 * |
| Underlying disease (n = 454) | ||||
| No underlying disease | 207 (83.8) | 40 (16.2) | 1 | |
| Other underlying disease | 158 (78.2) | 44 (21.8) | 1.44 (0.90–2.32) | 0.132 |
| Chronic kidney disease with other underlying disease | 1 (20) | 4 (80) | 20.7 (2.54–190.1) | 0.007 * |
| NSAIDs (n = 433) | ||||
| Never use | 288 (81.6) | 65 (18.4) | 1 | |
| Using | 60 (75.0) | 20 (25.0) | 1.48 (0.83–2.62) | 0.182 |
| Illegal combined drugs (n = 433) | ||||
| Never used | 318 (80.7) | 76 (19.3) | 1 | |
| Using | 30 (76.9) | 9 (23.1) | 1.25 (0.57–2.75) | 0.571 |
| Alcohol drinking before monkhood (n = 411) | ||||
| No | 167 (85.2) | 29 (14.8) | 1 | |
| Yes | 172 (80.0) | 43 (20.0) | 1.44 (0.86–2.41) | 0.167 |
| Smoking (n = 480) | ||||
| No smoking | 171 (87.2) | 25 (12.8) | 1 | |
| Ex- or current smoking | 219 (77.1) | 65 (22.9) | 2.03 (1.23–3.36) | 0.007 * |
| Education of parasitic infection (n = 404) | ||||
| Never | 165 (76.4) | 51 (23.6) | 1 | |
| Received | 164 (87.2) | 24 (12.8) | 0.47 (0.28–0.80) | 0.006 * |
| Anthelmintic drugs used ≥ 1 time/year (n = 331) | ||||
| No | 63 (82.9) | 13 (17.1) | 1 | |
| Yes | 213 (83.5) | 42 (16.5) | 0.96 (0.48–1.89) | 0.896 |
| Wearing shoes outside (except during alms work) (n = 430) | ||||
| Do not always wear | 94 (79.0) | 25 (21.0) | 1 | |
| Always wear | 253 (81.4) | 58 (18.6) | 0.86 (0.51–1.46) | 0.579 |
| Defecation on ground soil (n = 376) | ||||
| No | 182 (80.2) | 45 (19.8) | 1 | |
| Yes | 119 (79.9) | 30 (20.1) | 1.02 (0.60–1.71) | 0.940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekobol, N.; Boonjaraspinyo, S.; Artchayasawat, A.; Boonmars, T. Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths. Trop. Med. Infect. Dis. 2023, 8, 135. https://doi.org/10.3390/tropicalmed8030135
Ekobol N, Boonjaraspinyo S, Artchayasawat A, Boonmars T. Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths. Tropical Medicine and Infectious Disease. 2023; 8(3):135. https://doi.org/10.3390/tropicalmed8030135
Chicago/Turabian StyleEkobol, Nuttapon, Sirintip Boonjaraspinyo, Atchara Artchayasawat, and Thidarut Boonmars. 2023. "Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths" Tropical Medicine and Infectious Disease 8, no. 3: 135. https://doi.org/10.3390/tropicalmed8030135
APA StyleEkobol, N., Boonjaraspinyo, S., Artchayasawat, A., & Boonmars, T. (2023). Monks: A Population at Risk for Liver Fluke and Skin-Penetrating Helminths. Tropical Medicine and Infectious Disease, 8(3), 135. https://doi.org/10.3390/tropicalmed8030135






