A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective Comparisons
2.2. Adjusted Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loyola, S.; Cano-Pérez, E.; Torres-Pacheco, J.; Malambo-Garcia, D.; Gomez, R.; Gomez-Camargo, D. Epidemiology of COVID-19 in Individuals under 18 Years Old in Cartagena, Colombia: An Ecological Study of the First 14 Months of the Pandemic. Trop. Med. Infect. Dis. 2022, 7, 107. [Google Scholar] [CrossRef]
- Jaya, A.M.; Harries, A.D.; Rahman, A.; Khogali, M.; Chinnakali, P.; Gopalakrishnan, L.G.; Pillai, M.N. Epidemiology and Response to the COVID-19 Pandemic in Kerala, India, 2020–2021: A Cross-Sectional Study. Trop. Med. Infect. Dis. 2022, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Oyelade, T.; Alqahtani, J.S.; Hjazi, A.M.; Li, A.; Kamila, A.; Raya, R.P. Global and Regional Prevalence and Outcomes of COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 22. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alhmeed, N.; Zaidi, A.R.Z.; Tobaiqy, M. Efficacy and Safety of Lopinavir/Ritonavir for Treatment of COVID-19: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 180. [Google Scholar] [CrossRef]
- Raudenbush, S.W. Analyzing effect sizes: Random-effectsmodels. In The Handbook of Research Synthesis and Meta-Analysis; Cooper, H., Hedges, L.V., Valentine, J.C., Eds.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 295–315. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Rabe-Hesketh, S.; Choi, I.H. Avoiding zero between-study variance estimates in random-effects meta-analysis. Stat. Med. 2013, 32, 4071–4089. [Google Scholar] [CrossRef] [PubMed]
- Kontopantelis, E.; Springate, D.A.; Reeves, D. A re-analysis of the Cochrane Library data: The dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 2013, 8, e69930. [Google Scholar] [CrossRef]
- Sidik, K.; Jonkman, J.N. A comparison of heterogeneity variance estimators in combining results of studies. Stat. Med. 2007, 26, 1964–1981. [Google Scholar] [CrossRef] [PubMed]
- Kulinskaya, E.; Dollinger, M.B. An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med. Res. Method 2015, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Hoaglin, D.C. Misunderstandings about Q and ‘Cochran’s Q test’ in meta-analysis. Stat. Med. 2016, 35, 485–495. [Google Scholar] [CrossRef]
- Hoaglin, D.C. Shortcomings of an approximate confidence interval for moment-based estimators of the between-study variance in random-effects meta-analysis. Res. Synth. Methods 2016, 7, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Shuster, J.J.; Walker, M.A. Low-event-rate meta-analyses of clinical trials: Implementing good practices. Stat. Med. 2016, 35, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; White, I.R. When should meta-analysis avoid making hidden normality assumptions? Biom. J. 2018, 60, 1040–1105. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001, 20, 3875–3889. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Lin, W.; Cai, W.; Wen, C.; Guan, Y.; Mo, X.; Wang, J.; Wang, Y.; Peng, P. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: An exploratory randomized controlled trial. Med 2020, 1, 105–113. [Google Scholar] [CrossRef]
- Wen, C.; Xie, Z.; Li, Y.; Deng, X.; Chen, X.; Cao, Y.; Ou, X.; Lin, W.; Li, F.; Cai, W. Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19: An observational cohort study. Zhonghua Nei Ke Za Zhi 2020, 59, E012. [Google Scholar]
- Jun, C.; Yun, L.; Xiuhong, X.; Ping, L.; Feng, L.; Tao, L.; Shang, Z.; Mei, W.; Yinzhong, S.; Hongzhou, L. Ecfficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin. J. Infect. Dis. 2020, 12, E008. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.-Y.; Zhu, Y.-N.; Huang, L.; Dan, B.-T.; Zhang, G.-J.; Gao, Y.-H. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur. Respir. J. 2020, 56, 2000799. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Lu, J.; Xue, Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Shao, C.; Zeng, X.; Wu, Z.; Xu, Y. Lopinavir-ritonavir alone or combined with arbidol in the treatment of 73 hospitalized patients with COVID-19: A pilot retrospective study. MedRxiv 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gonnermann, A.; Framke, T.; Großhennig, A.; Koch, A. No solution yet for combining two independent studies in the presence of heterogeneity. Stat. Med. 2015, 34, 2476–2480. [Google Scholar] [CrossRef]
- Tzeng, I.S.; Chen, J.H. Exploring Hepatocellular Carcinoma Mortality Using Weighted Regression Estimation for the Cohort Effect in Taiwan from 1976 to 2015. Int. J. Environ. Res. Public Health 2022, 19, 5573. [Google Scholar] [CrossRef] [PubMed]
- Van Aert, R.C.M.; Jackson, D. A new justification of the Hartung-Knapp method for random-effects meta-analysis based on weighted least squares regression. Res. Synth. Methods 2019, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, I.S. Dealing with the Problem of Monotone Likelihood in the Inflation of Estimated Effects in Clinical Studies. Comment on Hasegawa et al. Impact of Blood Type O on Mortality of Sepsis Patients: A Multicenter Retrospective Observational Study. Diagnostics 2020, 10, 826. [Google Scholar]

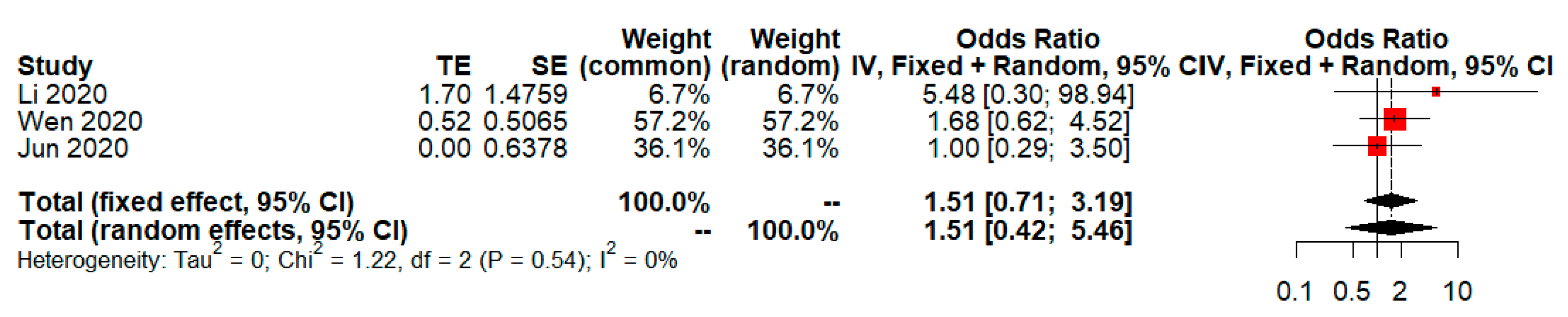
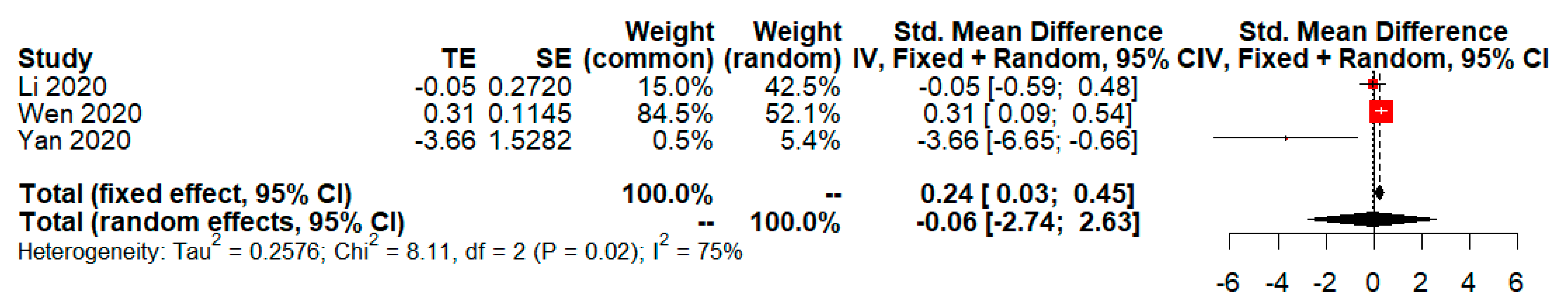

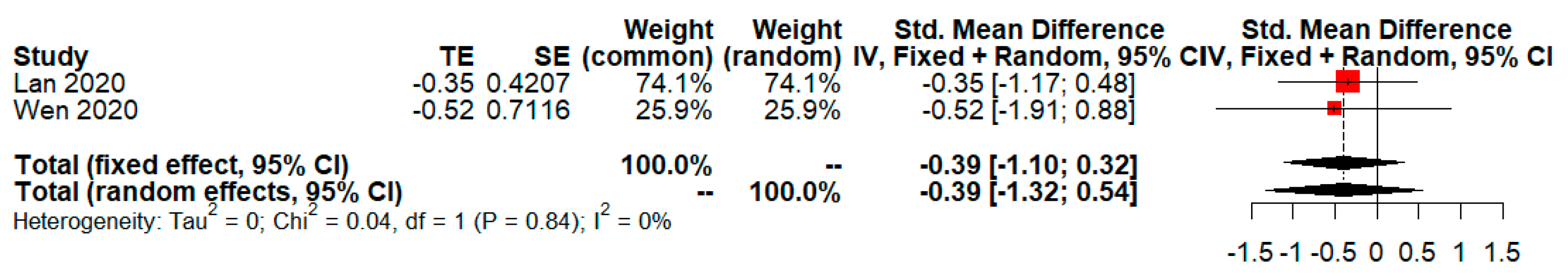
| Objectives or Intervention vs. Control | Included Studies (Year, Country) | Number of Events/Total in Intervention Group | Number of Events/Total in Control Group | Unadjusted OR (p-Value; 95%CI) Base on DL | Adjusted OR (p-Value; 95%CI) Base on GW and HK |
|---|---|---|---|---|---|
| (1) LPV/RTV vs. umifenovir | Li (2020, China) [19]; Wen (2020, China) [20] | 54/86 | 39/58 | 0.87 (p = 0.70; 0.42, 1.78) (Figure 5 in [6]) | 0.43 (p = 0.14; 0.04, 4.92) |
| (2) LPV/RTV vs. no antiviral treatment (conventional) | Li (2020, China) [19]; Wen (2020, China) [20] | 54/86 | 40/67 | 0.99 (p = 0.98; 0.49, 1.99) (Figure 6 in [6]) | 0.49 (p = 0.27; 0.01, 33.85) |
| (3) Rate of cough alleviation after 7 days of treatment (LPV/RTV vs. umifenovir) | Li (2020, China) [19]; Wen (2020, China) [20] | 11/80 | 13/61 | 0.62 (p = 0.69; 0.66, 6.53) (Figure 7 in [6]) | 0.31 (p = 0.51; 0.0001, 1,082,807) |
| (4) Rate of cough alleviation after 7 days of treatment (LPV/RTV vs. no antiviral treatment) | Li (2020, China) [19]; Wen (2020, China) [20] | 11/80 | 8/67 | 0.87 (p = 0.89; 0.10, 7.16) (Figure 8 in [6]) | 0.43 (p = 0.58; 0.0001, 417,569) |
| (5) Rate of improvement on chest CT after 7 days of treatment (LPV/RTV vs. umifenovir) | Li (2020, China) [19]; Wen (2020, China) [20] | 32/87 | 29/69 | 0.80 (p = 0.5; 0.42, 1.54) (Figure 9 in [6]) | 0.40 (p = 0.13;0.04, 4.04) |
| (6) Rate of improvement on chest CT after 7 days of treatment (LPV/RTV vs. no antiviral treatment or conventional) | Li (2020, China) [19]; Wen (2020, China) [20] | 32/87 | 34/74 | 0.69 (p = 0.26; 0.36, 1.31) (Figure 10 in [6]) | 0.34 (p = 0.14; 0.01, 8.11) |
| (7) Rate of adverse events of treatment (LPV/RTV vs. umifenovir) | Li (2020, China) [19]; Wen (2020, China) [20]; Jun (2020, China) [21] | 45/145 | 15/105 | 2.66 (p = 0.004; 1.36, 5.19) (Figure 13 in [6]) | 0.83 (p= 0.60; 0.23, 3.03) (Refer to Figure 1 in this study) |
| (8) Rate of adverse events of treatment (LPV/RTV vs. no antiviral treatment or conventional) | Li (2020, China) [19]; Wen (2020, China) [20]; Jun (2020, China) [21] | 45/145 | 10/123 | 4.6 (p = 0.0007; 1.91, 11.07) (Figure 14 in [6]) | 1.51 (p = 0.30; 0.42, 5.46) (Refer to Figure 2 in this study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, I.-S. A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity. Trop. Med. Infect. Dis. 2023, 8, 83. https://doi.org/10.3390/tropicalmed8020083
Tzeng I-S. A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity. Tropical Medicine and Infectious Disease. 2023; 8(2):83. https://doi.org/10.3390/tropicalmed8020083
Chicago/Turabian StyleTzeng, I-Shiang. 2023. "A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity" Tropical Medicine and Infectious Disease 8, no. 2: 83. https://doi.org/10.3390/tropicalmed8020083
APA StyleTzeng, I.-S. (2023). A Practical Approach in Refining Binary Outcome for Treatment Effect of COVID-19 According to Geographical Diversity. Tropical Medicine and Infectious Disease, 8(2), 83. https://doi.org/10.3390/tropicalmed8020083








