Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Source
2.2. Sample Collection
2.3. Extraction of Viral RNA
2.4. G/P Typing of Rotavirus
2.5. Statistical Processing
3. Results
3.1. Crowd Distribution
3.2. Time Distribution
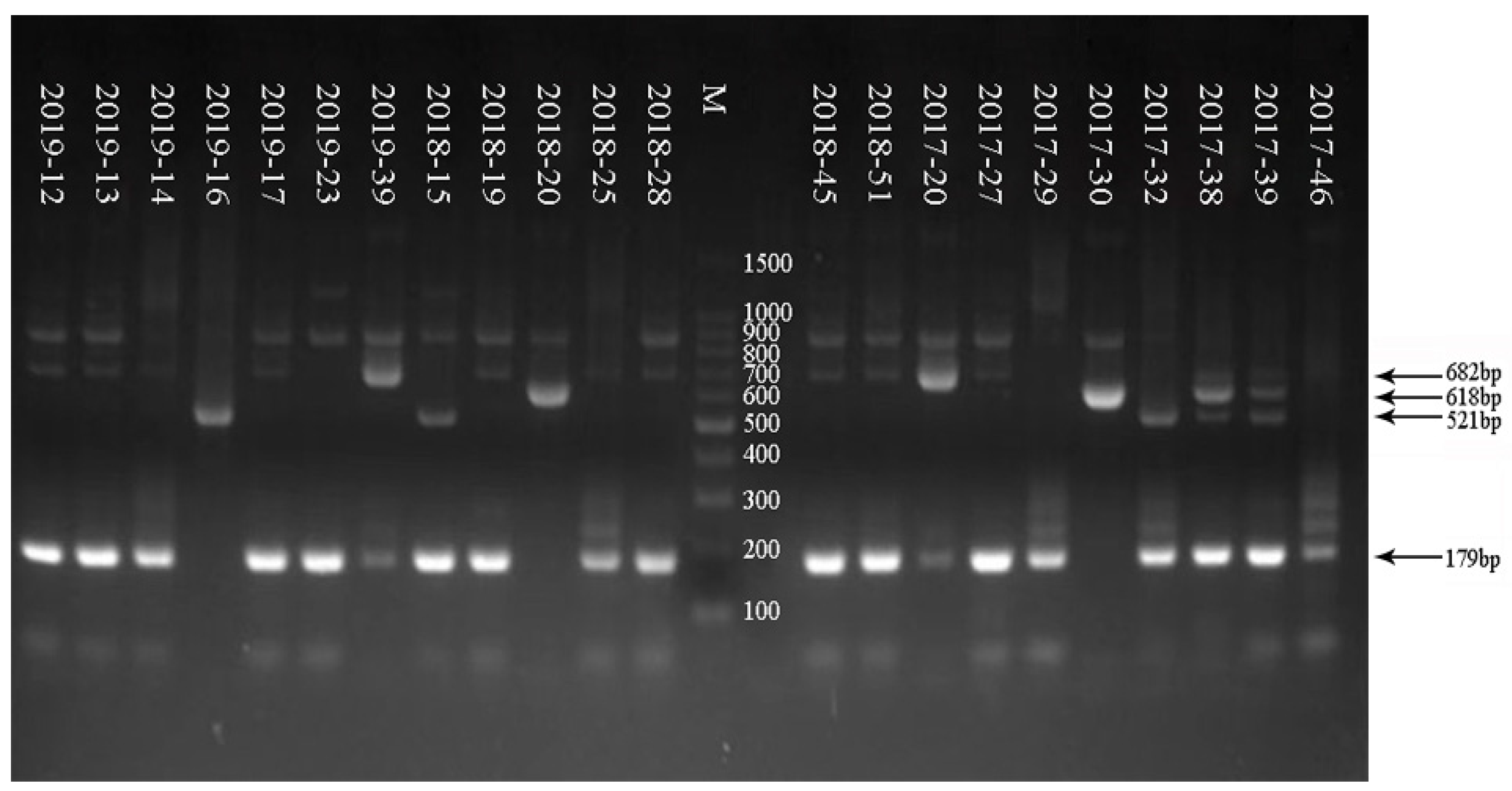
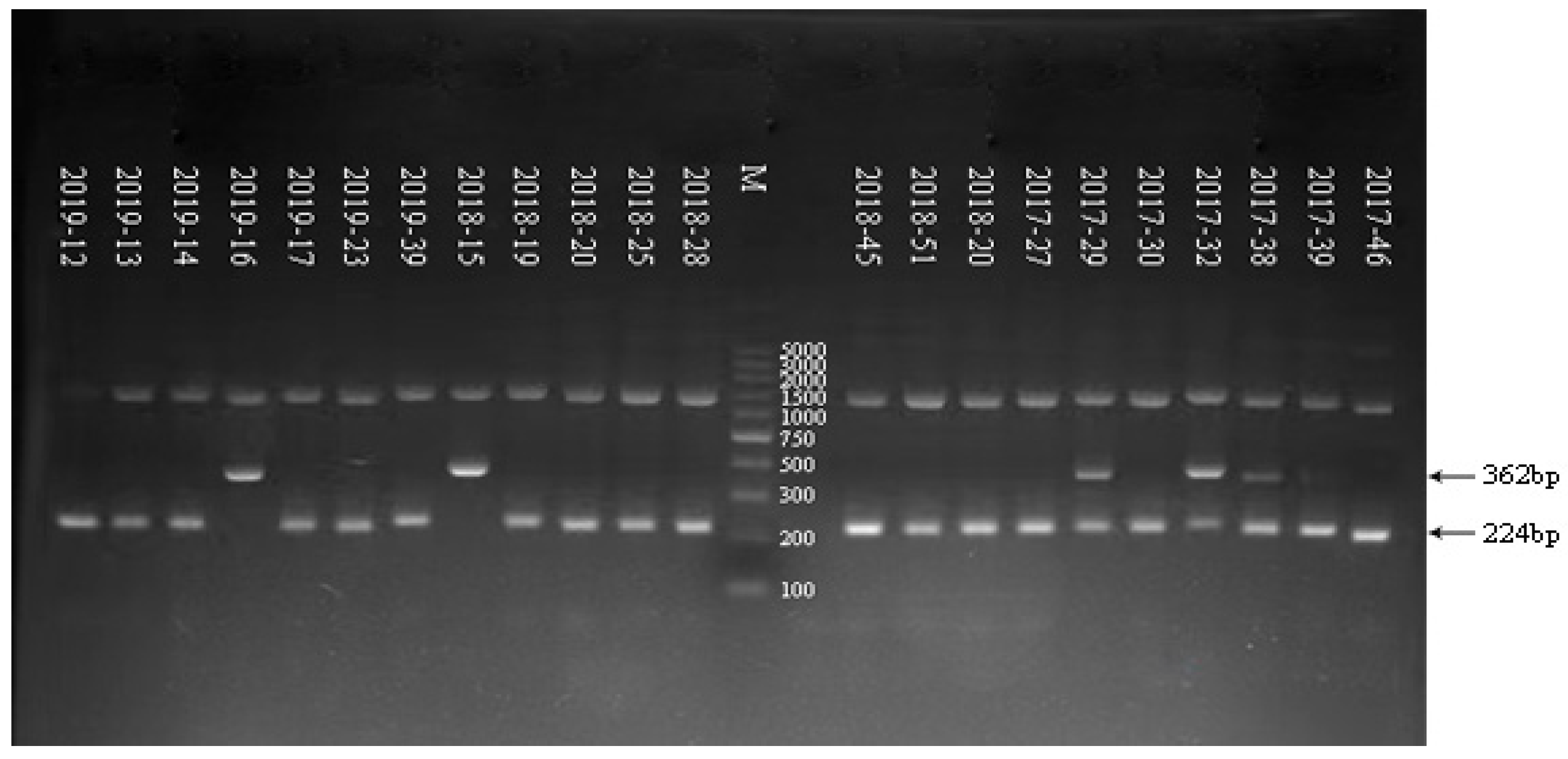
3.3. Electrophoresis Result of G/P Genotyping of RV in Some Samples from 2017 to 2019
3.4. Result of G/P Genotyping of RV in 2017
3.5. Result of G/P Genotyping of RV in 2018
3.6. Result of G/P Genotyping of RV in 2019
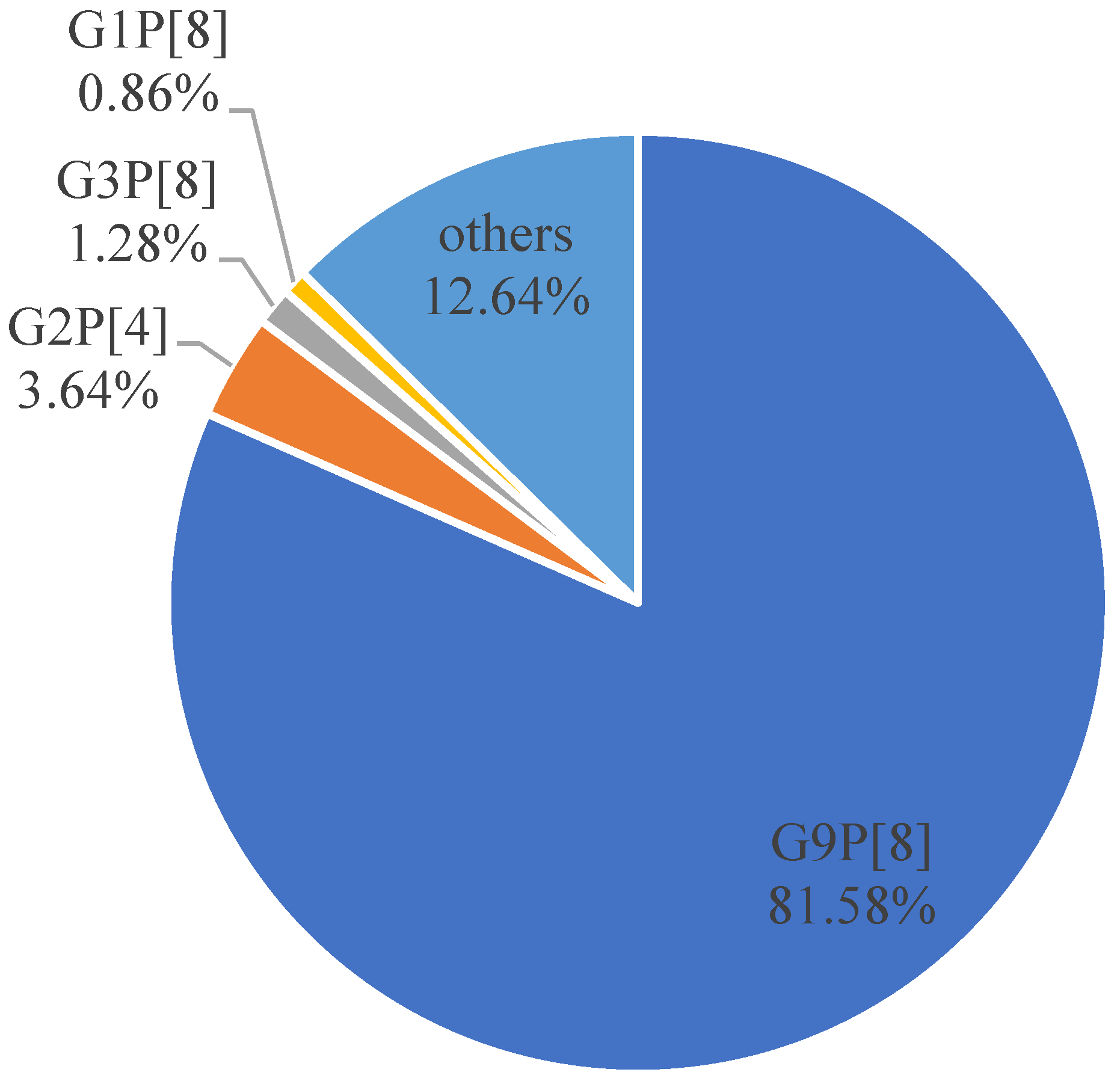
3.7. Comprehensive Analysis of G/P Genotyping of RV from 2017 to 2019
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estes, M.K.; Greenberg, H.B. Rotaviruses; Wolters Kluwer Health/Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Chen, J.Z.; Settembre, E.C.; Aoki, S.T.; Zhang, X.; Bellamy, A.R.; Dormitzer, P.R.; Harrison, S.C.; Grigorieff, N. Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Proc. Natl. Acad. Sci. USA 2009, 106, 10644–10648. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Baker, M.L.; Jiang, W.; Estes, M.K.; Prasad, B.V. Rotavirus architecture at subnanometer resolution. J. Virol. 2009, 83, 1754–1766. [Google Scholar] [CrossRef] [PubMed]
- Settembre, E.C.; Chen, J.Z.; Dormitzer, P.R.; Grigorieff, N.; Harrison, S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011, 30, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, C.D. Genetic and antigenic diversity of human rotaviruses: Potential impact on vaccination programs. J. Infect. Dis. 2010, 202, S43–S48. [Google Scholar] [CrossRef]
- RCWG. List of Accepted Genoserotype. [EB/OL]. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 26 September 2019).
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Dodet, B.; Heseltine, E.; Saliou, P. Rotaviruses in human and veterinary medicine. Sante 1997, 7, 195–199. [Google Scholar] [CrossRef]
- World Health Organization. Rotavirus vaccines. Relev. Epidemiol. Hebd. 2007, 82, 285–295. [Google Scholar]
- Global Rotavirus Surveillance Network and Rotavirus Age Study Collaborators. Global Review of the Age Distribution of Rotavirus Disease in Children Aged <5 Years before the Introduction of Rotavirus Vaccination. Clin. Infect. Dis. 2019, 69, 1071–1078. [Google Scholar]
- Isanaka, S.; Langendorf, C.; McNeal, M.M.; Meyer, N.; Plikaytis, B.; Garba, S.; Sayinzoga-Makombe, N.; Soumana, I.; Guindo, O.; Makarimi, R.; et al. Rotavirus Vaccine Efficacy up to 2 Years of Age and against Diverse Circulating Rotavirus Strains in Niger: Extended Follow-up of a Randomized Controlled Trial. PLoS Med. 2021, 18, e1003655. [Google Scholar] [CrossRef]
- Bergman, H.; Henschke, N.; Hungerford, D.; Pitan, F.; Ndwandwe, D.; Cunliffe, N.; Soares-Weiser, K. Vaccines for PreventingRotavirus Diarrhoea: Vaccines in Use. Cochrane Database Syst. Rev. 2021, 11, CD008521. [Google Scholar]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D.; World Health Organization–Coordinated Global Rotavirus Surveillance Network; Agocs, M.; Serhan, F.; de Oliveira, L.; Mwenda, J.M.; Mihigo, R.; et al. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S96–S105. [Google Scholar] [PubMed]
- Ministry of Health of the People’s Republic of China. WS 271- 2007 Diagnostic Criteria for Infections Diarrhea; People’s Medical Publishing House: Beijing, China, 2007. [Google Scholar]
- Li, J.; Pan, H.; Xiao, W.J.; Gong, X.H.; Zhuang, Y.; Kuang, X.Z.; Wu, H.Y.; Yuan, Z.A. Epidemiological and etiological surveillance study of infectious diarrhea in Shanghai in 2013–2015. Chin. J. Prev. Med. 2017, 51, 1113–1117. [Google Scholar]
- Iturriza-Gómara, M.; Green, J.; Brown, D.W.; Desselberger, U.; Gray, J.J. Diversity within the VP4 gene of rotavirus P[8] strains: Implications for reverse transcription-PCR genotyping. J. Clin. Microbiol. 2000, 38, 898–901. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Van Ranst, M. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr. Opin. Virol. 2012, 2, 426–433. [Google Scholar] [CrossRef]
- Bányai, K.; László, B.; Duque, J.; Steele, A.D.; Nelson, E.A.S.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact ofrotavirus vaccination programs. Vaccine 2012, 30, A122–A130. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhoea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef]
- Malik, A.; Haldar, P.; Ray, A.; Shet, A.; Kapuria, B.; Bhadana, S.; Santosham, M.; Ghosh, R.S.; Steinglass, R.; Kumar, R. Introducing Rotavirus Vaccine in the Universal Immunization Programme in India: From Evidence to Policy to Implementation. Vaccine 2019, 37, 5817–5824. [Google Scholar] [CrossRef]
- Wang, F.; Jin, M.; Li, D.; Zhang, Q.; Li, Y. Molecular epidemiological analysis of viral diarrhea in children under 5 years in Lanzhou in 2017. Chin. J. Biol. 2019, 32, 1102–1107. [Google Scholar]
- Tian, Y.; Gao, Z.; Li, W.; Liu, B.; Chen, Y.; Jia, L.; Yan, H.; Wang, Q. Group A rotavirus prevalence and genotypes among adult outpatients with diarrhea in Beijing, China, 2011–2018. J. Med. Virol. 2021, 93, 6191–6199. [Google Scholar] [CrossRef]
- Kuang, X.; Gong, X.; Zhang, X.; Pan, H.; Teng, Z. Genetic diversity of group A rotavirus in acute gastroenteritis outpatients in Shanghai from2017 to 2018. BMC Infect. Dis. 2020, 20, 596. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, X.; Meng, D.; Guo, J.; Li, Y.; Liang, L.; Guo, X.; Tao, R.; Zhang, X.; Gao, R.; et al. Prevalence and genotype distribution of group A rotavirus circulating in Shanxi province, China during 2015–2019. BMC Infect. Dis. 2021, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Isanaka, S.; Garba, S.; Plikaytis, B.; McNeal, M.M.; Guindo, O.; Langendorf, C.; Adehossi, E.; Ciglenecki, I.; Grais, R.F. Immunogenicityof an Oral Rotavirus Vaccine Administered with Prenatal Nutritional Support in Niger: A Cluster Randomized Clinical Trial. PLoS Med. 2021, 18, e1003720. [Google Scholar]
- Soares-Weiser, K.; Bergman, H.; Henschke, N.; Pitan, F.; Cunliffe, N. Vaccines for Preventing Rotavirus Diarrhoea: Vaccines in Use. Cochrane Database Syst. Rev. 2019, 10, CD008521. [Google Scholar]
- Khagayi, S.; Omore, R.; Otieno, G.P.; Ogwel, B.; Ochieng, J.B.; Juma, J.; Apondi, E.; Bigogo, G.; Onyango, C.; Ngama, M.; et al. Effectiveness of Monovalent Rotavirus Vaccine against Hospitalization with Acute Rotavirus Gastroenteritis in Kenyan Children. Clin. Infect. Dis. 2020, 70, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, X.; Hou, H.; Lu, Y.; Yu, J.; Mao, L.; Mao, L.; Sun, Z. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: A hospital based study. BMC Infect. Dis. 2018, 18, 63. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, J.; Gong, S.T.; Xu, X.H.; Zhu, C.M.; Zhu, Q.R. Epidemiological surveillance of Norovirus and rotavirus diarrhea among outpatient children in five metropolitan cities. Chin. J. Pediatr. 2010, 48, 564–570. [Google Scholar]
- Mizan, M.F.R.; Jahid, I.K.; Kim, M.; Lee, K.H.; Kim, T.J.; Ha, S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling 2016, 32, 497–509. [Google Scholar] [CrossRef]
- Merten, S.; Schaetti, C.; Manianga, C.; Lapika, B.; Hutubessy, R.; Chaignat, C.L.; Weiss, M. Sociocultural determinants of anticipated vaccine acceptance for acute watery diarrhea in early childhood in Katanga Province, Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2013, 89, 419–425. [Google Scholar] [CrossRef]
- Salami, A.; Fakih, H.; Chakkour, M.; Salloum, L.; Bahmad, H.F.; Ghssein, G. Prevalence, risk factors and seasonal variations of different Enteropathogens in Lebanesehospitalized children with acute gastroenteritis. BMC Pediatr. 2019, 19, 137. [Google Scholar] [CrossRef]
- Ali, Z.; Harastani, H.; Hammadi, M.; Reslan, L.; Ghanem, S.; Hajar, F.; Sabra, A.; Haidar, A.; Inati, A.; Rajab, M.; et al. Rotavirus Genotypes and Vaccine Effectiveness from a Sentinel, Hospital-Based, Surveillance Study for Three Consecutive Rotavirus Seasons in Lebanon. PLoS ONE 2016, 11, e0161345. [Google Scholar] [CrossRef] [PubMed]
- Zaraket, R.; Salami, A.; Bahmad, M.; El Roz, A.; Khalaf, B.; Ghssein, G.; Bahmad, H.F. Prevalence, risk factors, and clinical characteristics of rotavirus and adenovirus among Lebanese hospitalized children with acute gastroenteritis. Heliyon 2020, 6, e04248. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-World Effectiveness of Rotavirus Vaccines, 2006–2019: A Literature Review and Meta-Analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef]
- Debellut, F.; Jaber, S.; Bouzya, Y.; Sabbah, J.; Barham, M.; Abu-Awwad, F.; Hjaija, D.; Ramlawi, A.; Pecenka, C.; Clark, A.; et al. Introduction of Rotavirus Vaccination in Palestine: An Evaluation of the Costs, Impact, and Cost-Effectiveness of ROTARIX andROTAVAC. PLoS ONE 2020, 15, e0228506. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. Update on Rotavirus Vaccine Underperformance in Low- to Middle-Income Countries and next-Generation Vaccines. Hum. Vaccines Immunother. 2021, 17, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Salami, A.; Salloum, L.; Chedid, P.; Joumaa, W.H.; Fakih, H. Surveillance Study of Acute Gastroenteritis Etiologies in Hospitalized Children in South Lebanon(SAGE study). Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.N.; Eghnatios, E.; El Roz, A.; Fardoun, T.; Ghssein, G. Prevalence, antimicrobial resistance and risk factors for campylobacteriosis in Lebanon. J. Infect. Dev. Ctries. 2019, 13, 11–20. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence 5′-3′ | Nucleotide Position (Expected Size cDNA 881 bp) |
|---|---|---|
| VP7F | ATG TAT GGT ATT GAA TAT ACC AC | 51–71 |
| VP7R | AAC TTG CCA CCA TTT TTT CC | 932–914 |
| Primer | Sequence 5′-3′ | Nucleotide Position (Expected Size cDNA is 663 bp) |
|---|---|---|
| VP4F | TAT GCT CCA GTN AAT TGG | 132–149 |
| VP4R | ATT GCA TTT CTT TCC ATA ATG | 775–795 |
| Type | Primer | Sequence 5′-3′ | Nucleotide Position | Size PCR Product (bp) |
|---|---|---|---|---|
| G1 | aBT1 | CAA GTA CTC AAA TCA ATG ATG G | 314–335 | 618 |
| G2 | aCT2 | CAA TGA TAT TAA CAC ATT TTC TGT G | 411–435 | 521 |
| G3 | G3 | ACG AAC TCA ACA CGA GAG G | 250–269 | 682 |
| G4 | aDT4 | CGT TTC TGG TGA GGA GTT G | 480–499 | 452 |
| G8 | aAT8 | GTC ACA CCA TTT GTA AAT TCG | 178–198 | 754 |
| G9 | G9 | CTT GAT GTG ACT AYA AAT AC | 757–776 | 179 |
| Type | Primer | Sequence 5′-3′ | Nucleotide Position | Size PCR Product (bp) |
|---|---|---|---|---|
| P[4] | 2T-1 | CTA TTG TTA GAG GTT AGA GTC | 492–474 | 362 |
| P[6] | 3T-1 | TGT TGA TTA GTT GGA TTC AA | 278–259 | 146 |
| P[8] | 1T-1D | TCT ACT GGR TTR ACN TGC | 356–339 | 224 |
| P[9] | 4T-1 | TGA GAC ATG CAA TTG GAC | 402–385 | 270 |
| P[10] | 5T-1 | ATC ATA GTT AGT AGT CGG | 594–575 | 462 |
| P[11] | P[11] | GTA AAC ATC CAG AAT GTG | 323–305 | 191 |
| Sample | G Serotype | Size PCR Product (bp) of G Serotype | P Serotype | Size PCR Product (bp) of P Serotype |
|---|---|---|---|---|
| 2019–12 | G9 | 179 | P[8] | 224 |
| 2019–13 | G9 | 179 | P[8] | 224 |
| 2019–14 | G9 | 179 | P[8] | 224 |
| 2019–16 | G2 | 521 | P[4] | 362 |
| 2019–17 | G9 | 179 | P[8] | 224 |
| 2019–23 | G9 | 179 | P[8] | 224 |
| 2019–39 | G3 | 682 | P[8] | 224 |
| 2018–15 | G2 + G9 | 521 + 179 | P[4] | 362 |
| 2018–19 | G9 | 179 | P[8] | 224 |
| 2018–20 | G1 | 618 | P[8] | 224 |
| 2018–25 | G9 | 179 | P[8] | 224 |
| 2018–28 | G9 | 179 | P[8] | 224 |
| 2018–39 | G9 | 179 | P[8] | 224 |
| 2018–45 | G9 | 179 | P[8] | 224 |
| 2018–51 | G9 | 179 | P[8] | 224 |
| 2017–20 | G3 | 682 | P[8] | 224 |
| 2017–27 | G9 | 179 | P[8] | 224 |
| 2017–29 | G9 | 179 | P[4] + P[8] | 362 + 224 |
| 2017–30 | G1 | 618 | P[8] | 224 |
| 2017–32 | G2 + G9 | 521 + 179 | P[4] + P[8] | 362 + 224 |
| 2017–38 | G1 + G9 | 618 + 179 | P[4] + P[8] | 362 + 224 |
| 2017–39 | G9 | 179 | P[4] + P[8] | 362 + 224 |
| 2017–46 | G9 | 179 | P[8] | 224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhang, G.; Li, C.; Niu, P.; Li, X.; Gao, Q.; Guo, K.; Zhang, R.; Wang, J.; Ma, X. Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019. Trop. Med. Infect. Dis. 2023, 8, 101. https://doi.org/10.3390/tropicalmed8020101
Sun Z, Zhang G, Li C, Niu P, Li X, Gao Q, Guo K, Zhang R, Wang J, Ma X. Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019. Tropical Medicine and Infectious Disease. 2023; 8(2):101. https://doi.org/10.3390/tropicalmed8020101
Chicago/Turabian StyleSun, Zhenlu, Guifang Zhang, Chunyan Li, Peihua Niu, Xia Li, Qiao Gao, Kai Guo, Ruiqing Zhang, Ji Wang, and Xuejun Ma. 2023. "Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019" Tropical Medicine and Infectious Disease 8, no. 2: 101. https://doi.org/10.3390/tropicalmed8020101
APA StyleSun, Z., Zhang, G., Li, C., Niu, P., Li, X., Gao, Q., Guo, K., Zhang, R., Wang, J., & Ma, X. (2023). Rotavirus Infection and Genotyping in Yantai, Shandong Province, 2017–2019. Tropical Medicine and Infectious Disease, 8(2), 101. https://doi.org/10.3390/tropicalmed8020101







