Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care
Abstract
1. Introduction
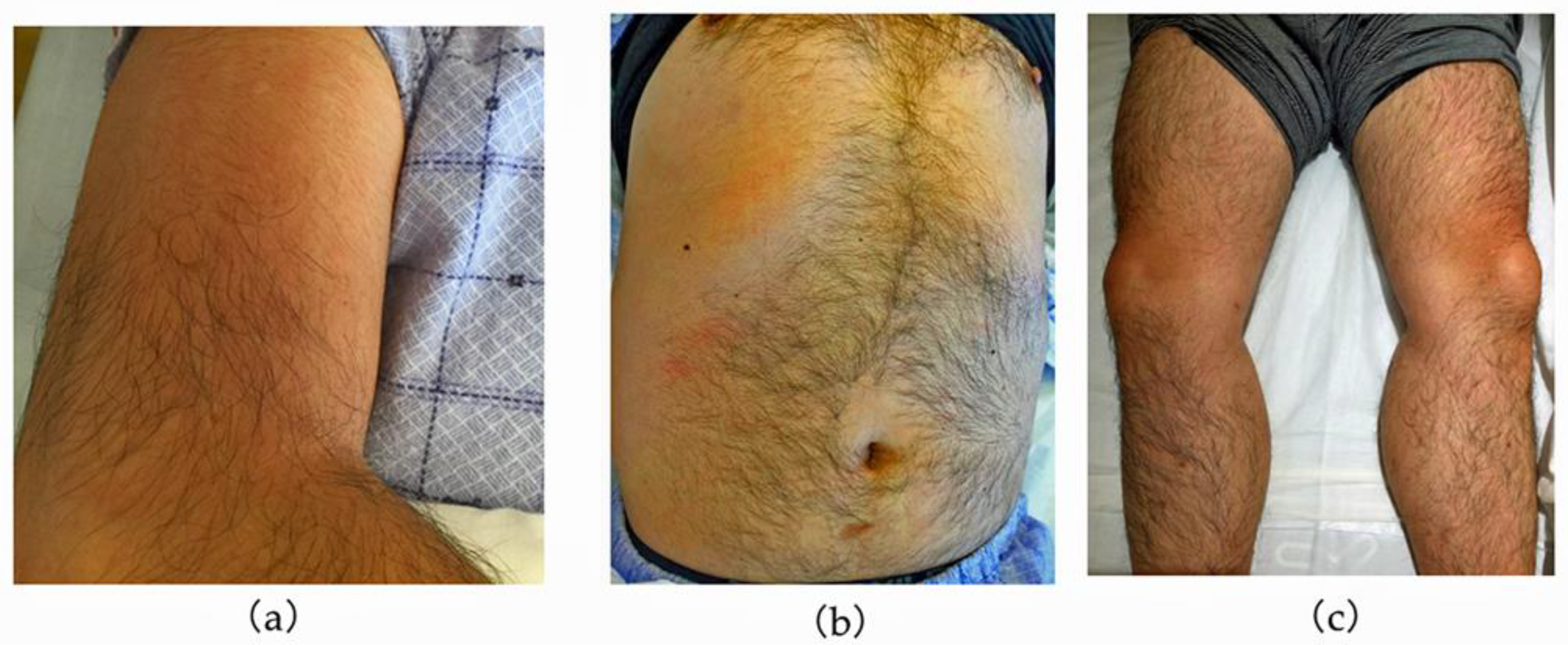
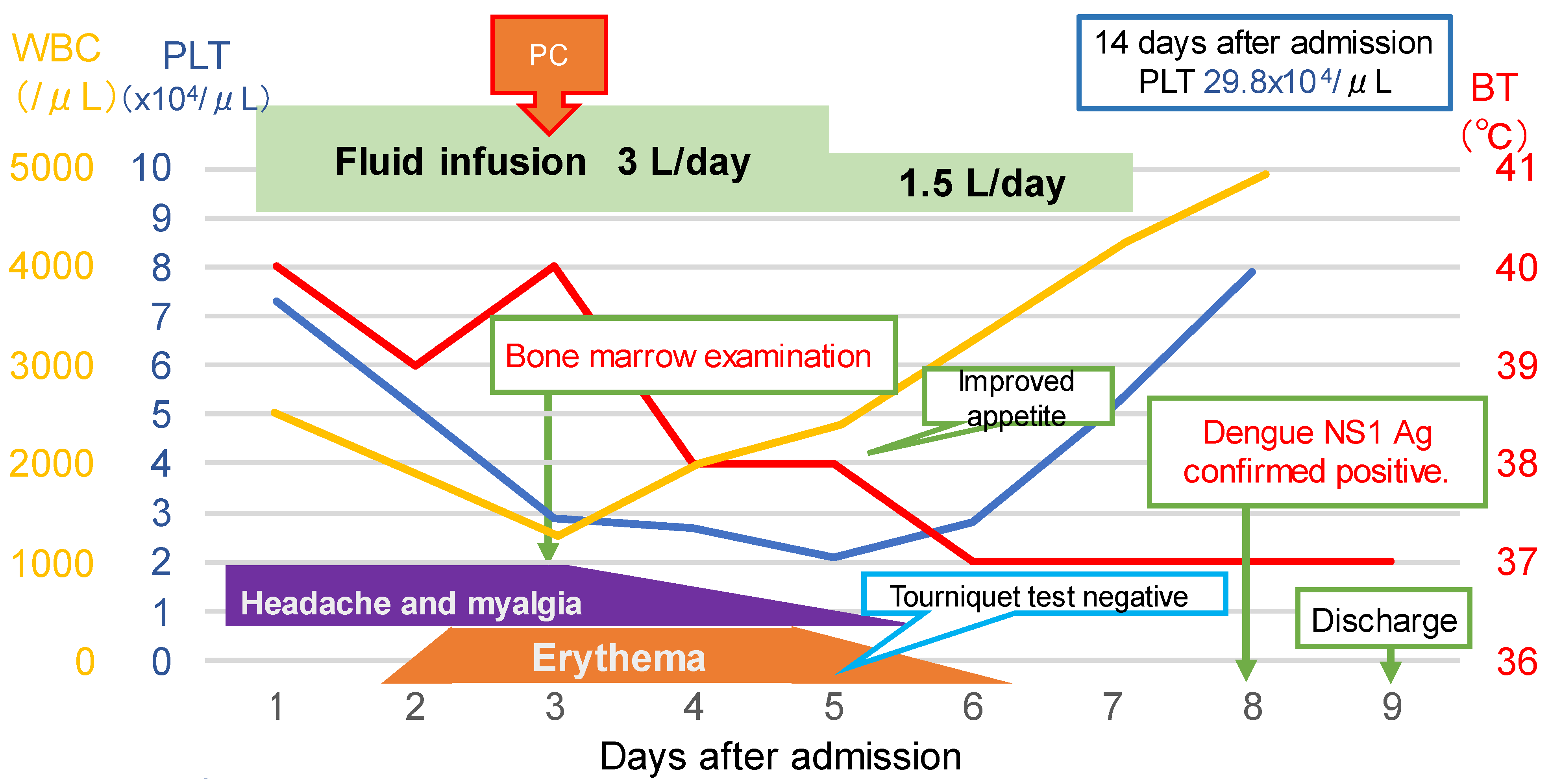
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simmons, C.P.; Farrar, J.J.; Nguyen, v.V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment. Available online: https://pubmed.ncbi.nlm.nih.gov/23762963/ (accessed on 1 September 2023).
- La Russa, V.F.; Innis, B.L. Mechanisms of dengue virus-induced bone marrow suppression. Bailliere’s Clin. Haematol. 1995, 8, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Oishi, K.; Inoue, S.; Dimaano, E.M.; Alera, M.T.; Robles, A.M.; Estrella, B.D., Jr.; Kumatori, A.; Moji, K.; Alonzo, M.T.; et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 2004, 138, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bandyopadhyay, D.; Saha, K.; Sarkar, S.; Ranjit, P.; Thiyagarajan, G.; Mondal, R.S.; Naskar, S.; Patra, S. Hemophagocytosis in dengue. Assam J. Intern. Med. 2013, 3, 27–29. [Google Scholar]
- Wan Jamaludin, W.F.; Periyasamy, P.; Wan Mat, W.R.; Abdul Wahid, S.F. Dengue infection associated hemophagocytic syndrome: Therapeutic interventions and outcome. J. Clin. Virol. 2015, 69, 91–95. [Google Scholar] [CrossRef]
- Chung, S.M.; Song, J.Y.; Kim, W.; Choi, M.J.; Jeon, J.H.; Kang, S.; Jung, E.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: A case report and literature review. Medicine 2017, 96, e6159. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Imashuku, S.; Ueda, I.; Teramura, T.; Mori, K.; Morimoto, A.; Sako, M.; Ishii, E. Occurrence of haemophagocytic lymphohistiocytosis at less than 1 year of age: Analysis of 96 patients. Eur. J. Pediatr. 2005, 164, 315–319. [Google Scholar] [CrossRef]
- Russell, J.H.; Ley, T.J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370. [Google Scholar] [CrossRef]
- Risma, K.A.; Frayer, R.W.; Filipovich, A.H.; Sumegi, J. Aberrant maturation of mutant perforin underlies the clinical diversity of hemophagocytic lymphohistiocytosis. J. Clin. Investig. 2006, 116, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Hildeman, D.; Kappler, J.; Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004, 104, 735–743. [Google Scholar] [CrossRef]
- Janka, G.E.; Schneider, E.M. Modern management of children with haemophagocytic lymphohistiocytosis. Br. J. Haematol. 2004, 124, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, R.A. Perforin deficiency: Fighting unarmed? Immunol. Today 2000, 21, 592. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L.; Moretta, A.; Hengartner, H.; Zinkernagel, R.M. On the pathogenesis of perforin defects and related immunodeficiencies. Immunol. Today 2000, 21, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Katano, H.; Cohen, J.I. Perforin and lymphohistiocytic proliferative disorders. Br. J. Haematol. 2005, 128, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Homchampa, P.; Sarasombath, S.; Suvatte, V.; Vongskul, M. Natural killer cells in dengue hemorrhagic fever/dengue shock syndrome. Asian Pac. J. Allergy Immunol. 1988, 6, 95–102. [Google Scholar]
- Matangkasombut, P.; Chan-In, W.; Opasawaschai, A.; Pongchaikul, P.; Tangthawornchaikul, N.; Vasanawathana, S.; Limpitikul, W.; Malasit, P.; Duangchinda, T.; Screaton, G.; et al. Invariant NKT cell response to dengue virus infection in human. PLOS Negl. Trop. Dis. 2014, 8, e2955. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; East, J.E.; Kimball, A.S.; Tkaczuk, K.; Kesmodel, S.; Strome, S.E.; Webb, T.J. Invariant natural killer T cells generated from human adult hematopoietic stem-progenitor cells are poly-functional. Cytokine 2015, 72, 48–57. [Google Scholar] [CrossRef]
- Ramanathan, M.; Duraisamy, G. Haemophagocytosis in dengue haemorrhagic fever: A case report. Ann. Acad. Med. Singap. 1991, 20, 803–804. [Google Scholar]
- Wong, K.F.; Chan, J.K.; Chan, J.C.; Lim, W.W.; Wong, W.K. Dengue virus infection-associated hemophagocytic syndrome. Am. J. Hematol. 1991, 38, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.L.; Hsiao, H.H.; Tsai, J.J.; Chen, T.C.; Feng, M.C.; Chen, T.P.; Lin, S.F. Dengue virus-associated hemophagocytic syndrome and dyserythropoiesis: A case report. Kaohsiung J. Med. Sci. 2005, 21, 34–39. [Google Scholar] [CrossRef]
- Srichaikul, T.; Punyagupta, S.; Kanchanapoom, T.; Chanokovat, C.; Likittanasombat, K.; Leelasiri, A. Hemophagocytic synrome in dengue hemorrhagic fever with severe multiorgan complications. J. Med. Assoc. Thai. 2008, 91, 104–109. [Google Scholar] [PubMed]
- Nakamura, I.; Nakamura-Uchiyama, F.; Komiya, N.; Ohnishi, K. A case of dengue fever with viral-associated hemophagocytic syndrome. Kansenshogaku Zasshi 2009, 83, 60–63. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Ray, S.; Kundu, S.; Saha, M.; Chakrabarti, P. Hemophagocytic syndrome in classic dengue fever. J. Glob. Infect. Dis. 2011, 3, 399–401. [Google Scholar] [CrossRef]
- Sorakhunpipitkul, L.; Punyagupta, S.; Srichaikul, T.; Tribuddharat, S. Thai adult dengue hemorrhagic fever during 2008–2010: Seven cases presented with severe multiorgan failure and successfully treated with high dose of corticosteroids and intravenous immunoglobulin G. J. Infect. Dis. Antimicrob. Agents 2011, 28, 99–103. [Google Scholar]
- Tan, L.H.; Lum, L.C.; Omar, S.F.; Kan, F.K. Hemophagocytosis in dengue: Comprehensive report of six cases. J. Clin. Virol. 2012, 55, 79–82. [Google Scholar] [CrossRef]
- Ribeiro, E.; Kassab, S.; Pistone, T.; Receveur, M.C.; Fialon, P.; Malvy, D. Primary dengue fever associated with hemophagocytic syndrome: A report of three imported cases, Bordeaux, France. Intern. Med. 2014, 53, 899–902. [Google Scholar] [CrossRef][Green Version]
- De Koninck, A.S.; Dierick, J.; Steyaert, S.; Taelman, P. Hemophagocytic lymphohistiocytosis and dengue infection: Rare case report. Acta Clin. Belg. 2014, 69, 210–213. [Google Scholar] [CrossRef]
- Sharp, T.M.; Gaul, L.; Muehlenbachs, A.; Hunsperger, E.; Bhatnagar, J.; Lueptow, R.; Santiago, G.A.; Muñoz-Jordan, J.L.; Blau, D.M.; Ettestad, P.; et al. Fatal hemophagocytic lymphohistiocytosis associated with locally acquired dengue virus infection—New Mexico and Texas, 2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 49–54. [Google Scholar]
- Arshad, U.; Ahmad, S.Q.; Khan, F. Hemophagocytic lymphohistiocytosis in a patient with dengue infection. Hematol. Oncol. Stem Cell Ther. 2015, 8, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, K.; Oshina, T.; Sonokawa, S.; Noguchi, Y.; Suzuki, S.; Tanaka, K.; Kumagai, T. Domestic dengue infection with hemophagocytic lymphohistiocytosis successfully treated by early steroid therapy. Rinsho Ketsueki 2016, 57, 864–868. (In Japanese) [Google Scholar] [CrossRef]
- Jasmine, Y.S.; Lee, S.L.; Kan, F.K. Infection associated haemophagocytic syndrome in severe dengue infection—A case series in a district hospital. Med. J. Malaysia 2017, 72, 62–64. [Google Scholar] [PubMed]
- Anam, A.M.; Rabbani, R.; Shumy, F. Expanded dengue syndrome: Three concomitant uncommon presentations in the same patient. Trop. Dr. 2017, 47, 167–170. [Google Scholar] [CrossRef] [PubMed]

| The diagnosis of HLH can be established if one of either 1 or 2 below is fulfilled. | |
| (1) A molecular diagnosis consistent with HLH | |
| (2) Diagnostic criteria for HLH fulfilled (five out of the eight criteria below): | |
| (A) Initial diagnostic criteria (to be evaluated in all patients with HLH): | |
| ✓ Fever | |
| ✓ Splenomegaly | |
| ✓ Cytopenias (affecting ≥2 of 3 lineages in the peripheral blood) | |
| Hemoglobin < 9.0 g/dL | |
| ✓ Platelets < 100 × 103/µL | |
| ✓ Neutrophils < 1.0 × 103/µL | |
| ✓ Hypertriglyceridemia and/or hypofibrinogenemia | |
| Fasting triglycerides ≥ 265 mg/dL | |
| ✓ Fibrinogen ≤ 150 mg/dL | |
| ✓ Hemophagocytosis in bone marrow or spleen or lymph nodes | |
| No evidence of malignancy | |
| (B) New diagnostic criteria: | |
| Low or absent NK cell activity (according to local laboratory reference) | |
| ✓ Ferritin ≥ 500 ng/mL | |
| Soluble interleukin-2 receptor ≥ 2.4 × 106 U/L | |
| Parameter | Criteria for Scoring |
|---|---|
| Known underlying Immunosuppression * | 0 (no) or 18 (yes) |
| Temperature (degrees of Celsius) | 0 (<38.4), 33 (38.4–39.4), or 49 (>39.4) |
| Organomegaly | 0 (no), 23 (hepatomegaly or splenomegaly), or 38 (hepatomegaly and splenomegaly) |
| No of cytopenias ** | 0 (1 lineage), 24 (2 lineages), or 34 (3 lineages) |
| Ferritin (ng/mL) | 0 (<2000), 35 (2000–6000), or 50 (>6000) |
| Triglycerides (mmoles/L) | 0 (<1.5), 44 (1.5–4), or 64 (>4) |
| Fibrinogen (gm/L) | 0 (>2.5) or 30 (≤2.5) |
| Serum glutamic oxaloacetic transaminase (IU/L) | 0 (<30) or 19 (≥30) |
| Hemophagocytosis features on bone marrow aspirate | 0 (no) or 35 (yes) |
| Parameter | Result | |
|---|---|---|
| On Admission | Day 3 before Bone Marrow Examination | |
| Known underlying immunosuppression | 0 (no) | 0 (no) |
| Temperature (degrees of Celsius) | 49 (>39.4) | 49 (>39.4) |
| Organomegaly | 38 (hepatomegaly and splenomegaly) | Unknown (not detected) |
| No of cytopenias | 24 (2 lineages) | 24 (2 lineages) |
| Ferritin (ng/mL) | Unknown (not detected) | 50 (>6000) |
| Triglycerides (mmoles/L) | Unknown (not detected) | Unknown (not detected) |
| Fibrinogen (gm/L) | Unknown (not detected) | 30 (≤2.5) |
| Serum glutamic oxaloacetic transaminase (IU/L) | 19 (≥30) | Unknown |
| Hemophagocytosis features on bone marrow aspirate | Unknown (not detected) | Unknown (not yet detected) |
| HScore | 130 | 153 |
| Probability of having HS (%) | 8.9 | 28.8 |
| Ref. | Year | Age (y) | Sex | Nationality | Epidemicin Country | Fever Duration (Days) | Hospital Visit since Fever Onset (days) | Infection Type | Virus Serotype | Underlying Diseases | Treatment for HLH | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [21] | 1991 | 19 | Female | Singapore | Yes | − | − | − | − | − | Symptomatic | Alive |
| [22] | 1991 | 53 | Male | China | Yes | − | − | Primary | 3 | − | Symptomatic | Alive |
| [23] | 2005 | 33 | Male | China | Yes | 10 | 4 | Primary | − | − | Symptomatic | Alive |
| [24] | 2008 | 46 | Female | Thailand | Yes | 4 | − | Secondary | 2 | None | MP, IVIg | Alive |
| [25] | 2009 | 32 | Female | Japan | No | 7 | 3 | Primary | − | None | Symptomatic | Alive |
| [26] | 2011 | 24 | Female | India | Yes | 7 | 4 | Primary | − | None | Dexa | Alive |
| [27] | 2011 | 22 | Female | Thailand | Yes | − | − | − | − | None | Dexa, MP, IVIg | Alive |
| [27] | 2011 | 43 | Female | Thailand | Yes | − | − | − | − | None | Dexa, MP, IVIg | Alive |
| [27] | 2011 | 45 | Female | Thailand | Yes | − | − | − | − | None | Dexa, IVIg | Died |
| [27] | 2011 | 65 | Male | Thailand | Yes | − | − | − | − | None | Dexa, IVIg | Died |
| [28] | 2012 | 43 | Female | Malaysia | Yes | 6 | 6 | Secondary | − | Diabetes | MP | Died |
| [28] | 2012 | 34 | Male | Malaysia | Yes | 4 | 4 | Primary | − | None | MP | Alive |
| [28] | 2012 | 36 | Female | Malaysia | Yes | 7 | 7 | Secondary | − | None | MP | Alive |
| [28] | 2012 | 20 | Male | Malaysia | Yes | 4 | 4 | Primary | − | G6PD deficiency | Symptomatic | Alive |
| [5] | 2013 | 22 | Female | India | Yes | 7 | − | Primary | − | None | Dexa | Alive |
| [29] | 2014 | 44 | Female | France | No | − | − | Primary | − | − | Steroid | Alive |
| [29] | 2014 | 38 | Male | France | No | ≤14 | 3 | Primary | 1 | − | Dexa, IVIg | Alive |
| [29] | 2014 | 25 | Female | France | No | ≤7 | − | Primary | − | − | MP | Alive |
| [30] | 2014 | 21 | Female | Belgium | No | 3 | 3 | Secondary | 3 | None | IVIg | Alive |
| [31] | 2014 | 63 | Female | USA | Yes | 1 | 1 | Primary | 3 | Crohn’s disease, CAD | IFN | Died |
| [6] | 2015 | 32 | Male | Malaysia | Yes | 9 | 5 | Primary | − | None | Dexa, IVIg | Alive |
| [6] | 2015 | 19 | Male | Malaysia | Yes | 5 | 4 | Secondary | − | None | Dexa | Alive |
| [32] | 2015 | 26 | Male | Pakistan | No | 17 | 14 | − | − | − | Symptomatic | Died |
| [33] | 2016 | 34 | Male | Japan | No | 6 | 0 | Primary | − | Cholesteatoma | MP | Alive |
| [7] | 2017 | 33 | Female | Korea | No | 8 | 3 | Primary | 3 | None | Dexa | Alive |
| [34] | 2017 | 56 | Male | China | Yes | 8 | 6 | − | − | − | MP | Alive |
| [34] | 2017 | 35 | Female | Malaysia | Yes | − | 3 | − | − | Obesity | MP | Alive |
| [35] | 2017 | 44 | Male | Bangladesh | Yes | ≤18 | 4 | − | − | None | Symptomatic | Alive |
| Present case | 2020 | 47 | Male | Japan | No | 6 | 3 | − | − | Diabetes, reflux esophagitis | Symptomatic | Alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, N.; Kenzaka, T.; Nishisaki, H. Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care. Trop. Med. Infect. Dis. 2023, 8, 497. https://doi.org/10.3390/tropicalmed8110497
Mizutani N, Kenzaka T, Nishisaki H. Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care. Tropical Medicine and Infectious Disease. 2023; 8(11):497. https://doi.org/10.3390/tropicalmed8110497
Chicago/Turabian StyleMizutani, Naoya, Tsuneaki Kenzaka, and Hogara Nishisaki. 2023. "Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care" Tropical Medicine and Infectious Disease 8, no. 11: 497. https://doi.org/10.3390/tropicalmed8110497
APA StyleMizutani, N., Kenzaka, T., & Nishisaki, H. (2023). Dengue Fever Complicated with Hemophagocytic Lymphohistiocytosis: A Case Report of Resolution with Steroid-Sparing Supportive Care. Tropical Medicine and Infectious Disease, 8(11), 497. https://doi.org/10.3390/tropicalmed8110497






