Using Regional Sero-Epidemiology SARS-CoV-2 Anti-S Antibodies in the Dominican Republic to Inform Targeted Public Health Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Data Source, Study Design, and Study Procedures
2.3. Statistical Analysis
2.3.1. Anti-S Prevalence
2.3.2. Correlates of Protective Immunity against Symptomatic Infection
2.3.3. Logistic Regression
- A national multivariable model that includes the ten regions (administrative divisions) as a covariate, as well as the sociodemographic covariates indicated above. The weights, calculated based on the survey design, were incorporated into the regression. This model was built to assess differences in ORs between regions.
- Multilevel survey-weighted mixed effects logistic regression model fitted at national and regional levels:
- 2.1
- The national level included region, cluster, and household as random effects, gender, age, area of residence, number of household members, work environment, smoking status, educational level, socioeconomic score, comorbidities/risk factors, and number of vaccine doses as fixed effects. To account for the sampling design, the weights of the selection probability were calculated in three stages. First, the probability of a cluster being selected (pc) was calculated based on the total number of clusters in each category and the weight (wc) was the inverse of the probability of selection for each category (wc = 1/pc). Second, the probability of a household being selected (ph) was calculated based on the total number of households in each cluster and the weight (wh) was the inverse of the probability of household selection (wh = 1/ph). Third, the weights (wf) from the first two steps were multiplied (wf = wc × wp) and corrected for a finite population. The full description of the weight calculation can be found in the Supplementary Materials.
- 2.2
- The regional level included cluster and household as random effects, gender, age, area of residence, number of household members, work environment, smoking status, educational level, socioeconomic score, comorbidities/risk factors, and number of vaccine doses as fixed effects, and incorporated level-specific sampling weights to account for sampling design. This model was built to identify variations in strength and significance of association across regions. Due to sample size, Cibao Sur was combined with Cibao Nordeste, and El Valle with Cibao Noroeste.
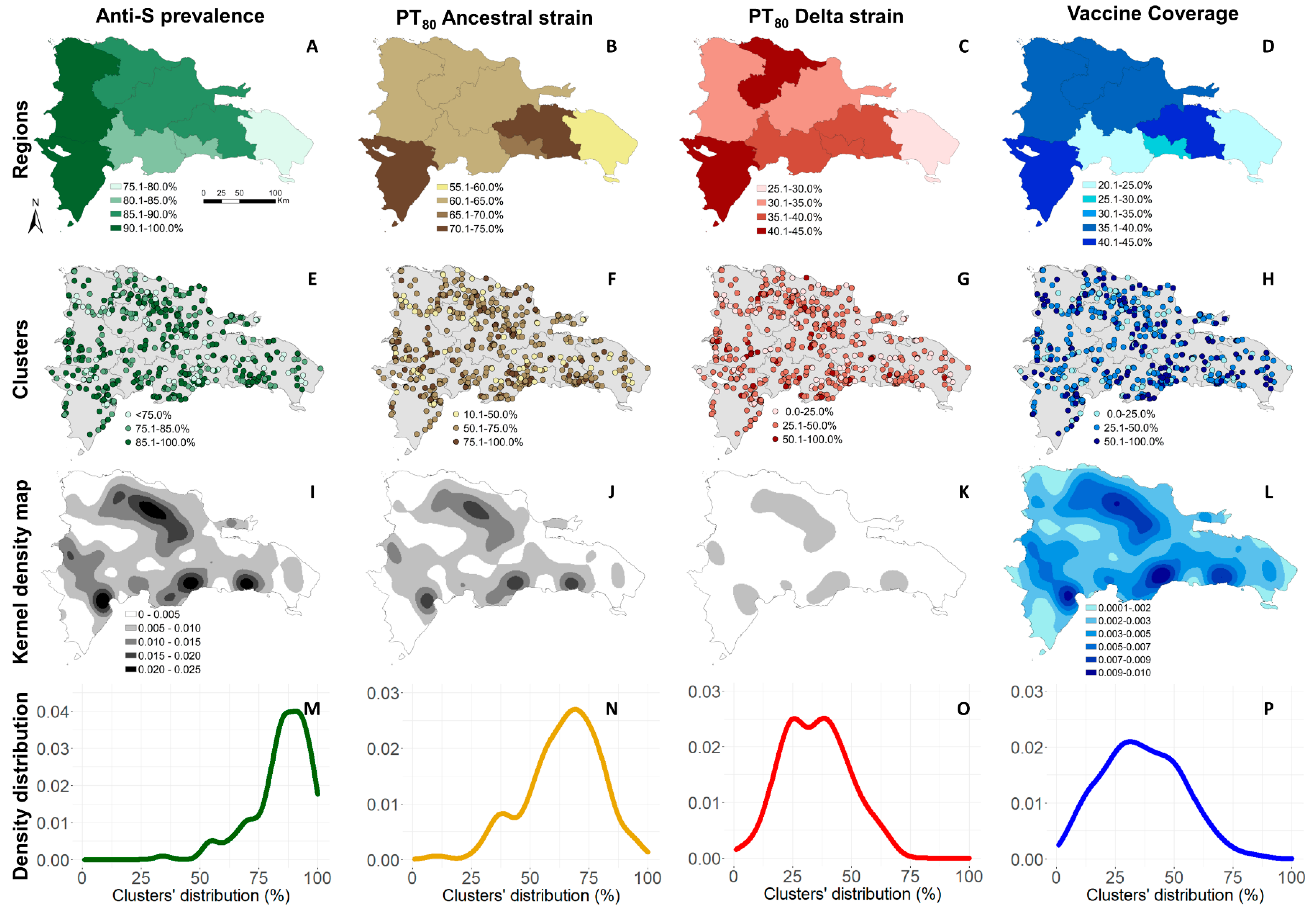
2.3.4. Kernel Density Maps
3. Results
3.1. Participants and Demographics
3.2. Anti-S Seroprevalence and PT80 for the Ancestral and Delta Strains
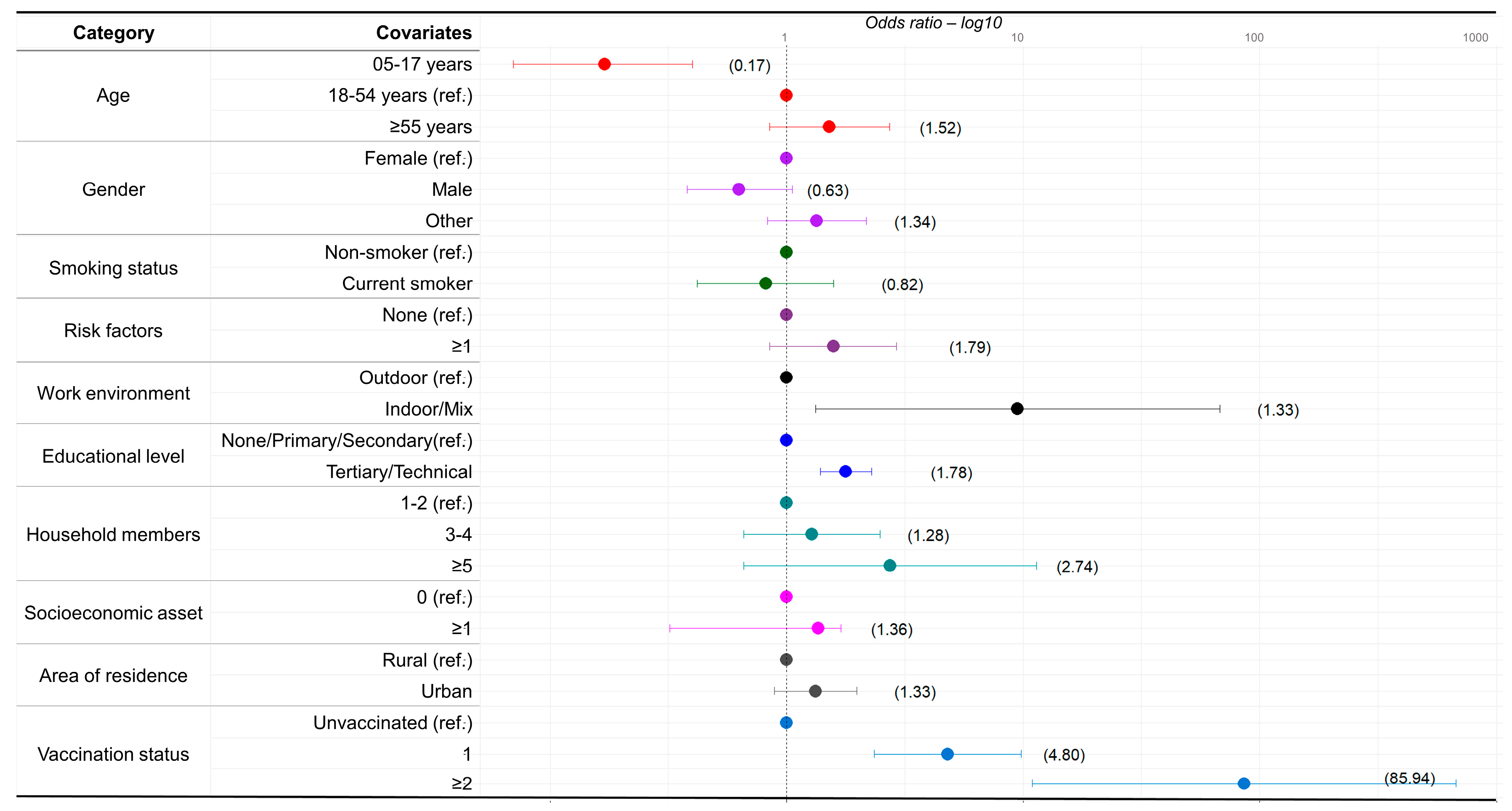
3.3. Logistic Regression
3.3.1. Multivariable Model for Anti-S, and PT80 for the Ancestral and Delta Strains
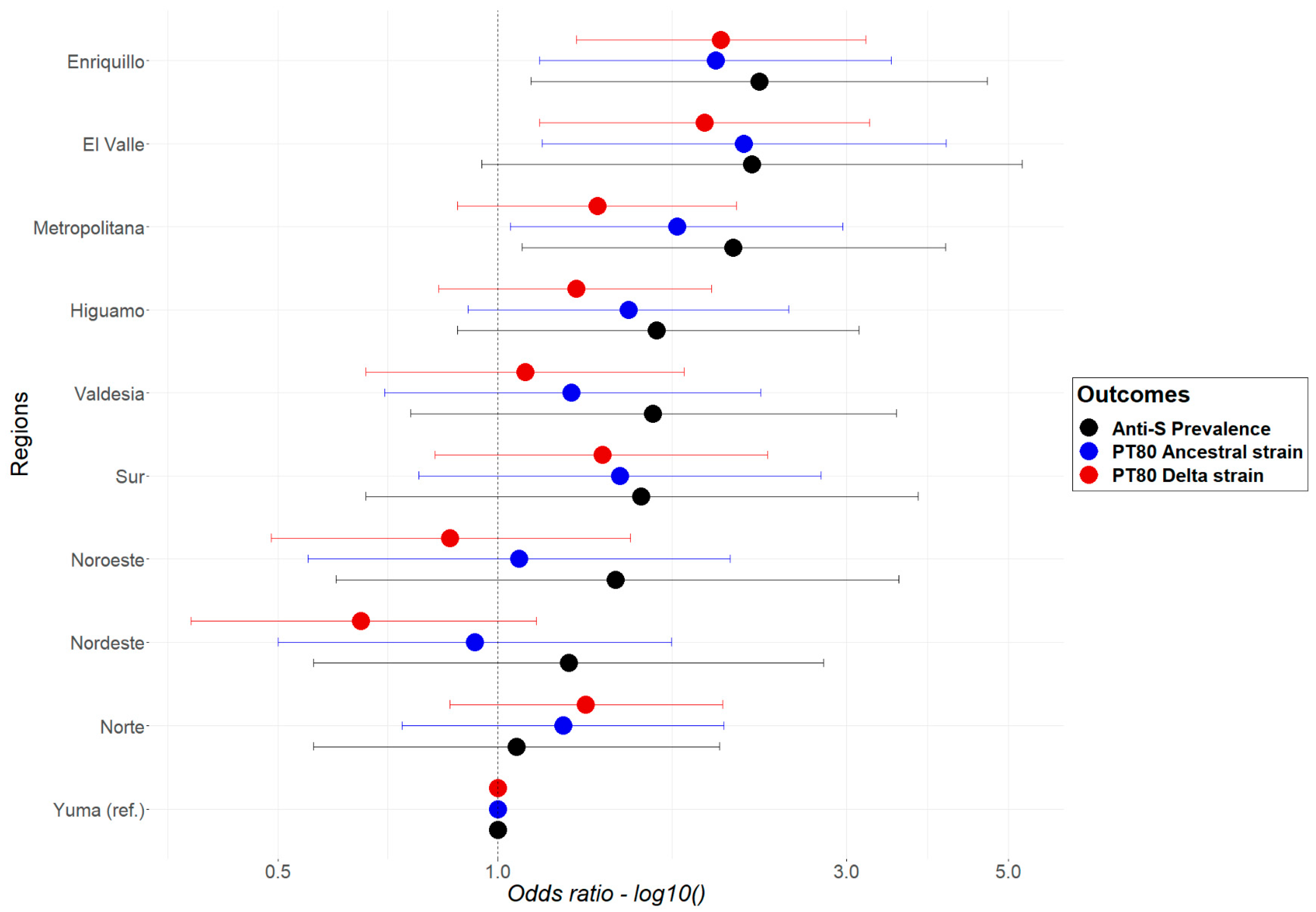
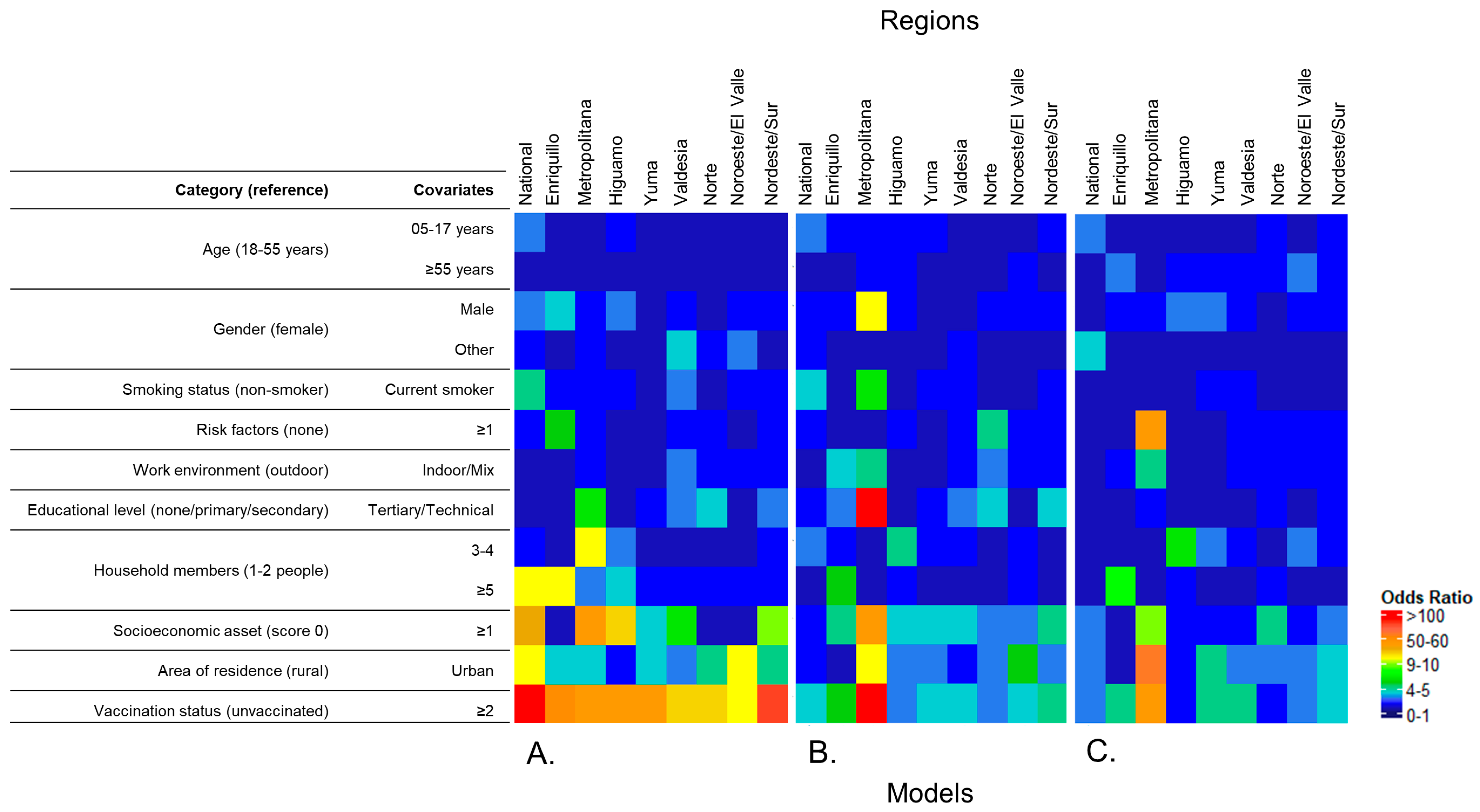
3.3.2. Multilevel Logistic Regression Models
National Models
Regional Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burki, T.K. COVID-19 in the Caribbean. Lancet Respir. Med. 2021, 9, e46. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; Meyer, P.J. Latin America and the Caribbean: Impact of COVID-19; In focus; Congressional Research Service Reports: Washington, DC, USA, 2022. [Google Scholar]
- Worldometers.info. Available online: https://www.worldometers.info/coronavirus/ (accessed on 24 October 2021).
- Paulino-Ramírez, R.; López, P.; Mueses, S.; Cuevas, P.; Jabier, M.; Rivera-Amill, V. Genomic Surveillance of SARS-CoV-2 Variants in the Dominican Republic and Emergence of a Local Lineage. Int. J. Environ. Res. Public Health 2023, 20, 5503. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Mumtaz, G.R.; Seedat, S.; Makhoul, M.; Chemaitelly, H.; Abu-Raddad, L.J. Estimates of global SARS-CoV-2 infection exposure, infection morbidity, and infection mortality rates in 2020. Glob. Epidemiol. 2021, 3, 100068. [Google Scholar] [CrossRef] [PubMed]
- Nilles, E.J.; Paulino, C.T.; Aubin, M.d.S.; Restrepo, A.C.; Mayfield, H.; Dumas, D.; Finch, E.; Garnier, S.; Etienne, M.C.; Iselin, L.; et al. SARS-CoV-2 seroprevalence, cumulative infections, and immunity to symptomatic infection—A multistage national household survey and modelling study, Dominican Republic, June–October 2021. Lancet Reg. Health. Am. 2022, 16, 100390. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Perez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Abate, D.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Janke, A.T.; Mei, H.; Rothenberg, C.; Becher, R.D.; Lin, Z.; Venkatesh, A.K. Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States. J. Hosp. Med. 2021, 16, 211–214. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chang, A.; Chiang, T.P.Y.; Massie, A.B.; Segev, D.L.; Makary, M.A. Prevalence and Durability of SARS-CoV-2 Antibodies Among Unvaccinated US Adults by History of COVID-19. JAMA 2022, 327, 1085. [Google Scholar] [CrossRef]
- Ferreira, C.E.d.S.; Gómez-Dantés, H.; Bellei, N.C.J.; López, E.; Crespo, K.A.N.; O’ryan, M.; Villegas, J. The Role of Serology Testing in the Context of Immunization Policies for COVID-19 in Latin American Countries. Viruses 2021, 13, 2391. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; di Ruffano, L.F.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef]
- Wu, T. The socioeconomic and environmental drivers of the COVID-19 pandemic: A review. AMBIO 2021, 50, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.; Wang, J.; Qiao, J.; Yan, M.; Zhu, Q. Spatiotemporal heterogeneity and its determinants of COVID-19 transmission in typical labor export provinces of China. BMC Infect. Dis. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Gunzler, D.D.; Jain, V.; Perzynski, A.T.; Dawson, N.V.; Einstadter, D.; Tarabichi, Y.; Imrey, P.B.; Lewis, M.; Kattan, M.W.; et al. Mechanisms of socioeconomic differences in COVID-19 screening and hospitalizations. PLoS ONE 2021, 16, e0255343. [Google Scholar] [CrossRef]
- Martin, C.A.; Jenkins, D.R.; Minhas, J.S.; Gray, L.J.; Tang, J.; Williams, C.; Sze, S.; Pan, D.; Jones, W.; Verma, R.; et al. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: Results from an observational cohort study. eClinicalMedicine 2020, 25, 100466. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Heap, S.; Crerar-Gilbert, A.; Albuquerque, W.; Haywood, S.; Avila, Z.; Hartopp, R.; Ball, J.; Hutt, K.; Kennea, N. Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: A hospital case note review. BMJ Open 2020, 10, e040638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Wen, H.; Shi, F.; Wang, F.; Zhao, Y.; Hong, Q.; Yu, C. What matters: Non-pharmaceutical interventions for COVID-19 in Europe. Antimicrob. Resist. Infect. Control. 2022, 11, 1–9. [Google Scholar] [CrossRef]
- Portal del SICA. Decretos y Medidas Adoptadas por República Dominicana. Available online: https://www.sica.int/coronavirus (accessed on 11 June 2022).
- Gurram, M.K.; Wang, M.X.; Wang, Y.-C.; Pang, J. Impact of urbanisation and environmental factors on spatial distribution of COVID-19 cases during the early phase of epidemic in Singapore. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Oficina Nacional de Estadistica. MapSession 2022. Available online: One.gob.do/#mapSeccion (accessed on 26 October 2022).
- Oficina Nacional de Estadistica. Division Territorial 2021-Cratograficas DdGDdO. 2020. Available online: One.gob.do/#mapSeccion (accessed on 26 October 2022).
- World Bank–microdata library. Dominican Republic-World Bank Group Country Survey (WBCS) 2019. Available online: https://microdata.worldbank.org/index.php/catalog/3407 (accessed on 6 November 2022).
- United Nations–Department of Economics and Social Affairs/Statistics. Available online: https://unstats.un.org/unsd/demographic-social/products/dyb/documents/dyb2017/table07.pdf (accessed on 6 November 2022).
- Diggle, P.; Lophaven, S. Bayesian Geostatistical Design. Scand. J. Stat. 2006, 33, 53–64. [Google Scholar] [CrossRef]
- Nilles, E.J.; Aubin, M.D.S.; Dumas, D.; Duke, W.; Etienne, M.C.; Abdalla, G.; Paulino, C.T. Integrated SARS-CoV-2 serological and virological screening across an acute fever surveillance platform to monitor temporal changes in anti-spike antibody levels and risk of infection during sequential waves of variant transmission—Dominican Republic, March 2021 to August 2022. medRxiv 2022. [Google Scholar] [CrossRef]
- Ainsworth, M.; Andersson, M.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Beveridge, A.; Bibi, S.; Blackwell, L.; Borak, M.; et al. Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Nilles, E.J.; Karlson, E.W.; Norman, M.; Gilboa, T.; Fischinger, S.; Atyeo, C.; Zhou, G.; Bennett, C.L.; Tolan, N.V.; Oganezova, K.; et al. Evaluation of Three Commercial and Two Non-Commercial Immunoassays for the Detection of Prior Infection to SARS-CoV-2. J. Appl. Lab. Med. 2021, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Seroepidemiology Technical Working Group ROSES-S: Statement from the World Health Organization on the reporting of seroepidemiologic studies for SARS-CoV-2. Influ. Other Respir. Viruses 2021, 15, 561–568. [CrossRef] [PubMed]
- A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC.: College Station, TX, USA, 2023. [Google Scholar]
- Rabe-Hesketh, S.; Pickles, A.; Taylor, C. Generalized linear latent and mixed models. Stata Tech. Bull. 2000, 53, 49–57. [Google Scholar]
- ESRI. ArcMap 2022. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 10 November 2022).
- Paulino-Ramirez, R.; Báez, A.A.; Vallejo Degaudenzi, A.; Tapia, L. Seroprevalence of Specific Antibodies against SARS-CoV-2 from Hotspot Codmmunities in the Dominican Republic. Am. J. Trop. Med. Hyg. 2020, 103, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Lessler, J.; Eckerle, I.; Lauer, S.A.; Kaiser, L.; Vuilleumier, N.; Cummings, D.A.T.; Flahault, A.; Petrovic, D.; Guessous, I.; et al. Insights into household transmission of SARS-CoV-2 from a population-based serological survey. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Household Transmission of SARS-CoV-2. JAMA Netw. Open 2020, 3, e2031756. [Google Scholar] [CrossRef]
- Lelieveld, J.; Helleis, F.; Borrmann, S.; Cheng, Y.; Drewnick, F.; Haug, G.; Klimach, T.; Sciare, J.; Su, H.; Pöschl, U. Model Calculations of Aerosol Transmission and Infection Risk of COVID-19 in Indoor Environments. Int. J. Environ. Res. Public Health 2020, 17, 8114. [Google Scholar] [CrossRef]
- Chen, B.; Jia, P.; Han, J. Role of indoor aerosols for COVID-19 viral transmission: A review. Environ. Chem. Lett. 2021, 19, 1953–1970. [Google Scholar] [CrossRef]
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 1–16. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2020, 223, 550–561. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Jaafarzadeh, N.; Martínez, S.S.; Mirzaee, S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2020, 193, 110612. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Choe, Y.J.; Lim, D.S.; Lee, H.R.; Kim, J.; Kim, Y.-Y.; Kim, R.K.; Jang, E.J.; Lee, S.; Park, E.; et al. Impact of national COVID-19 vaccination Campaign, South Korea. Vaccine 2022, 40, 3670–3675. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Nilles, E.J.; Paulino, C.T.; Aubin, M.d.S.; Duke, W.; Jarolim, P.; Sanchez, I.M.; O Murray, K.; Lau, C.L.; Gutiérrez, E.Z.; Ramm, R.S.; et al. Tracking immune correlates of protection for emerging SARS-CoV-2 variants. Lancet Infect. Dis. 2023, 23, 153–154. [Google Scholar] [CrossRef]
- Li, X.-N.; Huang, Y.; Wang, W.; Jing, Q.-L.; Zhang, C.-H.; Qin, P.-Z.; Guan, W.-J.; Gan, L.; Li, Y.-L.; Liu, W.-H.; et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case–control real-world study. Emerg. Microbes Infect. 2021, 10, 1751–1759. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Eng. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Xie, F.; Ackerson, B.K.; McLaughlin, J.M. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: A test-negative case–control study. Lancet Respir. Med. 2022, 10, 689–699. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639. [Google Scholar] [CrossRef]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of mRNA Vaccines against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Moriyama, S.; Adachi, Y.; Sato, T.; Tonouchi, K.; Sun, L.; Fukushi, S.; Yamada, S.; Kinoshita, H.; Nojima, K.; Kanno, T.; et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 2021, 54, 1841–1852.e4. [Google Scholar] [CrossRef] [PubMed]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Kreutmair, S.; Kauffmann, M.; Unger, S.; Ingelfinger, F.; Núñez, N.G.; Alberti, C.; De Feo, D.; Krishnarajah, S.; Friebel, E.; Ulutekin, C.; et al. Preexisting comorbidities shape the immune response associated with severe COVID-19. J. Allergy Clin. Immunol. 2022, 150, 312–324. [Google Scholar] [CrossRef]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Dozier, A.M. Tobacco use in the Dominican Republic: Understanding the culture first. Tob. Control 2006, 15, i30–i36. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.; Ogun, O.A.; Simmons, S.; Zamparo, J.M.; et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect. Dis. 2022. [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]

| Yuma 330 (%) | Nordeste/Sur 533 (%) | Valdesia 489 (%) | Noroeste/El Valle 602 (%) | Norte 1306 (%) | Higuamo 1717 (%) | Metropolitana 999 (%) | Enriquillo 707 (%) | National 6683 (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | |||||||||
| Female | 128 (38.8) | 185 (34.7) | 188 (38.4) | 234 (38.9) | 489 (37.4) | 602 (35.1) | 391 (39.1) | 277 (39.2) | 2512 (37.6) |
| Male | 198 (60) | 347 (65.1) | 295 (60.3) | 367 (61) | 811 (62.1) | 1099 (64.0) | 598 (59.9) | 429 (60.7) | 4171 (62.4) |
| Other | 4 (1.2) | 1 (0.2) | 6 (1.2) | 1 (0.2) | 6 (0.5) | 16 (0.9) | 10 (1.0) | 1 (0.1) | 45 (0.7) |
| Age | |||||||||
| 05–17 y | 47 (14.2) | 25 (4.7) | 62 (12.7) | 78 (13.0) | 120 (9.2) | 274 (16.0) | 164 (16.4) | 142 (20.1) | 912 (13.6) |
| 18–54 y | 183 (55.5) | 295 (55.3) | 286 (58.5) | 328 (54.5) | 745 (57.0) | 1007 (58.6) | 553 (55.4) | 398 (56.3) | 3975 (59.5) |
| >55 y | 100 (30.3) | 213 (40) | 141 (28.8) | 196 (32.6) | 441 (33.8) | 436 (25.4) | 282 (28.2) | 167 (23.6) | 1976 (29.6) |
| Educational level | |||||||||
| None/Primary/Secondary | 288 (87.3) | 390 (73.2) | 343 (70.1) | 438 (72.8) | 1059 (81.1) | 1406 (81.9) | 733 (73.4) | 539 (76.2) | 5196 (77.7) |
| Tertiary/Technical | 42 (12.7) | 143 (26.8) | 146 (29.9) | 164 (27.2) | 247 (18.9) | 311 (18.1) | 266 (26.6) | 168 (23.8) | 1487 (22.3) |
| Socioeconomic score | |||||||||
| 0 pts | 33 (10.0) | 6 (1.1) | 6 (1.2) | 18 (3.0) | 26 (2.0) | 81 (4.7) | 37 (3.7) | 78 (11.0) | 285 (4.3) |
| 1–5 pts | 294 (89.1) | 521 (97.7) | 474 (96.9) | 581 (96.5) | 1263 (96.7) | 1624 (94.6) | 952 (95.3) | 628 (88.8) | 6337 (94.8) |
| Area of residence | |||||||||
| Rural | 169 (51.2) | 245 (46.0) | 259 (53.0) | 288 (47.8) | 732 (56.0) | 729 (42.5) | 434 (43.4) | 230 (32.5) | 3086 (46.2) |
| Urban | 161 (48.8) | 288 (54.0) | 230 (47.0) | 560 (93.0) | 574 (44.0) | 988 (57.5) | 565 (56.6) | 477 (67.5) | 3597 (53.8) |
| Household members | |||||||||
| 1–2p | 64 (19.4) | 163 (30.6) | 57 (11.7) | 106 (17.6) | 335 (25.7) | 373 (21.7) | 210 (21.0) | 119 (16.8) | 1427 (21.4) |
| 3–4p | 133 (40.3) | 232 (43.5) | 206 (42.1) | 250 (41.5) | 579 (44.3) | 716 (41.7) | 407 (40.7) | 253 (35.8) | 2776 (41.5) |
| >5p | 133 (40.3) | 138 (25.9) | 226 (46.2) | 246 (40.9) | 392 (30.0) | 628 (36.6) | 382 (38.2) | 335 (47.4) | 2480 (37.1) |
| Work environment | |||||||||
| Indoor/Mix | 63 (19.1) | 167 (31.3) | 95 (19.4) | 141 (23.4) | 382 (29.2) | 315 (18.3) | 233 (23.3) | 135 (19.1) | 1531 (22.9) |
| Outdoor | 38 (11.5) | 38 (7.1) | 53 (10.8) | 52 (8.6) | 68 (5.2) | 123 (7.2) | 90 (9.0) | 65 (9.2) | 527 (7.9) |
| Smoking status | |||||||||
| Current smoker | 28 (8.5) | 43 (8.1) | 45 (9.2) | 41 (6.8) | 90 (6.9) | 98 (5.7) | 70 (7.0) | 54 (7.6) | 469 (7.0) |
| Non-smoker | 302 (91.5) | 490 (91.9) | 444 (90.8) | 561 (93.2) | 1216 (93.1) | 1619 (94.3) | 929 (93.0) | 653 (92.4) | 6214 (93.0) |
| Risk factors | |||||||||
| None | 189 (57.3) | 253 (47.5) | 278 (56.9) | 331 (55.0) | 663 (50.8) | 1011 (58.9) | 578 (57.9) | 440 (62.2) | 3743 (56.0) |
| >1 | 141 (42.7) | 280 (52.5) | 211 (43.1) | 271 (45.0) | 643 (49.2) | 706 (41.1) | 421 (42.1) | 267 (37.8) | 2940 (44.0) |
| Vaccine doses | |||||||||
| Unvaccinated | 182 (55.2) | 186 (34.9) | 222 (45.4) | 213 (35.4) | 398 (30.5) | 691 (40.2) | 406 (40.6) | 278 (39.3) | 2576 (38.5) |
| 1 | 53 (16.1) | 123 (23.1) | 81 (16.6) | 90 (15.0) | 176 (13.5) | 213 (12.4) | 143 (14.3) | 73 (10.3) | 952 (14.2) |
| >2 | 95 (28.8) | 224 (42.0) | 186 (38.0) | 299 (49.7) | 732 (56.0) | 813 (47.4) | 450 (45.0) | 356 (50.4) | 3155 (47.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mario Martin, B.; Cadavid Restrepo, A.; Mayfield, H.J.; Then Paulino, C.; De St Aubin, M.; Duke, W.; Jarolim, P.; Zielinski Gutiérrez, E.; Skewes Ramm, R.; Dumas, D.; et al. Using Regional Sero-Epidemiology SARS-CoV-2 Anti-S Antibodies in the Dominican Republic to Inform Targeted Public Health Response. Trop. Med. Infect. Dis. 2023, 8, 493. https://doi.org/10.3390/tropicalmed8110493
Mario Martin B, Cadavid Restrepo A, Mayfield HJ, Then Paulino C, De St Aubin M, Duke W, Jarolim P, Zielinski Gutiérrez E, Skewes Ramm R, Dumas D, et al. Using Regional Sero-Epidemiology SARS-CoV-2 Anti-S Antibodies in the Dominican Republic to Inform Targeted Public Health Response. Tropical Medicine and Infectious Disease. 2023; 8(11):493. https://doi.org/10.3390/tropicalmed8110493
Chicago/Turabian StyleMario Martin, Beatris, Angela Cadavid Restrepo, Helen J. Mayfield, Cecilia Then Paulino, Micheal De St Aubin, William Duke, Petr Jarolim, Emily Zielinski Gutiérrez, Ronald Skewes Ramm, Devan Dumas, and et al. 2023. "Using Regional Sero-Epidemiology SARS-CoV-2 Anti-S Antibodies in the Dominican Republic to Inform Targeted Public Health Response" Tropical Medicine and Infectious Disease 8, no. 11: 493. https://doi.org/10.3390/tropicalmed8110493
APA StyleMario Martin, B., Cadavid Restrepo, A., Mayfield, H. J., Then Paulino, C., De St Aubin, M., Duke, W., Jarolim, P., Zielinski Gutiérrez, E., Skewes Ramm, R., Dumas, D., Garnier, S., Etienne, M. C., Peña, F., Abdalla, G., Lopez, B., de la Cruz, L., Henríquez, B., Baldwin, M., Sartorius, B., ... Lau, C. L. (2023). Using Regional Sero-Epidemiology SARS-CoV-2 Anti-S Antibodies in the Dominican Republic to Inform Targeted Public Health Response. Tropical Medicine and Infectious Disease, 8(11), 493. https://doi.org/10.3390/tropicalmed8110493









