Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Occurrence Data
2.2. Climate Data
2.3. Ecological Niche Model
2.4. Model Construction
2.4.1. Model Construction under Current Climate Conditions
2.4.2. Model Construction under the SSP1-2.6 Climate Scenario
2.4.3. Model Evaluation
3. Results
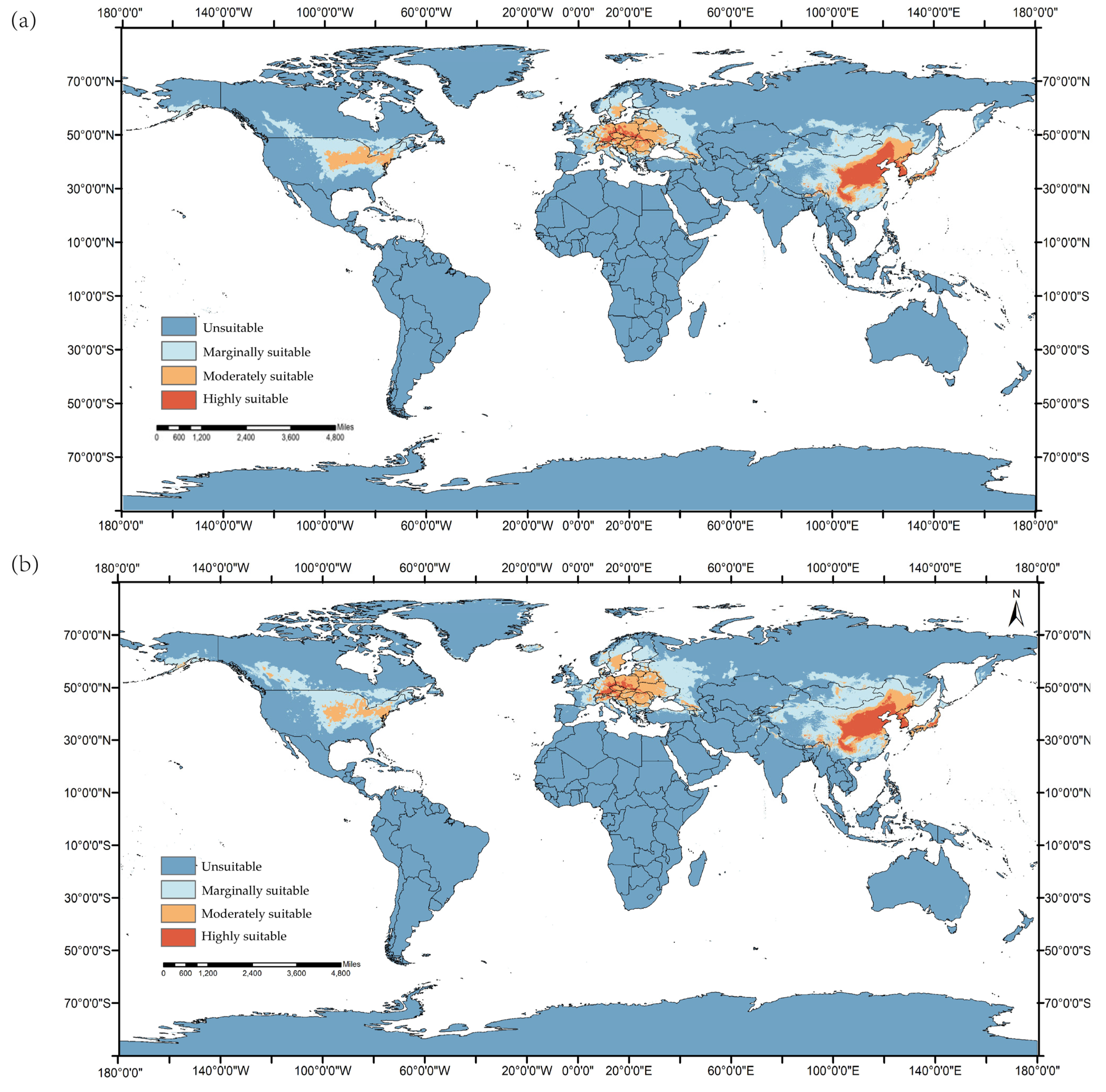
3.1. Global Predicted Suitable Areas under the Current Climatic Conditions for Ae. koreicus
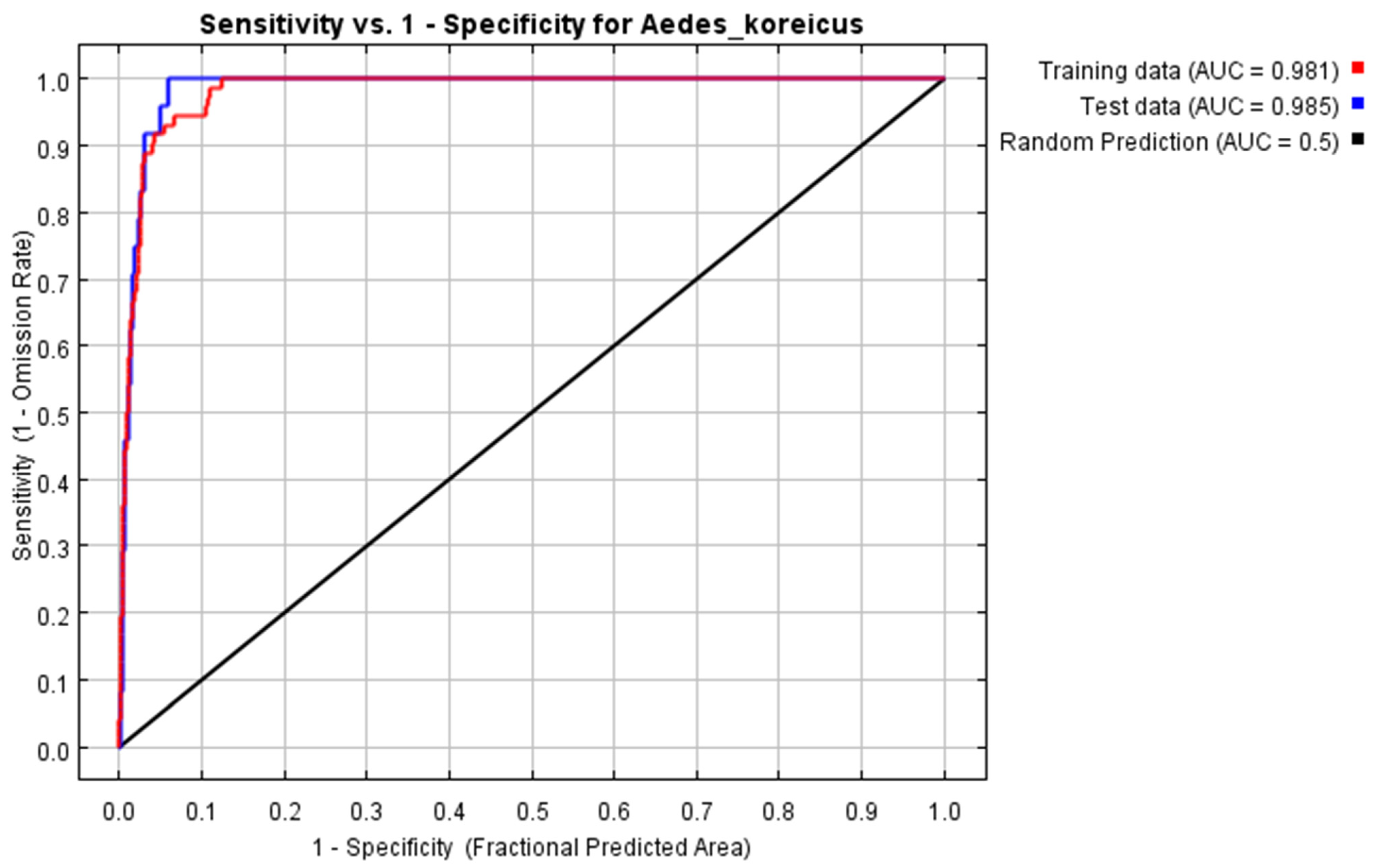
3.2. Model Evaluation
3.3. Global Predicted Suitable Areas the SSP1-2.6 Climate Scenario for Ae. koreicus
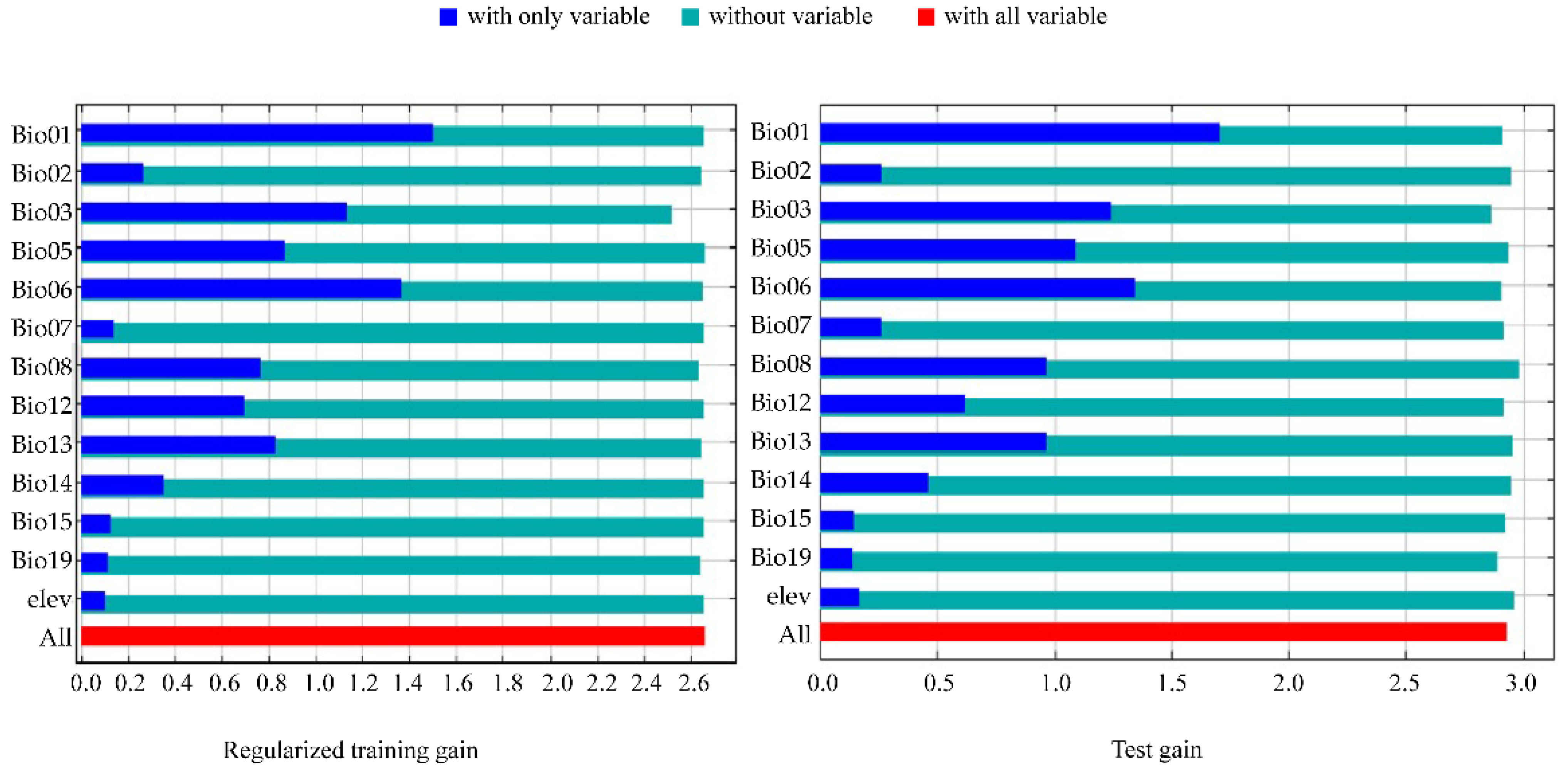
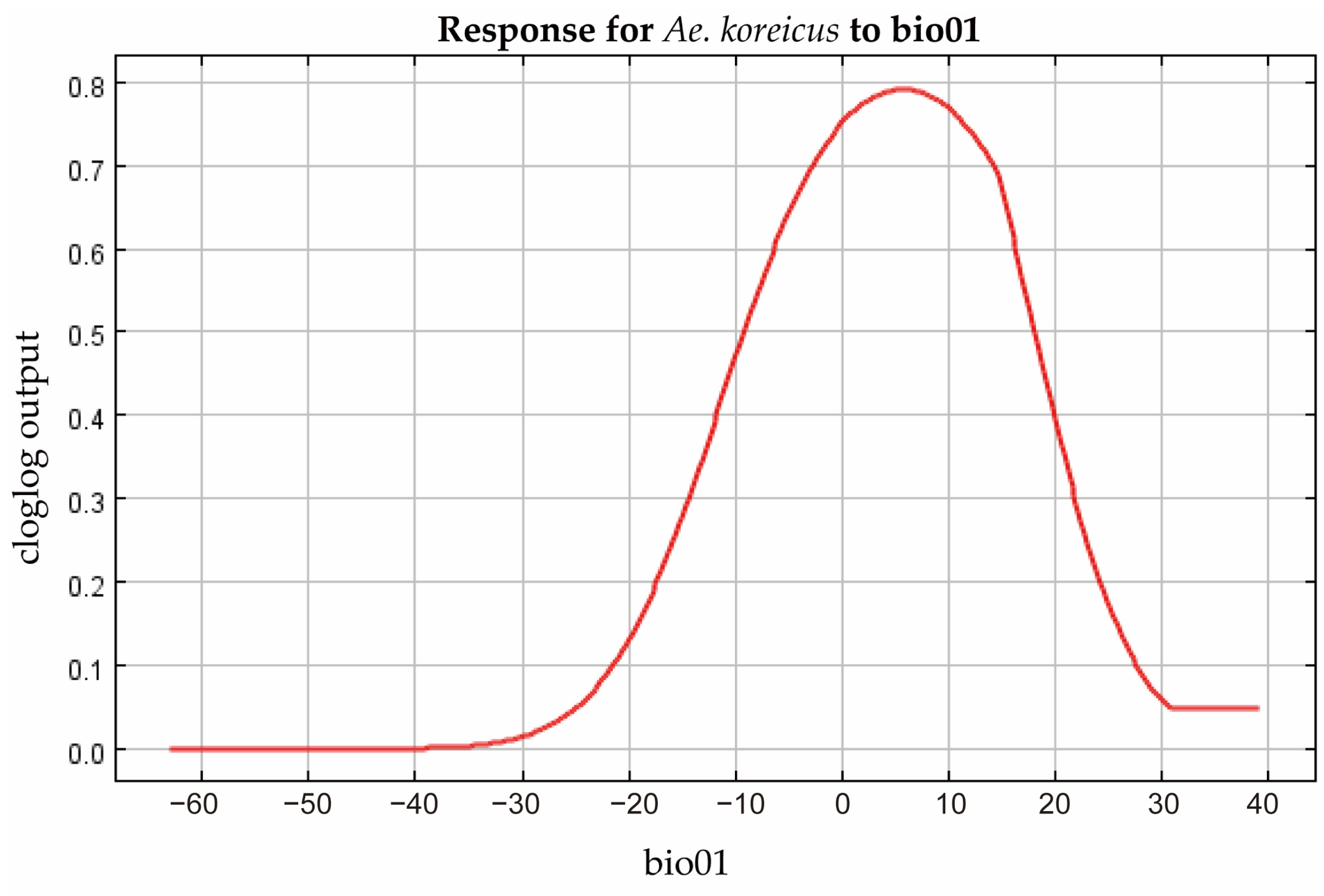
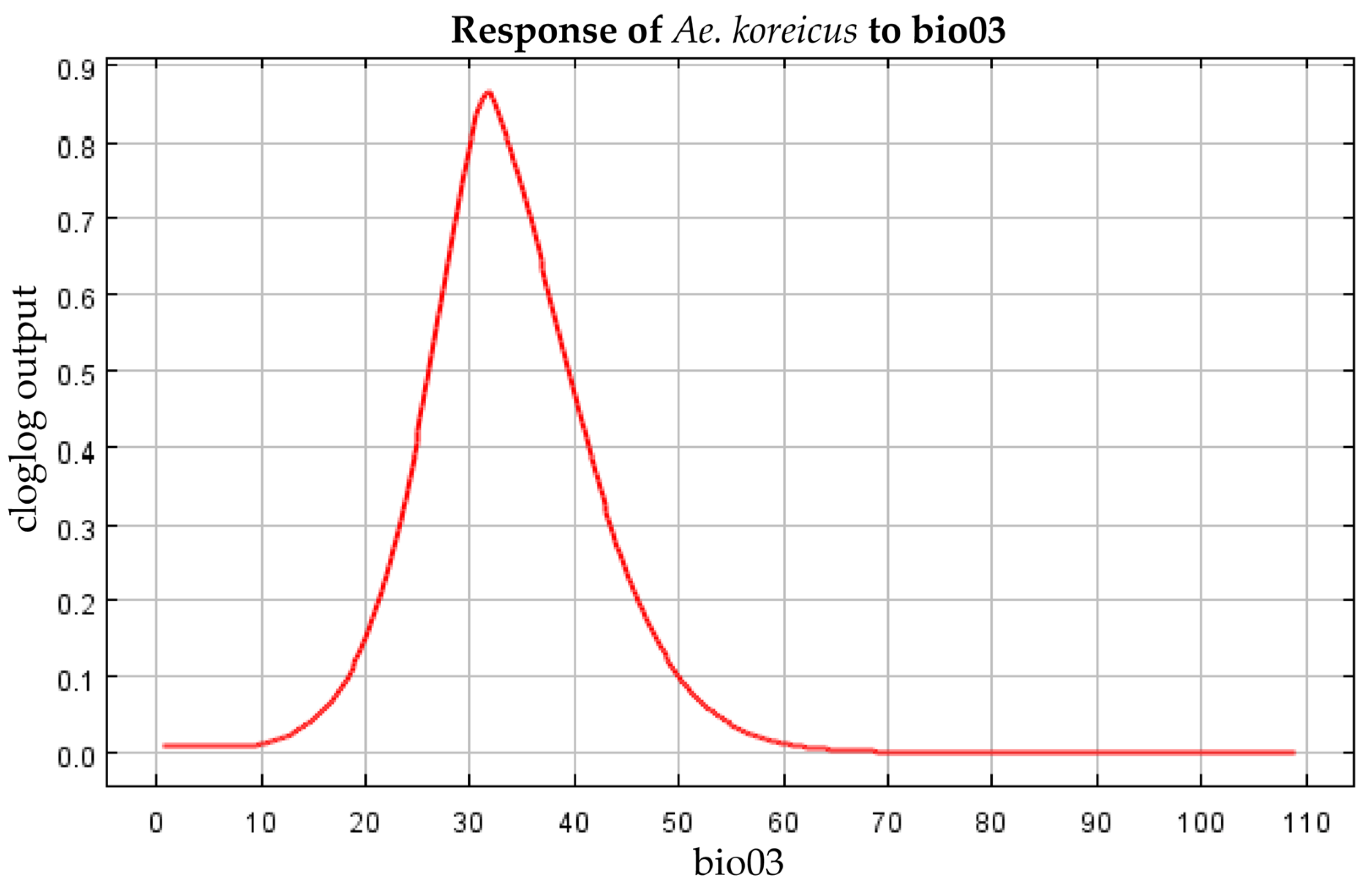
3.4. Filtering for Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montarsi, F.; Drago, A.; Pont, M.D.; Delai, N.; Carlin, S.; Cazzin, S.; Ciocchetta, S.; Arnoldi, D.; Baldacchino, F.; Rizzoli, A.; et al. Current Knowledge on the Distribution and Biology of the Recently Introduced Invasive Mosquito Aedes Koreicus (Diptera: Culicidae). Atti Accad. Naz. Ital. Entomol. 2014, 62, 169–174. [Google Scholar]
- Cebrián-Camisón, S.; Martínez-de la Puente, J.; Figuerola, J. A Literature Review of Host Feeding Patterns of Invasive Aedes Mosquitoes in Europe. Insects 2020, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.A. Some Ecological Aspects of the Problem of Arthropod-Borne Animal Viruses in the Western Pacific and South-east Asia Regions. Bull. World Health Organ. 1964, 30, 197–210. [Google Scholar] [PubMed]
- Montarsi, F.; Ciocchetta, S.; Devine, G.; Ravagnan, S.; Mutinelli, F.; Frangipane di Regalbono, A.; Otranto, D.; Capelli, G. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasites Vectors 2015, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Heitmann, A.; Lühken, R.; Jöst, H.; Helms, M.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by Aedes japonicus japonicus from southwestern Germany. Emerg. Microbes Infect. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Ciocchetta, S.; Prowa, N.A.; Darbroa, J.M.; Frentiu, F.D.; Savino, S.; Montarsi, F.; Capelli, G.; Aaskov, J.G.; Devine, G.J. The new European invader Aedes (Finlaya) koreicus: A potential vector of chikungunya virus. Pathog. Glob. Health 2018, 112, 107–114. [Google Scholar] [CrossRef]
- Baldacchino, F.; Arnoldi, D.; Lapère, C.; Rosà, R.; Montarsi, F.; Capelli, G.; Rizzoli, A. Weak Larval Competition between Two Invasive Mosquitoes Aedes koreicus and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2017, 54, 1266–1272. [Google Scholar] [CrossRef]
- Ganassi, S.; De Cristofaro, A.; Di Criscio, D.; Petrarca, S.; Leopardi, C.; Guarnieri, A.; Pietrangelo, L.; Venditti, N.; Di Marco, R.; Petronio Petronio, G.; et al. The new invasive mosquito species Aedes koreicus as vector-borne diseases in the European area, a focus on Italian region: What we know from the scientific literature. Front. Microbiol. 2022, 13, 931994. [Google Scholar] [CrossRef]
- Tanaka, K.; Mizusawa, K.; Saugstad, E.S. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contrib. Am. Inst. 1979, 16, 1–987. [Google Scholar]
- Lu, B.L. Fauna Sinica: Insecta. Diptera: Culicidae 1; China Science Press: Beijing, China, 1997; Volume 8. [Google Scholar]
- Versteirt, V.; Pecor, J.E.; Fonseca, D.M.; Coosemans, M.; Bortel, W.V. Confirmation of Aedes koreicus (Diptera: Culicidae) in Belgium and description of morphological differences between Korean and Belgian specimens validated by molecular identification. Zootaxa 2012, 3191, 21–32. [Google Scholar] [CrossRef]
- Montarsi, F.; Drago, A.; Martini, S.; Calzolari, M.; Filippo, F.D.; Bianchi, A.; Mazzucato, M.; Ciocchetta, S.; Arnoldi, D.; Baldacchino, F.; et al. Current distribution of the invasive mosquito species, Aedes koreicus [Hulecoeteomyia koreica] in northern Italy. Parasites Vectors 2015, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Hohmeister, N.; Werner, D.; Kampen, H. The invasive Korean bush mosquito Aedes koreicus (Diptera: Culicidae) in Germany as of 2020. Parasites Vectors 2021, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, K.; Kiss, V.; Zana, B.; Schmieder, V.; Kepner, A.; Jakab, F.; Kemenesi, G. Emergence of Aedes koreicus (Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitol. Res. 2016, 115, 4687–4689. [Google Scholar] [CrossRef] [PubMed]
- Kalan, K.; Šušnjar, J.; Ivović, V.; Buzan, E. First record of Aedes koreicus (Diptera, Culicidae) in Slovenia. Parasitol. Res. 2017, 116, 2355–2358. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Schoener, E.; Weiler, S.; Barogh, B.S.; Zittra, C.; Walder, G. Monitoring of alien mosquitoes in Western Austria (Tyrol, Austria, 2018). PLoS Negl. Trop. Dis. 2020, 14, e0008433. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, Y.V.; Khrabrova, N.V.; Alekseeva, S.S.; Abylkassymova, G.M.; Simakova, A.V.; Sibataev, A.K. First record of the invasive mosquito species Aedes koreicus (Diptera, Culicidae) in the Republic of Kazakhstan. Parasite 2021, 28, 52. [Google Scholar] [CrossRef]
- Vojtíšek, J.; Šebesta, O.; Šikutová, S.; Kampen, H.; Rudolf, I. First record of the invasive mosquito species Aedes koreicus (Diptera: Culicidae) in the Czech Republic. Parasitol. Res. 2022, 121, 3701–3704. [Google Scholar] [CrossRef]
- Teekema, S.; Stroo, A.; Uiterwijk, M.; Vossenberg, B.; Jacobs, F.; Ibáñez-Justicia, A. First finding of Aedes koreicus (Diptera: Culicidae) in the Netherlands. J. Eur. Mosq. Control Assoc. 2022, 40, 3–9. [Google Scholar] [CrossRef]
- Ibañez-Justicia, A.; Smitz, N.; den Hartog, W.; van de Vossenberg, B.; De Wolf, K.; Deblauwe, I.; Van Bortel, W.; Jacobs, F.; Vaux, A.G.C.; Medlock, J.M.; et al. Detection of Exotic Mosquito Species (Diptera: Culicidae) at International Airports in Europe. Int. J. Environ. Res. Public Health 2020, 17, 3450. [Google Scholar] [CrossRef]
- Kampen, H.; Schuhbauer, A.; Walther, D. Emerging mosquito species in Germany—A synopsis after 6 years of mosquito monitoring (2011–2016). Parasitol. Res. 2017, 116, 3253–3263. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Van Bortel, W.; Schaffner, F. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Wieser, A.; Reuss, F.; Niamir, A.; Müller, R.; O Hara, R.B.; Pfenninger, M. Modelling seasonal dynamics, population stability, and pest control in Aedes japonicus japonicus (Diptera: Culicidae). Parasites Vectors 2019, 12, 142. [Google Scholar] [CrossRef]
- Reuss, F.; Wieser, A.; Niamir, A.; Bálint, M.; Kuch, U.; Pfenninger, M.; Müller, R. Thermal experiments with the Asian bush mosquito (Aedes japonicus japonicus) (Diptera: Culicidae) and implications for its distribution in Germany. Parasites Vectors 2018, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Peng, H.H.; Peng, S.Z. The development and evaluation of species distribution models. Acta Ecol. Sin. 2015, 35, 557–567. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kusia, E.S.; Borgemeister, C.; Khamis, F.M.; Copeland, R.S.; Tanga, C.M.; Ombura, F.L.; Subramanian, S. Diversity, Host Plants and Potential Distribution of Edible Saturniid Caterpillars in Kenya. Insects 2021, 12, 600. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.; Chen, G.; Xie, T.; Liu, Z.; Ma, Z.; Du, J.; Chao, B.; et al. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Khan, J.; Guo, J.; Feng, Q.; Sun, Y.; Li, B.; Wu, Y.; Wu, Z.; Zheng, X. Predicting the Impact of Climate Change on the Distribution of a Neglected Arboviruses Vector (Armigeres subalbatus) in China. Trop. Med. Infect. Dis. 2022, 7, 431. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Stanley, G.; Misinzo, G.; Mboera, L.E.G. Climate Change Influences Potential Distribution of Infected Aedes aegypti Co-Occurrence with Dengue Epidemics Risk Areas in Tanzania. PLoS ONE 2016, 11, e0162649. [Google Scholar] [CrossRef]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.D.; Xing, D.; Jia, N.; Du, Y.T.; Xie, J.W.; Wang, M.; Li, C.X.; Zhao, T.; Jiang, Y.T.; et al. The predicted potential distribution of Aedes albopictus in China under the shared socioeconomic pathway (SSP)1-2.6. Acta Trop. 2023, 248, 107001. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Montarsi, F.; Martini, S.; Dal Pont, M.; Delai, N.; Ferro Milone, N.; Mazzucato, M.; Soppelsa, F.; Cazzola, L.; Cazzin, S.; Ravagnan, S.; et al. Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern Italy. Parasit Vectors 2013, 6, 292. [Google Scholar] [CrossRef]
- Suter, T.; Flacio, E.; Fariña, B.F.; Engeler, L.; Tonolla, M.; Müller, P. First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasit Vectors 2015, 8, 402. [Google Scholar] [CrossRef]
- Bezzhonova, O.V.; Bezzhonova, O.V.; Patraman, I.V.; Ganushkina, L.A.; Vyshemirskiĭ, O.I.; Sergiev, V.P. The first finding of invasive species Aedes (Finlaya) koreicus (Edwards, 1917) in European Russia. Meditsinskaia Parazitol. Parazit. Bolezn. 2014, 1, 16–19. [Google Scholar]
- Negri, A.; Arnoldi, I.; Brilli, M.; Bandi, C.; Gabrieli, P.; Epis, S. Evidence for the spread of the alien species Aedes koreicus in the Lombardy region, Italy. Parasit Vectors 2021, 14, 534. [Google Scholar] [CrossRef]
- Spanoudis, C.G.; Andreadis, S.S.; Tsaknis, N.K.; Petrou, A.P.; Gkeka, C.D.; Savopoulou-Soultani, M. Effect of Temperature on Biological Parameters of the West Nile Virus Vector Culex pipiens form ‘molestus’ (Diptera: Culicidae) in Greece: Constant vs. Fluctuating Temperatures. J. Med. Entomol. 2019, 56, 641–650. [Google Scholar] [CrossRef]
- Arnoldi, I.; Negri, A.; Soresinetti, L.; Brambilla, M.; Carraretto, D.; Montarsi, F.; Roberto, P.; Mosca, A.; Rubolini, D.; Bandi, C.; et al. Assessing the distribution of invasive Asian mosquitoes in Northern Italy and modelling the potential spread of Aedes koreicus in Europe. Acta Trop. 2022, 232, 106536. [Google Scholar] [CrossRef]
- Marcantonio, M.; Metz, M.; Baldacchino, F.; Arnoldi, D.; Montarsi, F.; Capelli, G.; Carlin, S.; Neteler, M.; Rizzoli, A. First assessment of potential distribution and dispersal capacity of the emerging invasive mosquito Aedes koreicus in Northeast Italy. Parasit Vectors 2016, 9, 63. [Google Scholar] [CrossRef]
- Kurucz, K.; Zeghbib, S.; Arnoldi, D.; Marini, G.; Manica, M.; Michelutti, A.; Montarsi, F.; Deblauwe, I.; Van Bortel, W.; Smitz, N.; et al. Aedes koreicus, a vector on the rise: Pan-European genetic patterns, mitochondrial and draft genome sequencing. PLoS ONE 2022, 17, e0269880. [Google Scholar] [CrossRef]
- Marini, G.; Arnoldi, D.; Baldacchino, F.; Capelli, G.; Guzzetta, G.; Merler, S.; Montarsi, F.; Rizzoli, A.; Rosà, R. First report of the influence of temperature on the bionomics and population dynamics of Aedes koreicus, a new invasive alien species in Europe. Parasit Vectors 2019, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Marini, G.; Arnoldi, D.; Inama, E.; Rizzoli, A. Diapause characterization in the invasive alien mosquito species Aedes koreicus: A laboratory experiment. Parasit Vectors 2022, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Hausfather, Z.; Marvel, K.; Schmidt, G.A.; Nielsen-Gammon, J.W.; Zelinka, M. Climate simulations: Recognize the ‘hot model’ problem. Nature 2022, 605, 26–29. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Chapter 4: Future Global Climate: Scenario-Based Projections and Near-Term Information. Available online: https://www.ipcc.ch/report/ar6/wg1/chapter/chapter-4/ (accessed on 26 August 2023).
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Guillén Bolaños, T.; Bindi, M.; Brown, S.; Camilloni, I.A.; Diedhiou, A.; Djalante, R.; Ebi, K.; et al. The human imperative of stabilizing global climate change at 1.5 °C. Science 2019, 365, eaaw6974. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef]
- Miner, J.J.; Aw-Yeang, H.X.; Fox, J.M.; Taffner, S.; Malkova, O.N.; Oh, S.T.; Kim, A.H.J.; Diamond, M.S.; Lenschow, D.J.; Yokoyama, W.M. Chikungunya viral arthritis in the United States: A mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 1214–1220. [Google Scholar] [CrossRef]
- Kuehn, B.M. Chikungunya virus transmission found in the United States: US health authorities brace for wider spread. JAMA 2014, 312, 776–777. [Google Scholar] [CrossRef]
- Gubler, D.J. The continuing spread of West Nile virus in the western hemisphere. Clin. Infect. Dis. 2007, 45, 1039–1046. [Google Scholar] [CrossRef]
- Stoops, C.A.; Kim, M.S.; Mahabir, S.; Chong, S.T.; Cinkovich, S.S.; Carder, J.B. CDC Bottle Bioassays for Detection of Insecticide Resistance in Culex pipiens, Aedes albopictus, and Aedes koreicus Collected on US Army Garrisons, Republic of Korea. J. Am. Mosq. Control Assoc. 2017, 39, 208–211. [Google Scholar] [CrossRef]
- Damiani, C.; Cappelli, A.; Comandatore, F.; Montarsi, F.; Serrao, A.; Michelutti, A.; Bertola, M.; Mancini, M.V.; Ricci, I.; Bandi, C.; et al. Wolbachia in Aedes koreicus: Rare Detections and Possible Implications. Insects 2022, 13, 216. [Google Scholar] [CrossRef] [PubMed]


| Bioclimate | Description |
|---|---|
| Bio01 | Annual mean temperature |
| Bio02 | Mean diurnal range (mean of monthly (max temp–min temp)) |
| Bio03 | Isothermality (Bio2/Bio7) (×100) |
| Bio04 | Temperature seasonality (standard deviation ×100) |
| Bio05 | Max temperature of warmest month |
| Bio06 | Min temperature of coldest month |
| Bio07 | Temperature annual range (Bio5–Bio6) |
| Bio08 | Mean temperature of wettest quarter |
| Bio09 | Mean temperature of driest quarter |
| Bio10 | Mean temperature of warmest quarter |
| Bio11 | Mean temperature of coldest quarter |
| Bio12 | Annual precipitation |
| Bio13 | Precipitation of wettest month |
| Bio14 | Precipitation of driest month |
| Bio15 | Precipitation seasonality (coefficient of variation) |
| Bio16 | Precipitation of wettest quarter |
| Bio17 | Precipitation of driest Quarter |
| Bio18 | Precipitation of warmest quarter |
| Bio19 | Precipitation of coldest quarter |
| Elev | Elevation |
| Level | 104 km | % |
|---|---|---|
| Unsuitable | 13,615.60 | 91.38 |
| Marginally suitable | 789.08 | 5.30 |
| Moderately suitable | 352.92 | 2.37 |
| Highly suitable | 142.40 | 0.96 |
| Level | 2021–2040 (104 km2) | 2041–2060 (104 km2) |
|---|---|---|
| Unsuitable | 133,369.00 | 13,108.62 |
| Lowly suitable | 1002.71 | 1233.59 |
| Moderately suitable | 373.39 | 394.24 |
| Highly suitable | 154.91 | 163.55 |
| Environmental Variables | Percent Contribution |
|---|---|
| Bio01 | 24.3 |
| Bio13 | 23.9 |
| Bio03 | 14.8 |
| Bio06 | 13.5 |
| Bio19 | 7.5 |
| Bio08 | 7.1 |
| Bio14 | 5.9 |
| Bio02 | 2.1 |
| Bio15 | 0.5 |
| Elev | 0.2 |
| Bio05 | 0.1 |
| Bio12 | 0 |
| Bio07 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xie, J.-W.; Wang, M.; Du, Y.-T.; Yin, Z.-G.; Zhou, N.-X.; Zhao, T.-Y.; Huang, E.-J.; Zhang, H.-D. Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate. Trop. Med. Infect. Dis. 2023, 8, 471. https://doi.org/10.3390/tropicalmed8100471
Liu Q, Xie J-W, Wang M, Du Y-T, Yin Z-G, Zhou N-X, Zhao T-Y, Huang E-J, Zhang H-D. Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate. Tropical Medicine and Infectious Disease. 2023; 8(10):471. https://doi.org/10.3390/tropicalmed8100471
Chicago/Turabian StyleLiu, Qing, Jing-Wen Xie, Ming Wang, Yu-Tong Du, Zi-Ge Yin, Ning-Xin Zhou, Tong-Yan Zhao, En-Jiong Huang, and Heng-Duan Zhang. 2023. "Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate" Tropical Medicine and Infectious Disease 8, no. 10: 471. https://doi.org/10.3390/tropicalmed8100471
APA StyleLiu, Q., Xie, J.-W., Wang, M., Du, Y.-T., Yin, Z.-G., Zhou, N.-X., Zhao, T.-Y., Huang, E.-J., & Zhang, H.-D. (2023). Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate. Tropical Medicine and Infectious Disease, 8(10), 471. https://doi.org/10.3390/tropicalmed8100471






