Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals
2.3. In Vitro Experiments
2.3.1. Parasite Maintenance and Isolation
2.3.2. Drug Treatment and Viability Assays
2.3.3. Ultrastructural Analysis via Scanning Electron Microscopy
2.3.4. In Toto Immunohistochemistry
2.3.5. In Vitro Culture of Bone Marrow-Derived Dendritic Cells from E. multilocularis Infected Mice
2.3.6. Flow Cytometry
2.3.7. Gene Expression Analysis via Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. In Vivo Assays
2.4.1. Experimental Animals and Determination of the Efficacy of In Vivo Treatment
2.4.2. Therapeutic Effectiveness of Resveratrol in Experimental Models of Echinococcosis
2.4.3. Determination of Resveratrol Levels
2.5. Statistics
3. Results
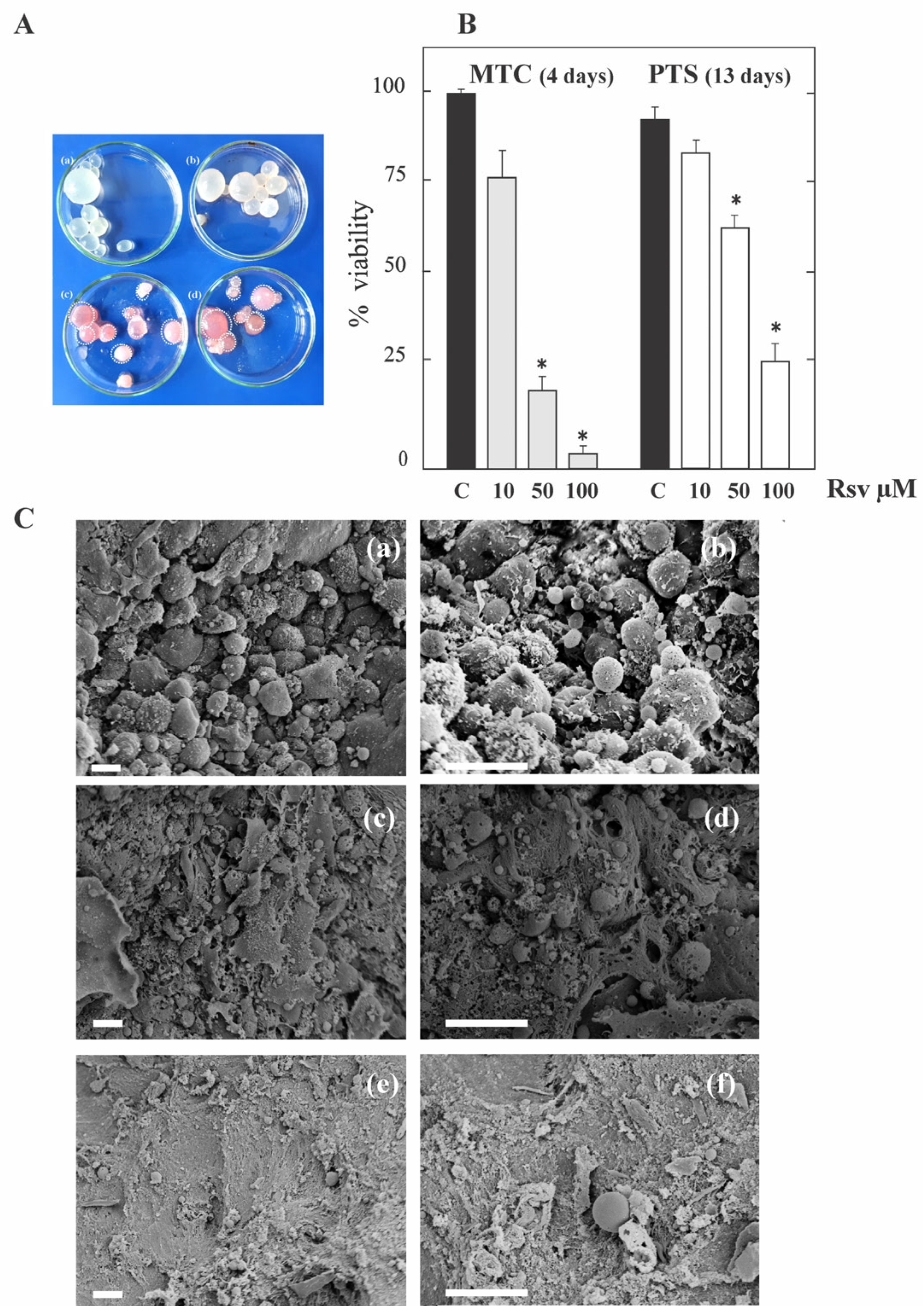
3.1. Resveratrol Exerts Anti-Echinococcal Activity In Vitro by Inhibiting TOR in E. granulosus Larval Stage
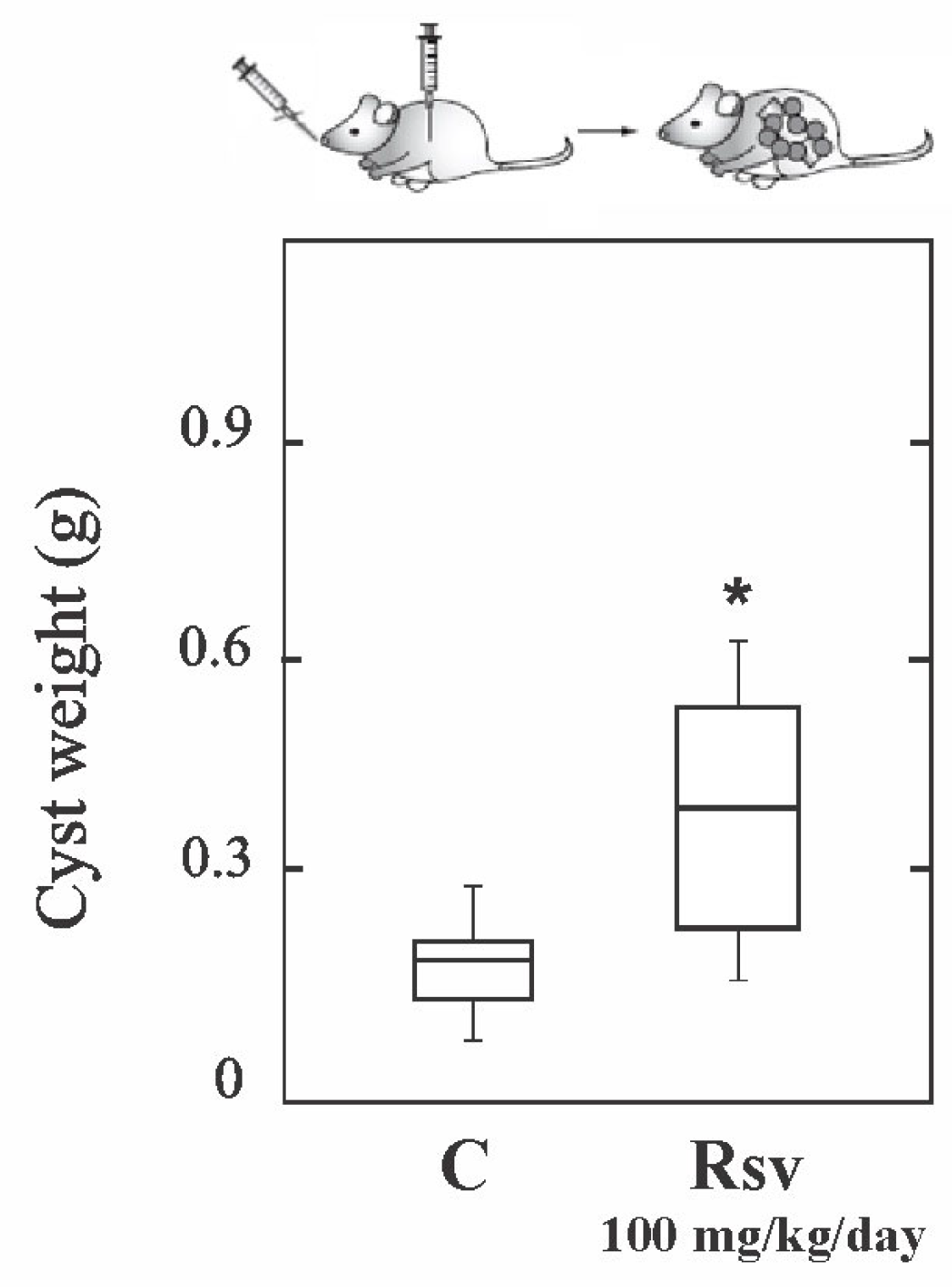
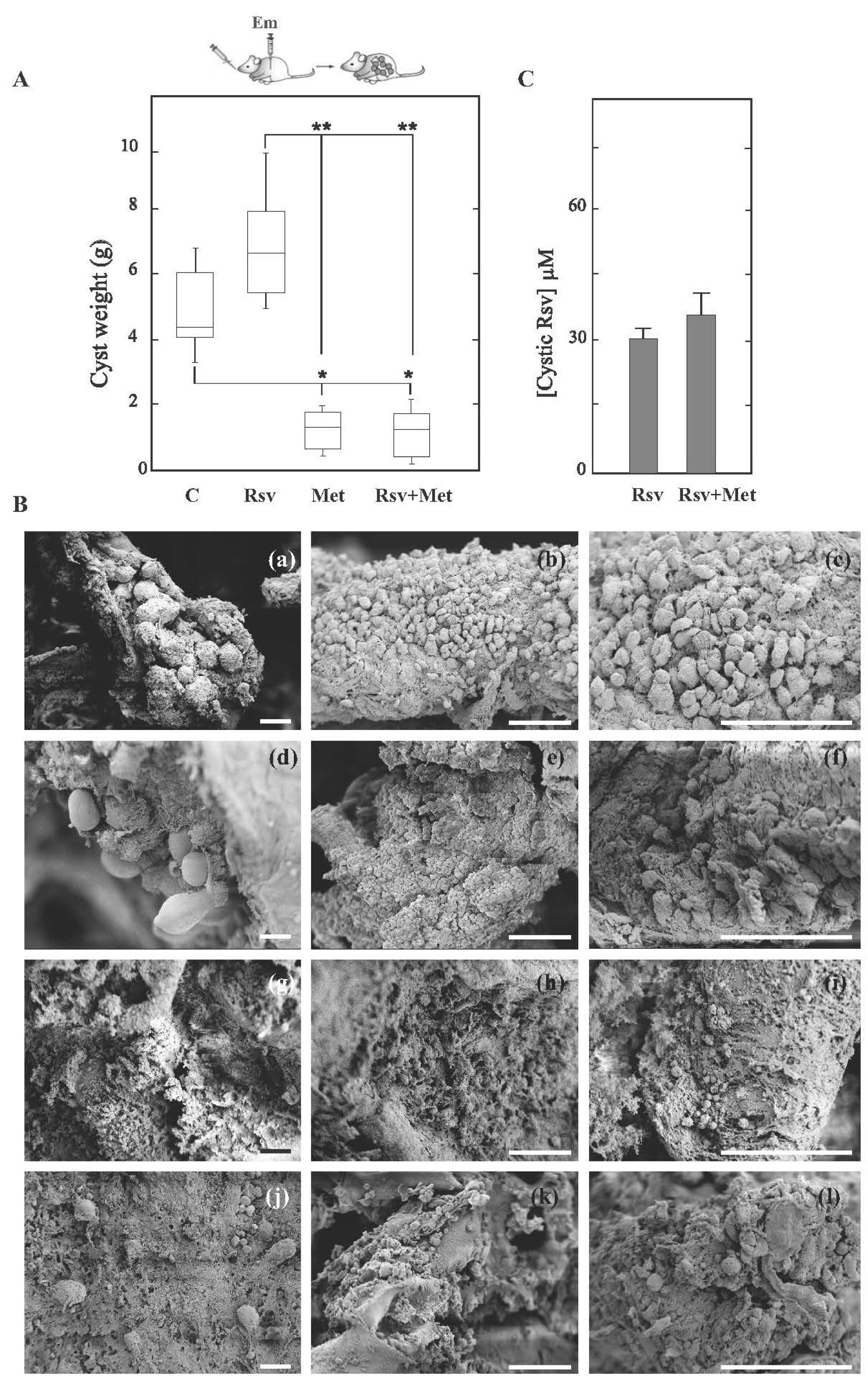
3.2. Resveratrol Shows Therapeutic Ineffectiveness in Early Infection Models of Cyst and Alveolar Echinococcosis
3.3. E. multilocularis Induces the Expansion of Hematopoietic Stem Cells in the Bone Marrow of Mice
3.4. E. multilocularis Infection and Pharmacological Treatments Promotes BMDC Maturation
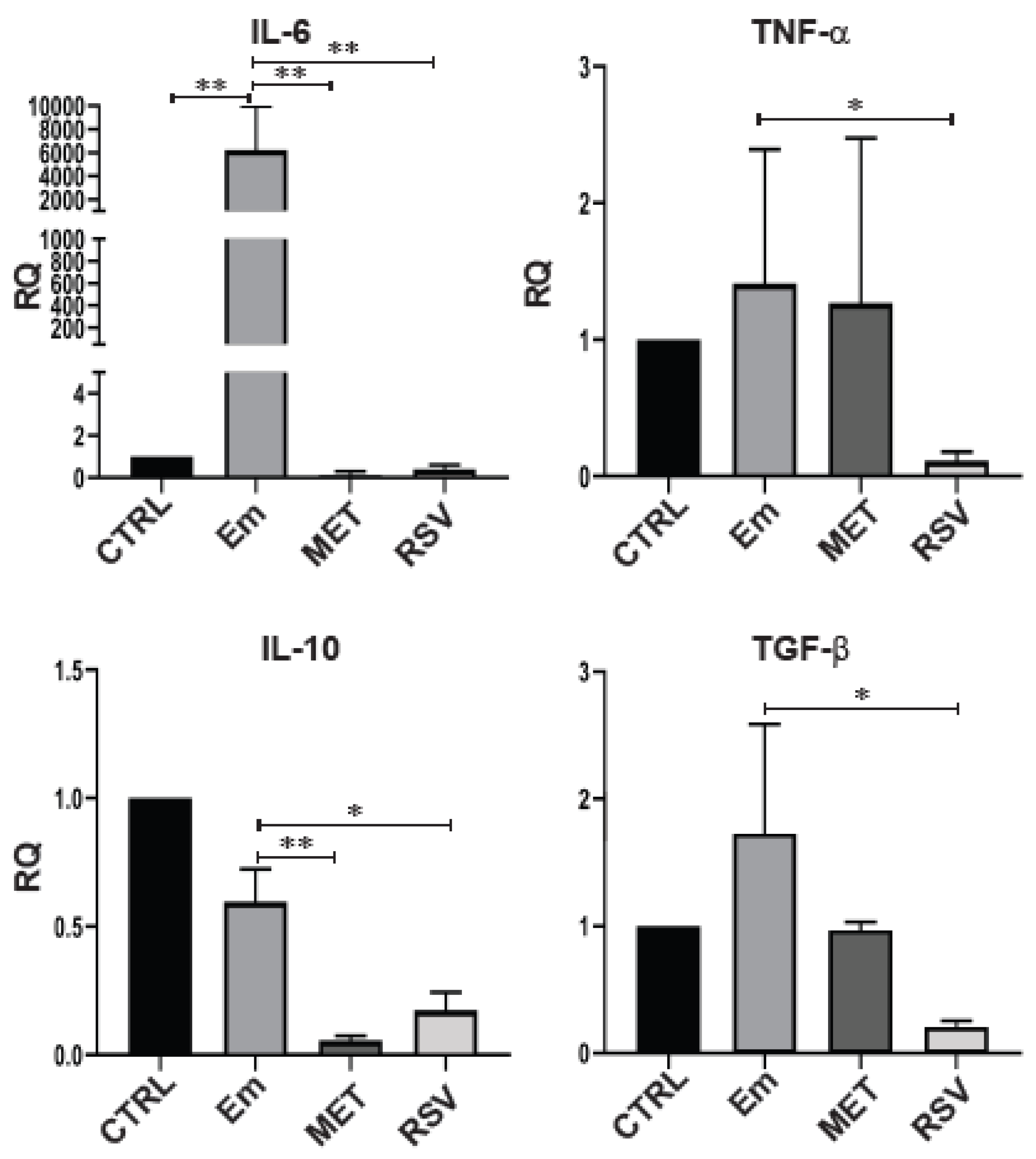
3.5. Resveratrol Modifies the Cytokine Production of BMDCs from E. multilocularis-Infected Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Gray, D.J.; Zhang, W.; Yang, Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012, 11, e3866. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Menezes da Silva, A.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, B.; Rufener, R.; Aeschbacher, D.; Spiliotis, M.; Gottstein, B.; Hemphill, A. Screening of the Open Source Malaria Box Reveals an Early Lead Compound for the Treatment of Alveolar Echinococcosis. PLoS Negl. Trop. Dis. 2016, 11, e0004535. [Google Scholar] [CrossRef] [PubMed]
- Brehm, K. The role of evolutionarily conserved signalling systems in Echinococcus multilocularis development and host-parasite interaction. Med. Microbiol. Immunol. 2010, 199, 247–259. [Google Scholar] [CrossRef]
- Parkinson, J.; Wasmuth, J.D.; Salinas, G.; Bizarro, C.V.; Sanford, C.; Berriman, M.; Ferreira, H.B.; Zaha, A.; Blaxter, M.L.; Maizels, R.M.; et al. A transcriptomic analysis of Echinococcus granulosus larval stages: Implications for parasite biology and host adaptation. PLoS Negl. Trop. Dis. 2012, 6, e1897. [Google Scholar] [CrossRef]
- Ritler, D.; Rufener, R.; Li, J.V.; Kämpfer, U.; Müller, J.; Bühr, C.; Schürch, S.; Lundström-Stadelmann, B. In vitro metabolomic footprint of the Echinococcus multilocularis metacestode. Sci. Rep. 2019, 9, 19438. [Google Scholar] [CrossRef]
- Enkai, S.; Inaoka, D.K.; Kouguchi, H.; Irie, T.; Yagi, K.; Kita, K. Mitochondrial complex III in larval stage of Echinococcus multilocularis as a potential chemotherapeutic target and in vivo efficacy of atovaquone against primary hydatid cysts. Parasitol. Int. 2020, 75, 102004. [Google Scholar] [CrossRef]
- Enkai, S.; Kouguchi, H.; Inaoka, D.K.; Shiba, T.; Hidaka, M.; Matsuyama, H.; Sakura, T.; Yagi, K.; Kita, K. Killing Two Birds with One Stone: Discovery of Dual Inhibitors of Oxygen and Fumarate Respiration in Zoonotic Parasite, Echinococcus multilocularis. Antimicrob. Agents Chemother. 2023, 67, e0142822. [Google Scholar] [CrossRef]
- Rufener, R.; Dick, L.; D’Ascoli, L.; Ritler, D.; Hizem, A.; Wells, T.N.C.; Hemphill, A.; Lundström-Stadelmann, B. Repurposing of an old drug: In vitro and in vivo efficacies of buparvaquone against Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 440–450. [Google Scholar] [CrossRef]
- Enkai, S.; Kouguchi, H.; Inaoka, D.K.; Irie, T.; Yagi, K.; Kita, K. In vivo efficacy of combination therapy with albendazole and atovaquone against primary hydatid cysts in mice. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1815–1820. [Google Scholar] [CrossRef]
- Chaudhry, S.; Zurbriggen, R.; Preza, M.; Kämpfer, T.; Kaethner, M.; Memedovski, R.; Scorrano, N.; Hemphill, A.; Doggett, J.S.; Lundström-Stadelmann, B. Dual inhibition of the Echinococcus multilocularis energy metabolism. Front. Vet. Sci. 2022, 9, 981664. [Google Scholar] [CrossRef]
- Loos, J.A.; Dávila, V.A.; Rodrígues, C.R.; Petrigh, R.; Zoppi, J.A.; Crocenzi, F.A.; Cumino, A.C. Metformin exhibits preventive and therapeutic efficacy against experimental cystic echinococcosis. PLoS Negl. Trop. Dis. 2017, 11, e0005370. [Google Scholar] [CrossRef]
- Loos, J.A.; Dávila, V.A.; Brehm, K.; Cumino, A.C. Metformin Suppresses Development of the Echinococcus multilocularis Larval Stage by Targeting the TOR Pathway. Antimicrob. Agents Chemother. 2020, 64, e01808-19. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.A.; Coccimiglio, M.; Nicolao, M.C.; Rodrigues, C.R.; Cumino, A.C. Metformin improves the therapeutic efficacy of low-dose albendazole against experimental alveolar echinococcosis. Parasitology 2022, 149, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.A.; Cumino, A.C. In Vitro Anti-Echinococcal and Metabolic Effects of Metformin Involve Activation of AMP-Activated Protein Kinase in Larval Stages of Echinococcus granulosus. PLoS ONE 2015, 10, e0126009. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.A.; Nicolao, M.C.; Cumino, A.C. Metformin promotes autophagy in Echinococcus granulosus larval stage. Mol. Biochem. Parasitol. 2018, 224, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.A.; Negro, P.; Cumino, A.C. In vitro anti-echinococcal activity of octreotide: Additive effect of metformin linked to autophagy. Acta Trop. 2020, 203, 105312. [Google Scholar] [CrossRef]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209-25. [Google Scholar] [CrossRef]
- Alavi, M.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int. 2021, 21, 579. [Google Scholar] [CrossRef]
- Fonseca, J.; Moradi, F.; Maddalena, L.A.; Ferreira-Tollstadius, B.; Selim, S.; Stuart, J.A. Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells-involvement of prolyl hydroxylase and hypoxia inducible factor-1. Oncol. Lett. 2019, 17, 697–705. [Google Scholar] [CrossRef]
- Ozkoc, S.; Tuncay, S.; Delibas, S.B.; Akisu, C. In vitro effects of resveratrol on Trichinella spiralis. Parasitol. Res. 2009, 105, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Bharti, R.R.; Roy, B. In vivo anthelmintic activity of Carex baccans and its active principle resveratrol against Hymenolepis diminuta. Parasitol. Res. 2015, 114, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Valera Vera, E.A.; Sayé, M.; Reigada, C.; Damasceno, F.S.; Silber, A.M.; Miranda, M.R.; Pereira, C.A. Resveratrol inhibits Trypanosoma cruzi arginine kinase and exerts a trypanocidal activity. Int. J. Biol. Macromol. 2016, 87, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Dong, K.; Qin, H.X.; Yang, Y.K.; He, J.L.; Li, J.; Zheng, Z.W.; Chen, D.L.; Chen, J.P. Direct and Indirect Inhibition Effects of Resveratrol against Toxoplasma gondii Tachyzoites In Vitro. Antimicrob. Agents Chemother. 2019, 63, e01233-18. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.J.; Brindley, P.J.; Azevedo, C.; Gärtner, F.; da Costa, J.M.C.; Vale, N. The antioxidants resveratrol and N-acetylcysteine enhance anthelmintic activity of praziquantel and artesunate against Schistosoma mansoni. Parasites Vectors 2019, 12, 309. [Google Scholar] [CrossRef]
- Cumino, A.C.; Lamenza, P.; Denegri, G.M. Identification of functional FKB protein in Echinococcus granulosus: Its involvement in the protoscolicidal action of rapamycin derivates and in calcium homeostasis. Int. J. Parasitol. 2010, 40, 651–661. [Google Scholar] [CrossRef]
- Spiliotis, M.; Brehm, K. Axenic in vitro cultivation of Echinococcus multilocularis metacestode vesicles and the generation of primary cell cultures. Methods Mol. Biol. 2009, 470, 245–262. [Google Scholar] [CrossRef]
- Spiliotis, M.; Lechner, S.; Tappe, D.; Scheller, C.; Krohne, G.; Brehm, K. Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int. J. Parasitol. 2008, 38, 1025–1039. [Google Scholar] [CrossRef]
- Loos, J.A.; Caparros, P.A.; Nicolao, M.C.; Denegri, G.M.; Cumino, A.C. Identification and pharmacological induction of autophagy in the larval stages of Echinococcus granulosus: An active catabolic process in calcareous corpuscles. Int. J. Parasitol. 2014, 44, 415–427. [Google Scholar] [CrossRef]
- Rodriguez Rodrigues, C.; Nicolao, M.C.; Chop, M.; Plá, N.; Massaro, M.; Loos, J.; Cumino, A.C. Modulation of the mTOR pathway plays a central role in dendritic cell functions after Echinococcus granulosus antigen recognition. Sci. Rep. 2021, 11, 17238. [Google Scholar] [CrossRef]
- Espínola, S.M.; Ferreira, H.B.; Zaha, A. Validation of suitable reference genes for expression normalization in Echinococcus spp. larval stages. PLoS ONE 2014, 9, e102228. [Google Scholar] [CrossRef] [PubMed]
- Noacco, N.; Rodenak-Kladniew, B.; de Bravo, M.G.; Castro, G.R.; Islan, G.A. Simple colorimetric method to determine the in vitro antioxidant activity of different monoterpenes. Anal. Biochem. 2018, 555, 59–66. [Google Scholar] [CrossRef] [PubMed]
- WHO Neglected Tropical Diseases. Available online: https://www.who.int/neglected_diseases/diseases/en/ (accessed on 1 April 2023).
- Xu, X.; Qian, X.; Gao, C.; Pang, Y.; Zhou, H.; Zhu, L.; Wang, Z.; Pang, M.; Wu, D.; Yu, W.; et al. Advances in the pharmacological treatment of hepatic alveolar echinococcosis: From laboratory to clinic. Front. Microbiol. 2022, 13, 953846. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta 2015, 1852, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2022, 80, 205–217. [Google Scholar] [CrossRef]
- Roy, B.; Giri, B.R. α-Viniferin and resveratrol induced alteration in the activities of some energy metabolism related enzymes in the cestode parasite Raillietina echinobothrida. Acta Trop. 2016, 154, 102–106. [Google Scholar] [CrossRef]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, C.; Lu, Q.; Cao, Y.; Yu, W.; Li, W.; Liu, B.; Gao, X.; Lü, J.; Pan, X. Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic. Biol. Med. 2022, 180, 108–120. [Google Scholar] [CrossRef]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef]
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverría, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Robert, G.; Fenouille, N.; Luciano, F.; Cassuto, J.P.; Raynaud, S.; Auberger, P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010, 70, 1042–1052. [Google Scholar] [CrossRef]
- Vilar-Pereira, G.; Carneiro, V.C.; Mata-Santos, H.; Vicentino, A.R.; Ramos, I.P.; Giarola, N.L.; Feijó, D.F.; Meyer-Fernandes, J.R.; Paula-Neto, H.A.; Medei, E.; et al. Resveratrol Reverses Functional Chagas Heart Disease in Mice. PLoS Pathog. 2016, 12, e1005947. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, P.; Rahimi Esboei, B.; Pourhajibagher, M.; Fakhar, M.; Shahmoradi, Z.; Hejazi, S.H.; Hassannia, H.; Nasrollahi Omran, A.; Hasanpour, H. Anti-leishmanial effects of resveratrol and resveratrol nanoemulsion on Leishmania major. BMC Microbiol. 2022, 22, 56. [Google Scholar] [CrossRef]
- Pais-Morales, J.; Betanzos, A.; García-Rivera, G.; Chávez-Munguía, B.; Shibayama, M.; Orozco, E. Resveratrol Induces Apoptosis-Like Death and Prevents In Vitro and In Vivo Virulence of Entamoeba histolytica. PLoS ONE 2016, 11, e0146287. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Zhong, L.X.; Wu, M.L.; Li, H.; Liu, J.; Lin, L.Z. Efficacy and safety of intraperitoneally administered resveratrol against rat orthotopic ovarian cancers. Cancer Manag. Res. 2019, 11, 6113–6124. [Google Scholar] [CrossRef]
- Shu, X.H.; Wang, L.L.; Li, H.; Song, X.; Shi, S.; Gu, J.Y.; Wu, M.L.; Chen, X.Y.; Kong, Q.Y.; Liu, J. Diffusion efficiency and bioavailability of resveratrol administered to rat brain by different routes: Therapeutic implications. Neurotherapeutics 2015, 12, 491–501. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Benedetti, F.; Buriani, A.; Fortinguerra, S.; Caudullo, G.; Davinelli, S.; Zella, D.; Scapagnini, G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals 2022, 15, 912. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D.A. Immunology of Alveolar and Cystic Echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Tartaglia, E.; Refolo, G.; Sacchi, A.; Grassi, G.; Antinori, A.; Fimia, G.M.; Agrati, C. Per2 Upregulation in Circulating Hematopoietic Progenitor Cells During Chronic HIV Infection. Front. Cell Infect. Microbiol. 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Downey, J.; Sanz, J.; Kaufmann, E.; Blankenhaus, B.; Pacis, A.; Pernet, E.; Ahmed, E.; Cardoso, S.; Nijnik, A.; et al. tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell 2020, 183, 752–770.e22. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.T.; Mills, K.H.G. Modulation of haematopoiesis by protozoal and helminth parasites. Parasite Immunol. 2023, 16, e12975. [Google Scholar] [CrossRef]
- Abidin, B.M.; Hammami, A.; Stä Ger, S.; Heinonen, K.M. Infection-adapted emergency hematopoiesis promotes visceral leishmaniasis. PLoS Pathog. 2017, 13, e1006422. [Google Scholar] [CrossRef]
- Babayan, S.A.; Sinclair, A.; Duprez, J.S.; Selman, C. Chronic helminth infection burden differentially affects haematopoietic cell development while ageing selectively impairs adaptive responses to infection. Sci. Rep. 2018, 8, 3802. [Google Scholar] [CrossRef]
- Romano, A.; Brown, N.; Ashwin, H.; Doehl, J.S.; Hamp, J.; Osman, M.; Dey, H.; Rani, G.F.; Ferreira, T.R.; Kaye, P.M. Interferon-γ-producing CD4+ T cells drive monocyte activation in the bone marrow during experimental Leishmania donovani infection. Front. Immunol. 2021, 12, 700501. [Google Scholar] [CrossRef]
- Margos, M.C.; Grandgirard, D.; Leib, S.; Gottstein, B. In vitro induction of lymph node cell proliferation by mouse bone marrow dendritic cells following stimulation with different Echinococcus multilocularis antigens. J. Helminthol. 2011, 85, 128–137. [Google Scholar] [CrossRef]
- Wei, X.L.; Xu, Q.; Rexiti, F.L.; Zhu, M.; Lin, R.Y.; Wen, H. Dynamic changes of DC and T cell subsets in mice during Echinococcus multilocularis infection. Cent. Eur. J. Immunol. 2014, 39, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Lv, S.; Zhang, S. Different protein of Echinococcus granulosus stimulates dendritic induced immune response. Parasitology 2015, 142, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Müller, S.; Lin, R.; Siffert, M.; Vuitton, D.A.; Wen, H.; Gottstein, B. Depletion of FoxP3+ Tregs improves control of larval Echinococcus multilocularis infection by promoting co-stimulation and Th1/17 immunity. Immun. Inflamm. Dis. 2017, 5, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cardoso, R.; Marreros, N.; Müller, N.; Lundström-Stadelmann, B.; Siffert, M.; Vuitton, D.A.; Boué, F.; Lin, R.; Wen, H.; et al. Foxp3+ T Regulatory Cells as a Potential Target for Immunotherapy against Primary Infection with Echinococcus multilocularis Eggs. Infect. Immun. 2018, 86, e00542-18. [Google Scholar] [CrossRef]
- Silva, A.M.; Oliveira, M.I.; Sette, L.; Almeida, C.R.; Oliveira, M.J.; Barbosa, M.A.; Santos, S.G. Resveratrol as a natural anti-tumor necrosis factor-α molecule: Implications to dendritic cells and their crosstalk with mesenchymal stromal cells. PLoS ONE 2014, 9, e91406. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Mejri, N.; Müller, J.; Gottstein, B. Intraperitoneal murine Echinococcus multilocularis infection induces differentiation of TGF-β-expressing DCs that remain immature. Parasite Immunol. 2011, 33, 471–482. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Zhao, H.; Pang, N.; Zhang, F.; Jiang, T.; Liu, X.; Mamuti, W.; Wen, H.; Ding, J. Th17 cells are associated with the Th1/Th2-cell balance during Echinococcus multilocularis infection. Mol. Med. Rep. 2014, 10, 236–240. [Google Scholar] [CrossRef]
- Linehan, J.L.; Dileepan, T.; Kashem, S.W.; Kaplan, D.H.; Cleary, P.; Jenkins, M.K. Generation of Th17 cells in response to intranasal infection requires TGF-β1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proc. Natl. Acad. Sci. USA 2015, 112, 12782–12787. [Google Scholar] [CrossRef]
- Fei, X.; Wang, A.; Wang, D.; Meng, X.; Ma, J.; Hong, L.; Qin, R.; Wang, A.; Dong, J.; Huang, Q.; et al. Establishment of malignantly transformed dendritic cell line SU3-ihDCTC induced by Glioma stem cells and study on its sensitivity to resveratrol. BMC Immunol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Delmas, D.; Limagne, E.; Ghiringhelli, F.; Aires, V. Immune Th17 lymphocytes play a critical role in the multiple beneficial properties of resveratrol. Food Chem. Toxicol. 2020, 137, 111091. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, Z.; Fu, Q.; Song, X.; Cui, Q.; Jia, R.; Zou, Y.; He, C.; Li, L.; Yin, Z. Resveratrol mitigates lipopolysaccharide-mediated acute inflammation in rats by inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci. Rep. 2017, 7, 45006. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Guo, N.H.; Fu, X.; Zi, F.M.; Song, Y.; Wang, S.; Cheng, J. The potential therapeutic benefit of resveratrol on Th17/Treg imbalance in immune thrombocytopenic purpura. Int. Immunopharmacol. 2019, 73, 181–192. [Google Scholar] [CrossRef]
- Yashiro, T.; Yura, S.; Tobita, A.; Toyoda, Y.; Kasakura, K.; Nishiyama, C. Pterostilbene reduces colonic inflammation by suppressing dendritic cell activation and promoting regulatory T cell development. FASEB J. 2020, 34, 14810–14819. [Google Scholar] [CrossRef]
- McManus, C.M.; Maizels, R.M. Regulatory T cells in parasite infections: Susceptibility, specificity and specialisation. Trends Parasitol. 2023, 39, 547–562. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loos, J.A.; Franco, M.; Chop, M.; Rodriguez Rodrigues, C.; Cumino, A.C. Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses. Trop. Med. Infect. Dis. 2023, 8, 460. https://doi.org/10.3390/tropicalmed8100460
Loos JA, Franco M, Chop M, Rodriguez Rodrigues C, Cumino AC. Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses. Tropical Medicine and Infectious Disease. 2023; 8(10):460. https://doi.org/10.3390/tropicalmed8100460
Chicago/Turabian StyleLoos, Julia A., Micaela Franco, Maia Chop, Christian Rodriguez Rodrigues, and Andrea C. Cumino. 2023. "Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses" Tropical Medicine and Infectious Disease 8, no. 10: 460. https://doi.org/10.3390/tropicalmed8100460
APA StyleLoos, J. A., Franco, M., Chop, M., Rodriguez Rodrigues, C., & Cumino, A. C. (2023). Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses. Tropical Medicine and Infectious Disease, 8(10), 460. https://doi.org/10.3390/tropicalmed8100460




