Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains and Culture
2.2. Expression and Purification of LRR Proteins
2.3. Preparation of Antigen-Adjuvant Mixtures for Immunization
2.4. Animal Model for Acute Leptospirosis, Immunization Protocols, and Lethal Leptospiral Challenge
2.4.1. The Animal Model for Acute Leptospirosis
2.4.2. Immunization Protocols
2.4.3. Challenge
2.5. Evaluation of Specific Humoral Immune Responses by ELISA
2.6. Serum Bactericidal Assay
2.7. Lymphoproliferation Assay in Hamsters Splenocytes
2.8. Evaluation of Splenocytes Cytokines Gene Expression by Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Quantification of Leptospira Load in Tissues by Quantitative Real-Time PCR
2.10. Culture
2.11. Histopathology
2.12. Statistical Analysis
3. Results
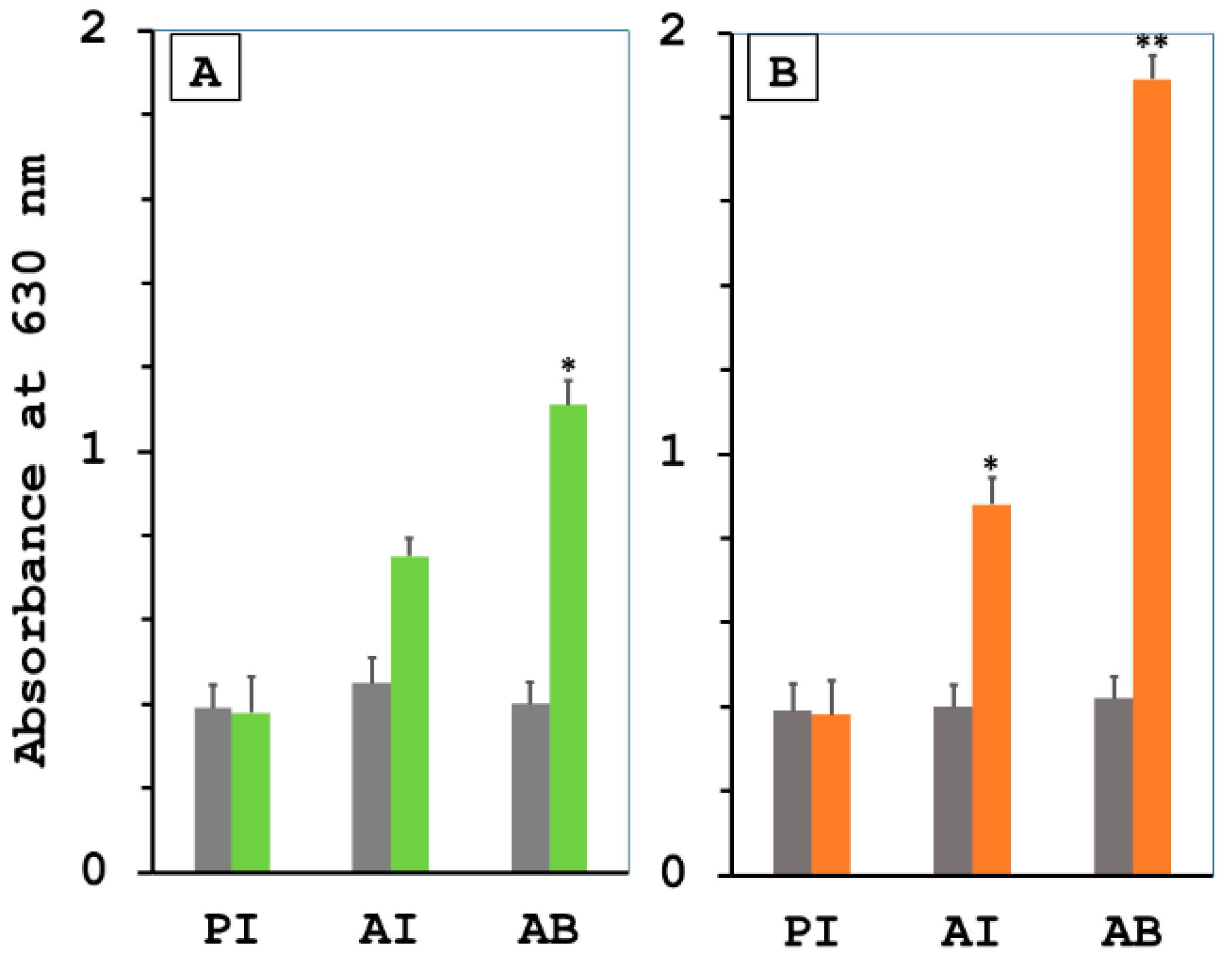
3.1. Stimulation of Specific Humoral Immune Responses by Letospiral LRR Proteins in the Immunized Hamsters
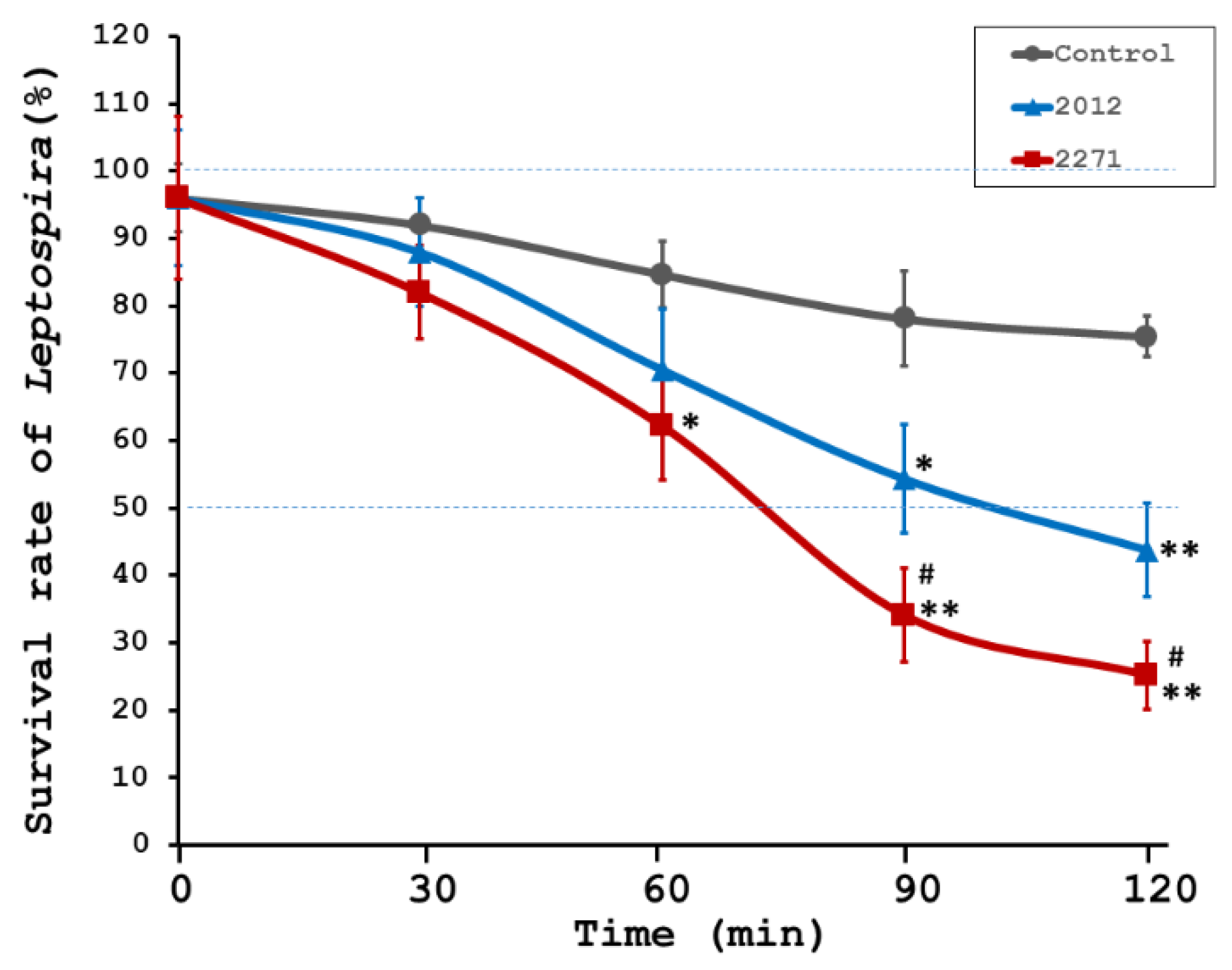
3.2. Bactericidal Activity of Sera from LRR Proteins Immunized Hamsters
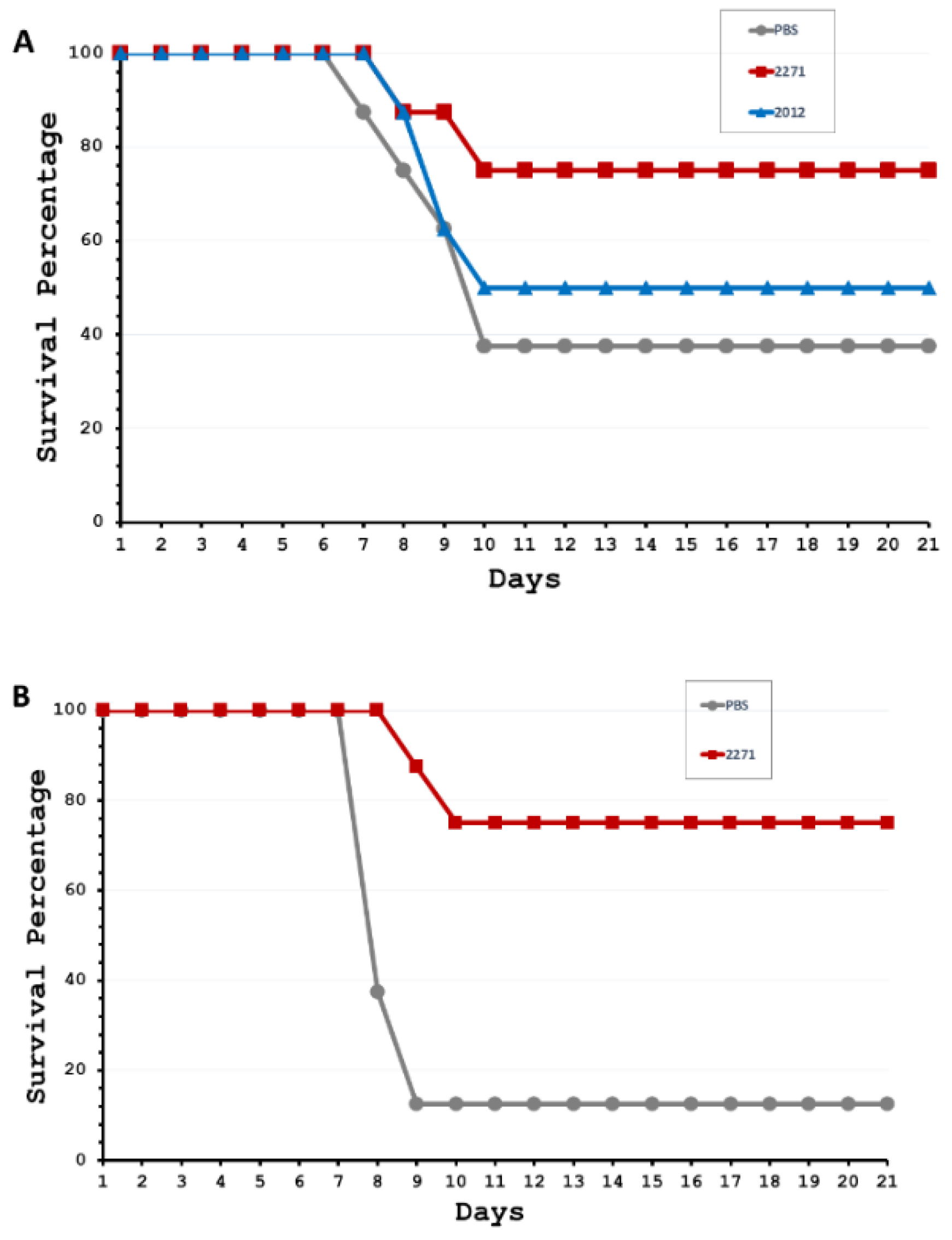
3.3. Protective Efficacy of LRR Proteins against Challenging Virulent L. interrogans Serovar Pomona in Hamsters
3.4. Role of the 2271 Protein as a Future Leptospiral Vaccine Candidate
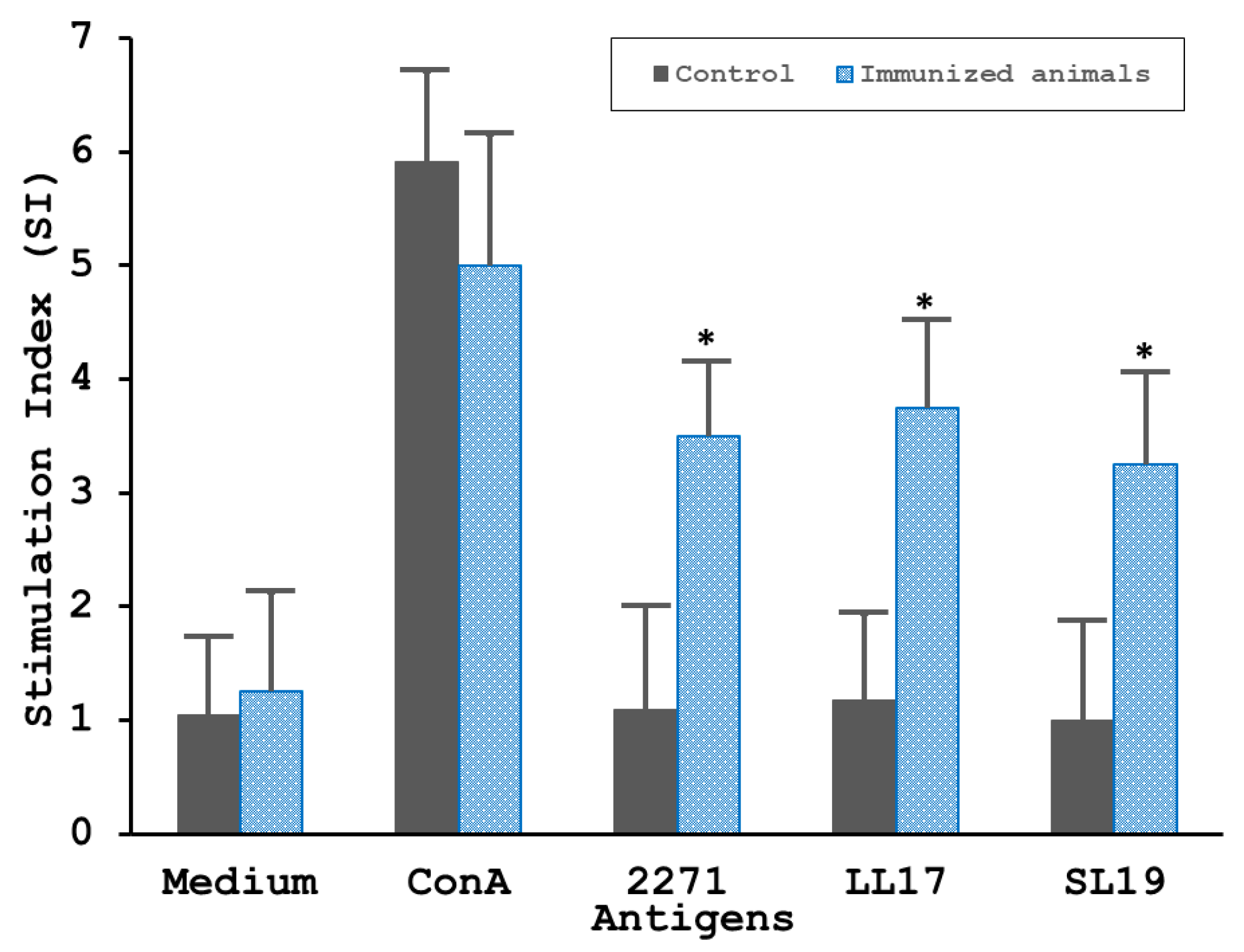
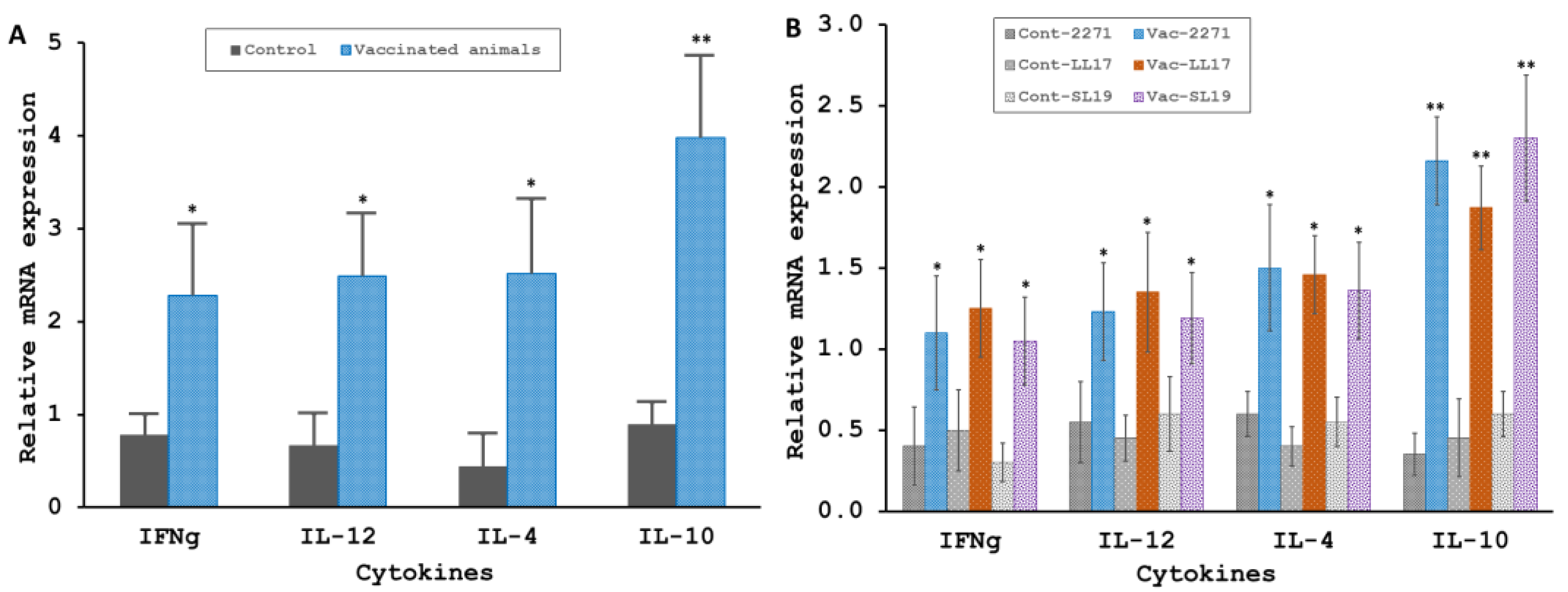
3.4.1. The 2271 Actions on Stimulating the Lymphoproliferation and the Cytokines Productions
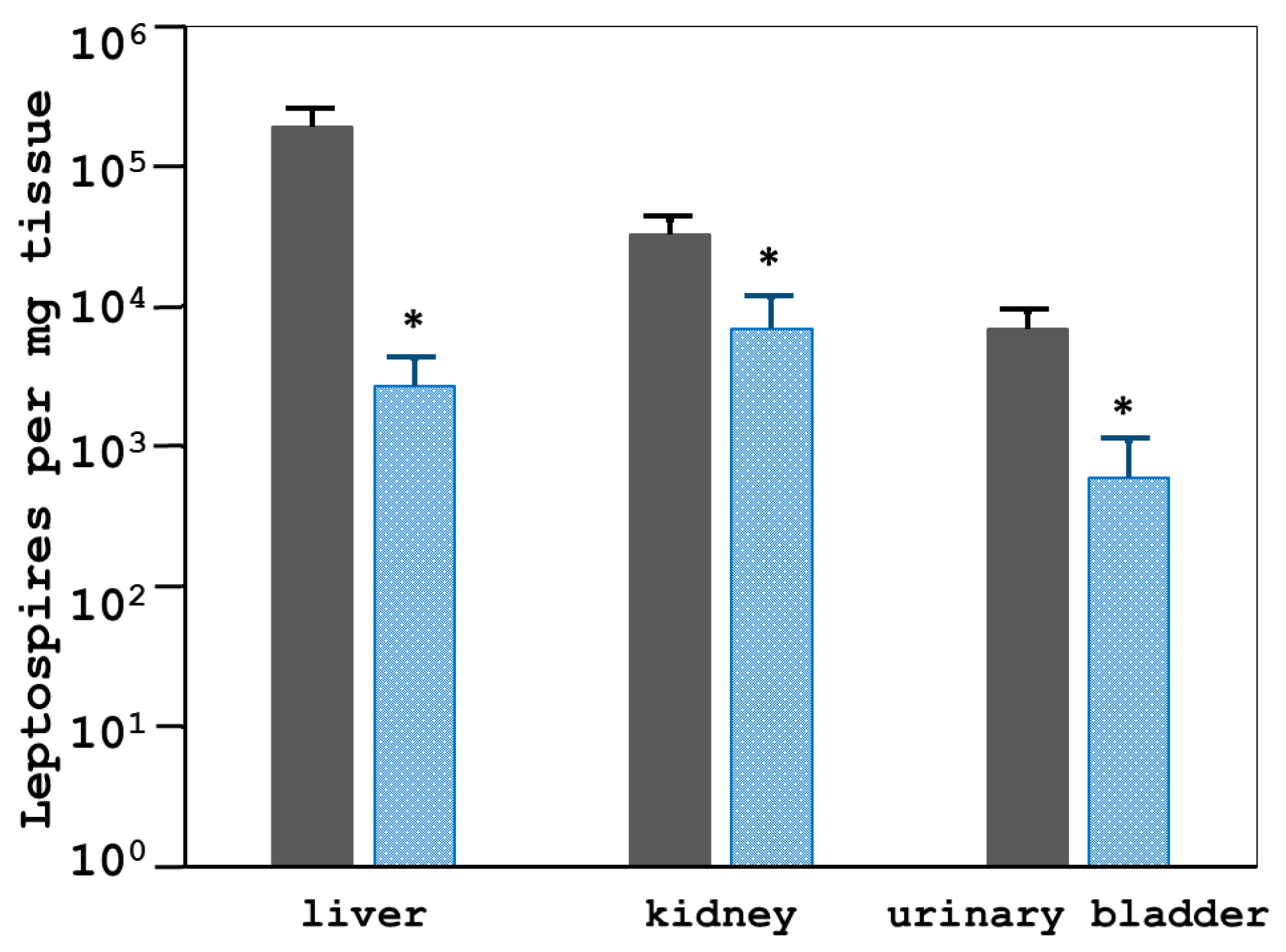
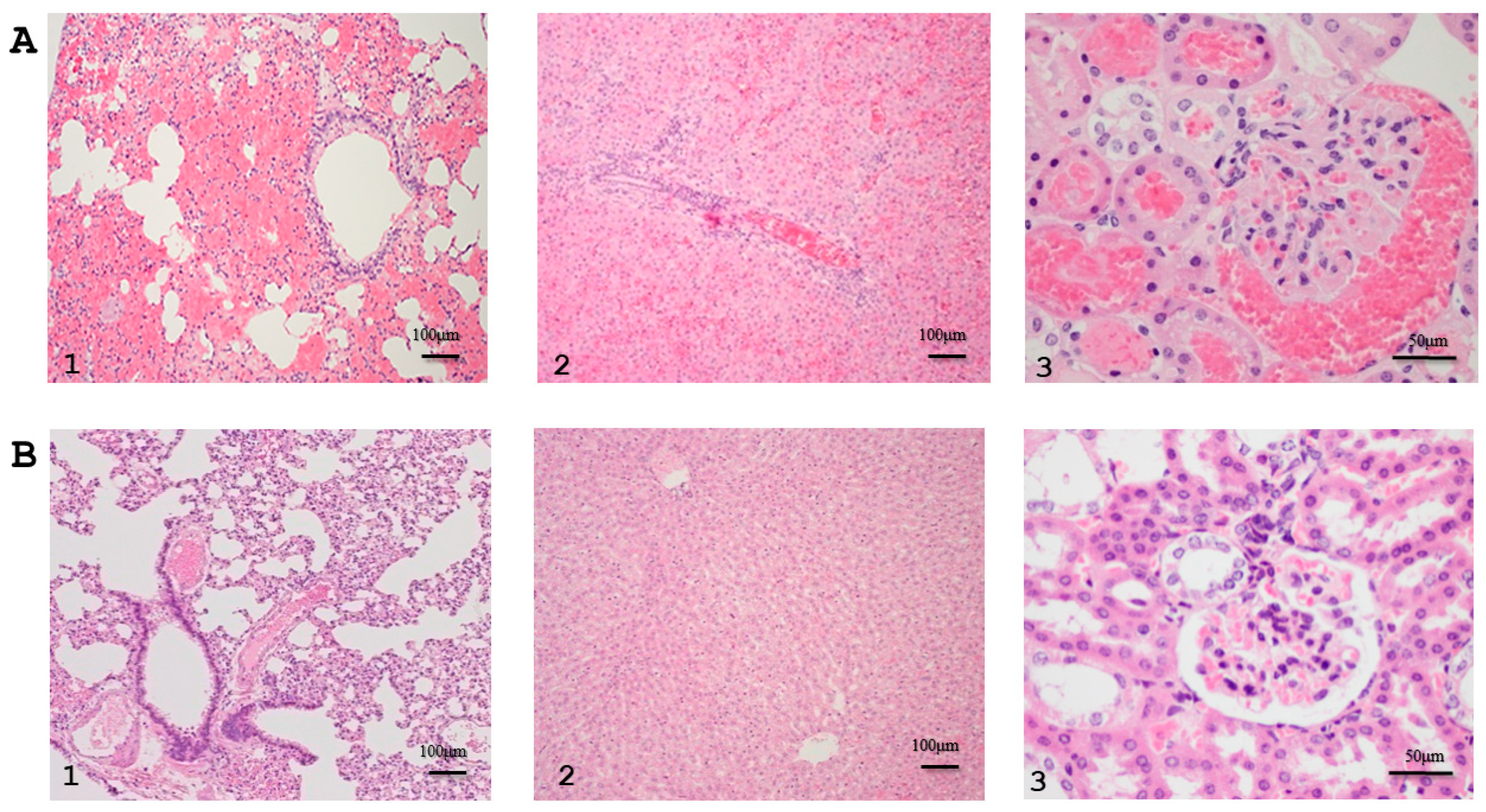
3.4.2. Tissues Bacterial Clearance and Tissues Inflammatory Responses of the 2271 Immunized Hamsters Challenged with L. interrogans Serovar Pomona
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, B.; De La Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Chadsuthi, S.; Bicout, D.J.; Wiratsudakul, A.; Suwancharoen, D.; Petkanchanapong, W.; Modchang, C.; Triampo, W.; Ratanakorn, P.; Chalvet-Monfray, K. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010–2015. PLoS Negl. Trop. Dis. 2017, 11, e0005228. [Google Scholar] [CrossRef]
- Hinjoy, S. Epidemiology of Leptospirosis from Thai National Disease Surveillance System, 2003–2012. Outbreak Surveill. Investig. Rep. (OSIR) E-J. 2014, 7, 1–5. Available online: http://www.osirjournal.net/index.php/osir/article/view/38 (accessed on 10 May 2022).
- Prapong, S.; Suwanchareon, D.; Tohmee, N. Genotyping survey the Leptospira in bovine urine samples in Thailand by PCR-based methos: A non matching with the antibody titer by MAT. In Proceedings of the 11th International Symposium of the World Association of Veterinary Laboratory Diagnosticians and OIE Seminar on Biotechnology, Sofitel Central Plaza, Bangkok, Thailand, 9–13 November 2003; pp. O33–O34. [Google Scholar]
- Martins, G.; Lilenbaum, W. Control of bovine leptospirosis: Aspects for consideration in a tropical environment. Res. Vet. Sci. 2017, 112, 156–160. [Google Scholar] [CrossRef]
- Tansiri, Y.; Sritrakul, T.; Saparpakorn, P.; Boondamnern, T.; Chimprasit, A.; Sripattanakul, S.; Hannongbua, S.; Prapong, S. New potent epitopes from Leptospira borgpetersenii for the stimulation of humoral and cell-mediated immune responses: Experimental and theoretical studies. Inform. Med. Unlocked 2021, 25, 100649. [Google Scholar] [CrossRef]
- Loureiro, A.P.; Lilenbaum, W. Genital bovine leptospirosis: A new look for an old disease. Theriogenology 2020, 141, 41–47. [Google Scholar] [CrossRef]
- Suwancharoen, D.; Chaisakdanugull, Y.; Thanapongtharm, W.; Yoshida, S. Serological survey of leptospirosis in livestock in Thailand. Epidemiol. Infect. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Sripattanakul, S.; Prapong, T.; Kamlangdee, A.; Katzenmeier, G.; Haltrich, D.; Hongprayoon, R.; Prapong, S. Leptospira borgpetersenii Leucine-Rich Repeat Proteins and Derived Peptides in an Indirect ELISA Development for the Diagnosis of Canine Leptospiral Infections. Trop. Med. Infect. Dis. 2022, 7, 311. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, A.; Blanda, V.; Di Marco Lo Presti, V.; Macaluso, G.; Galluzzo, P.; Bertasio, C.; Sciacca, C.; Arcuri, F.; D’Agostino, R.; Ippolito, D.; et al. Leptospira interrogans Serogroup Pomona in a Dairy Cattle Farm in a Multi-Host Zootechnical System. Vet. Sci. 2022, 9, 83. [Google Scholar] [CrossRef]
- Dellagostin, O.A.; Grassmann, A.A.; Rizzi, C.; Schuch, R.A.; Jorge, S.; Oliveira, T.L.; McBride, A.J.; Hartwig, D.D. Reverse Vaccinology: An Approach for Identifying Leptospiral Vaccine Candidates. Int. J. Mol. Sci. 2017, 18, 158. [Google Scholar] [CrossRef]
- Adler, B. Vaccines against leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 251–272. [Google Scholar] [CrossRef]
- Zuerner, R.L.; Alt, D.P.; Palmer, M.V.; Thacker, T.C.; Olsen, S.C. A Leptospira borgpetersenii Serovar Hardjo Vaccine Induces a Th1 Response, Activates NK Cells, and Reduces Renal Colonization. Clin. Vaccine Immunol. 2011, 18, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.; Nally, J.E.; Jones, C.; Sheoran, A.S. Passive immunization with Leptospira LPS-specific agglutinating but not non-agglutinating monoclonal antibodies protect guinea pigs from fatal pulmonary hemorrhages induced by serovar Copenhageni challenge. Vaccine 2011, 29, 4431–4434. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM small animal consensus statement on leptospirosis: Diagnosis, epidemiology, treatment, and prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Jacobs, A.A.C.; Harks, F.; Hoeijmakers, M.; Collell, M.; Segers, R.P.A.M. Safety and efficacy of a new octavalent combined Erysipelas, Parvo and Leptospira vaccine in gilts against Leptospira interrogans serovar Pomona associated disease and foetal death. Vaccine 2015, 33, 3963–3969. [Google Scholar] [CrossRef] [PubMed]
- Lauretti-Ferreira, F.; Silva, P.L.D.; Alcantara, N.M.; Silva, B.F.; Grabher, I.; Souza, G.O.; Nakajima, E.; Akamatsu, M.A.; Vasconcellos, S.A.; Abreu, P.A.E.; et al. New strategies for Leptospira vaccine development based on LPS removal. PLoS ONE 2020, 15, e0230460. [Google Scholar] [CrossRef]
- Wunder, E.A.; Adhikarla, H.; Hamond, C.; Owers Bonner, K.A.; Liang, L.; Rodrigues, C.B.; Bisht, V.; Nally, J.E.; Alt, D.P.; Reis, M.G.; et al. A live attenuated-vaccine model confers cross-protective immunity against different species of the Leptospira genus. elife 2021, 10, e64166. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Oliveira, N.; Jorge, S.; Andrade Colares Maia, M.; Thurow Bunde, T.; Kurz Pedra, A.C.; Pinto Seixas Neto, A.C.; Larré Oliveira, T.; Dellagostin, O.A. Protective efficacy of whole-cell inactivated Leptospira vaccines made using virulent or avirulent strains in a hamster model. Vaccine 2021, 39, 5626–5634. [Google Scholar] [CrossRef]
- Cao, Y.; Faisal, S.M.; Yan, W.; Chang, Y.-C.; McDonough, S.P.; Zhang, N.; Akey, B.L.; Chang, Y.-F. Evaluation Of Novel Fusion Proteins Derived From Extracellular Matrix Binding Domains Of Ligb As Vaccine Candidates Against Leptospirosis In A Hamster Model. Vaccine 2011, 29, 7379–7386. [Google Scholar] [CrossRef]
- Deveson Lucas, D.S.; Lo, M.; Bulach, D.M.; Quinsey, N.S.; Murray, G.L.; Allen, A.; Adler, B. Recombinant LipL32 stimulates interferon-gamma production in cattle vaccinated with a monovalent Leptospira borgpetersenii serovar Hardjo subtype Hardjobovis vaccine. Vet. Microbiol. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Haake, D.A.; Mazel, M.K.; McCoy, A.M.; Milward, F.; Chao, G.; Matsunaga, J.; Wagar, E.A. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 1999, 67, 6572–6582. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.L.; Rizzi, C.; Da Cunha, C.E.P.; Dorneles, J.; Seixas Neto, A.C.P.; Amaral, M.G.; Hartwig, D.D.; Dellagostin, O.A. Recombinant BCG strains expressing chimeric proteins derived from Leptospira protect hamsters against leptospirosis. Vaccine 2019, 37, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.K.; Da Silva, E.F.; Hartwig, D.D.; Cerqueira, G.M.; Amaral, M.; Fagundes, M.Q.; Dossa, R.G.; Dellagostin, O.A. Recombinant Mycobacterium bovis BCG expressing the LipL32 antigen of Leptospira interrogans protects hamsters from challenge. Vaccine 2007, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.K.; Fernandes, C.H.; Hartwig, D.D.; Conceição, F.R.; Aleixo, J.A.G.; Dellagostin, O.A. Evaluation of different ways of presenting LipL32 to the immune system with the aim of developing a recombinant vaccine against leptospirosis. Canadian J. Microbiol. 2007, 53, 472–479. [Google Scholar] [CrossRef]
- Faisal, S.M.; Yan, W.; McDonough, S.P.; Chang, C.-F.; Pan, M.-J.; Chang, Y.-F. Leptosome-entrapped leptospiral antigens conferred significant higher levels of protection than those entrapped with PC-liposomes in a hamster model. Vaccine 2009, 27, 6537–6545. [Google Scholar] [CrossRef]
- Faisal, S.M.; Yan, W.; McDonough, S.P.; Chang, Y.-F. Leptospira Immunoglobulin-Like Protein A Variable Region (Ligavar) Incorporated In Liposomes And Plga Microspheres Produces A Robust Immune Response Correlating To Protective Immunity. Vaccine 2009, 27, 378–387. [Google Scholar] [CrossRef]
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009, 7, 736–747. [Google Scholar] [CrossRef]
- Murray, G.L. The lipoprotein LipL32, an enigma of leptospiral biology. Vet. Microbiol. 2013, 162, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L.; Lo, M.; Bulach, D.M.; Srikram, A.; Seemann, T.; Quinsey, N.S.; Sermswan, R.W.; Allen, A.; Adler, B. Evaluation of 238 antigens of Leptospira borgpetersenii serovar Hardjo for protection against kidney colonisation. Vaccine 2013, 31, 495–499. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Chou, L.-F.; Hong, C.-H.; Chang, M.-Y.; Tsai, C.-Y.; Tian, Y.-C.; Yang, H.-Y.; Yang, C.-W. Crosstalk between E-Cadherin/β-Catenin and NF-κB Signaling Pathways: The Regulation of Host-Pathogen Interaction during Leptospirosis. Int. J. Mol. Sci. 2021, 22, 13132. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Yang, C.-W. Insight into the Structure, Functions, and Dynamics of the Leptospira Outer Membrane Proteins with the Pathogenicity. Membranes 2022, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Hniman, A.; Prapong, S. Development of Leptospira molecular markers by using bioinformation from predicted Leucine-Rich Repeat (LRR) protein genes. J. Thai Vet. Med. Assoc. 2007, 58, 65–78. [Google Scholar]
- Nitipan, S.; Sritrakul, T.; Kunjantarachot, A.; Prapong, S. Identification of epitopes in Leptospira borgpetersenii leucine-rich repeat proteins. Infect. Genet. Evol. 2013, 14, 46–57. [Google Scholar] [CrossRef]
- Sritrakul, T.; Nitipan, S.; Wajjwalku, W.; La-ard, A.; Suphatpahirapol, C.; Petkarnjanapong, W.; Ongphiphadhanakul, B.; Prapong, S. Leptospira borgpetersenii hybrid leucine-rich repeat protein: Cloning and expression, immunogenic identification and molecular docking evaluation. J. Microbiol. Methods 2017, 142, 52–62. [Google Scholar] [CrossRef]
- Suphatpahirapol, C.; Nguyen, T.-H.; Tansiri, Y.; Yingchutrakul, Y.; Roytrakul, S.; Nitipan, S.; Wajjwalku, W.; Haltrich, D.; Prapong, S.; Keawsompong, S. Expression of a leptospiral leucine-rich repeat protein using a food-grade vector in Lactobacillus plantarum, as a strategy for vaccine delivery. 3 Biotech. 2019, 9, 324. [Google Scholar] [CrossRef]
- Nitipan, S. Molecular Cloning and Sequence Analysis of the Genes Encoding Leucine-rich Repeat Proteins of Pathogenic Leptospira borgpetersenii. Ph.D. Thesis, Kasetsart University, Bangkok, Thailand, 2013. [Google Scholar]
- Gomes, C.K.; Guedes, M.; Potula, H.H.; Dellagostin, O.A.; Gomes-Solecki, M. Sex Matters: Male Hamsters Are More Susceptible to Lethal Infection with Lower Doses of Pathogenic Leptospira than Female Hamsters. Infect. Immun. 2018, 86, e00369-18. [Google Scholar] [CrossRef]
- Kunjantarachot, A.; Yan, W.; McDonough, S.P.; Prapong, S.; Theeragool, G.; Chang, Y.-F. Immunogenicity of Leptospira interrogans Outer Membrane Vesicles in a Hamster Model. J. Vaccines Vaccin. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Ptak, C.P.; Tseng, A.; Suguiura, I.M.d.S.; McDonough, S.P.; Sritrakul, T.; Li, T.; Lin, Y.-P.; Gillilan, R.E.; Oswald, R.E.; et al. Extended low-resolution structure of a Leptospira antigen offers high bactericidal antibody accessibility amenable to vaccine design. eLife 2017, 6, e30051. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.L.; Choy, H.A.; Kelley, M.M.; Matsunaga, J.; Babbitt, J.T.; Lewis, M.S.; Aleixo, J.A.G.; Haake, D.A. A LigA Three-Domain Region Protects Hamsters from Lethal Infection by Leptospira interrogans. PLoS Negl. Trop. Dis. 2011, 5, e1422. [Google Scholar] [CrossRef]
- Faisal, S.M.; Yan, W.; Chen, C.-S.; Palaniappan, R.U.M.; McDonough, S.P.; Chang, Y.-F. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 2008, 26, 277–287. [Google Scholar] [CrossRef]
- Khare, P.; Jaiswal, A.K.; Tripathi, C.D.P.; Sundar, S.; Dube, A. Immunoprotective responses of T helper type 1 stimulatory protein-S-adenosyl-L-homocysteine hydrolase against experimental visceral leishmaniasis. Clin. Exp. Immunol. 2016, 185, 165–179. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Blumerman, S.; Gay, C.; Bolin, C.; Duby, R.; Baldwin, C.L. Comparison of three different leptospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 2003, 21, 4448–4458. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, C.E.P.; Bettin, E.B.; Bakry, A.F.A.A.Y.; Seixas Neto, A.C.P.; Amaral, M.G.; Dellagostin, O.A. Evaluation of different strategies to promote a protective immune response against leptospirosis using a recombinant LigA and LigB chimera. Vaccine 2019, 37, 1844–1852. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Alt, D.P.; Nally, J.E.; Olsen, S.C.; Pascual, D.W. Bovine Immune Response to Vaccination and Infection with Leptospira borgpetersenii Serovar Hardjo. mSphere 2021, 6, e00988-20. [Google Scholar] [CrossRef] [PubMed]
- Khantaboot, A.; Setkit, T.; Panneum, S.; Bamrungkit, K.; Kamlers, S.; Wongsanit, J.; Tancharean, K.; Sakpuaram, T.; Pinyopummin, A. Leptospirosis outbreak in dairy cattle in Ratchaburi province: A case report. In Proceedings of the 47th Kasetsart University Annual Conference, Bangkok, Thailand, 17–20 March 2009; pp. 275–286. [Google Scholar]
- Kobe, B.; Kajava, A.V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2001, 11, 725–732. [Google Scholar] [CrossRef]
- Gaillard, J.L.; Berche, P.; Frehel, C.; Gouln, E.; Cossart, P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 1991, 65, 1127–1141. [Google Scholar] [CrossRef]
- Parida, S.K.; Domann, E.; Rohde, M.; Müller, S.; Darji, A.; Hain, T.; Wehland, J.; Chakraborty, T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol. 1998, 28, 81–93. [Google Scholar] [CrossRef]
- Sabet, C.; Lecuit, M.; Cabanes, D.; Cossart, P.; Bierne, H. LPXTG Protein InlJ, a Newly Identified Internalin Involved in Listeria monocytogenes Virulence. Infect. Immun. 2005, 73, 6912–6922. [Google Scholar] [CrossRef]
- Sawyer, R.T.; Drevets, D.A.; Campbell, P.A.; Potter, T.A. Internalin A can mediate phagocytosis of Listeria monocytogenes by mouse macrophage cell lines. J. Leukoc. Biol. 1996, 60, 603–610. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Vacratsis, P.O.; Bliska, J.B.; Dixon, J.E. The Yersinia Virulence Factor YopM Forms a Novel Protein Complex with Two Cellular Kinases. J. Biol. Chem. 2003, 278, 18514–18523. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Kerschen, E.J.; Cohen, D.A.; Kaplan, A.M.; Van Rooijen, N.; Straley, S.C. Gr1+ Cells Control Growth of YopM-Negative Yersinia pestis during Systemic Plague. Infect. Immun. 2009, 77, 3791–3806. [Google Scholar] [CrossRef]
- Helft, L.; Reddy, V.; Chen, X.; Koller, T.; Federici, L.; Fernández-Recio, J.; Gupta, R.; Bent, A. LRR Conservation Mapping to Predict Functional Sites within Protein Leucine-Rich Repeat Domains. PLoS ONE 2011, 6, e21614. [Google Scholar] [CrossRef]
- Loimaranta, V.; Hytönen, J.; Pulliainen, A.T.; Sharma, A.; Tenovuo, J.; Strömberg, N.; Finne, J. Leucine-rich Repeats of Bacterial Surface Proteins Serve as Common Pattern Recognition Motifs of Human Scavenger Receptor gp340*. J. Biol. Chem. 2009, 284, 18614–18623. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.W.; Faisal, S.M.; McDonough, S.P.; Divers, T.J.; Barr, S.C.; Chang, C.F.; Pan, M.J.; Chang, Y.F. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes Infect. 2009, 11, 230–237. [Google Scholar] [CrossRef]
- Conrad, N.L.; Cruz McBride, F.W.; Souza, J.D.; Silveira, M.M.; Felix, S.; Mendonca, K.S.; Santos, C.S.; Athanazio, D.A.; Medeiros, M.A.; Reis, M.G.; et al. LigB subunit vaccine confers sterile immunity against challenge in the hamster model of leptospirosis. PLoS Negl. Trop. Dis. 2017, 11, e0005441. [Google Scholar] [CrossRef]
- Fernandes, L.G.V.; Teixeira, A.F.; Filho, A.F.S.; Souza, G.O.; Vasconcellos, S.A.; Heinemann, M.B.; Romero, E.C.; Nascimento, A.L.T.O. Immune response and protective profile elicited by a multi-epitope chimeric protein derived from Leptospira interrogans. Int. J. Infect. Dis. 2017, 57, 61–69. [Google Scholar] [CrossRef]
- Murray, G.L.; Simawaranon, T.; Kaewraemruaen, C.; Adler, B.; Sermswan, R.W. Heterologous protection elicited by a live, attenuated, Leptospira vaccine. Vet. Microbiol. 2018, 223, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.M.; Varma, V.P.; Subathra, M.; Azam, S.; Sunkara, A.K.; Akif, M.; Baig, M.S.; Chang, Y.-F. Leptospira surface adhesin (Lsa21) induces Toll like receptor 2 and 4 mediated inflammatory responses in macrophages. Sci. Rep. 2016, 6, 39530. [Google Scholar] [CrossRef] [PubMed]
| Culture Results (Score) | Liver | Kidney | Urinary Bladder | |||
|---|---|---|---|---|---|---|
| PBS | 2271 | PBS | 2271 | PBS | 2271 | |
| Positive (+1) | 7 | 2 | 8 | 2 (#) | 7 | 1 |
| Negative (0) | 1 | 6 | 0 | 6 | 1 | 7 |
| Average score | 0.875 | 0.250 | 1.000 | 0.250 | 0.875 | 0.125 |
| Statistical differences | * | * | * | |||
| Score | Lung | Liver | Kidney | |||
|---|---|---|---|---|---|---|
| PBS | 2271 | PBS | 2271 | PBS | 2271 | |
| 0 | 1 | 3 | 0 | 1 | 0 | 3 |
| 1 | 2 | 3 | 2 | 3 | 6 | 3 |
| 2 | 5 | 1 | 0 | 1 | 2 | 1 |
| 3 | 0 | 1 | 6 | 3 | 0 | 1 |
| Average score | 1.50 | 1.00 | 2.50 | 1.75 | 1.25 | 1.00 |
| p-value | 0.12 | 0.17 | 0.09 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapong, S.; Tansiri, Y.; Sritrakul, T.; Sripattanakul, S.; Sopitthummakhun, A.; Katzenmeier, G.; Hsieh, C.-L.; McDonough, S.P.; Prapong, T.; Chang, Y.-F. Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates. Trop. Med. Infect. Dis. 2023, 8, 6. https://doi.org/10.3390/tropicalmed8010006
Prapong S, Tansiri Y, Sritrakul T, Sripattanakul S, Sopitthummakhun A, Katzenmeier G, Hsieh C-L, McDonough SP, Prapong T, Chang Y-F. Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates. Tropical Medicine and Infectious Disease. 2023; 8(1):6. https://doi.org/10.3390/tropicalmed8010006
Chicago/Turabian StylePrapong, Siriwan, Yada Tansiri, Tepyuda Sritrakul, Sineenat Sripattanakul, Aukkrimapann Sopitthummakhun, Gerd Katzenmeier, Chin-Lin Hsieh, Sean P. McDonough, Teerasak Prapong, and Yung-Fu Chang. 2023. "Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates" Tropical Medicine and Infectious Disease 8, no. 1: 6. https://doi.org/10.3390/tropicalmed8010006
APA StylePrapong, S., Tansiri, Y., Sritrakul, T., Sripattanakul, S., Sopitthummakhun, A., Katzenmeier, G., Hsieh, C.-L., McDonough, S. P., Prapong, T., & Chang, Y.-F. (2023). Leptospira borgpetersenii Leucine-Rich Repeat Proteins Provide Strong Protective Efficacy as Novel Leptospiral Vaccine Candidates. Tropical Medicine and Infectious Disease, 8(1), 6. https://doi.org/10.3390/tropicalmed8010006








