Immunoprofiling of Equine Plasma against Deinagkistrodon acutus in Taiwan: Key to Understanding Differential Neutralization Potency in Immunized Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Snake Venom and Hyperimmunized Horse Plasma
2.2. Animals
2.3. Animal Ethics Statement
2.4. Evaluation of the Neutralization Potency of Hyperimmunized Equine Plasma
2.5. Indirect-Enzyme-Linked Immunosorbent Assay (Indirect ELISA)
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Western Blot Analysis
2.8. C18 Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) Fractionation of D. acutus Venom
2.9. In-Gel Tryptic Digestion of Protein
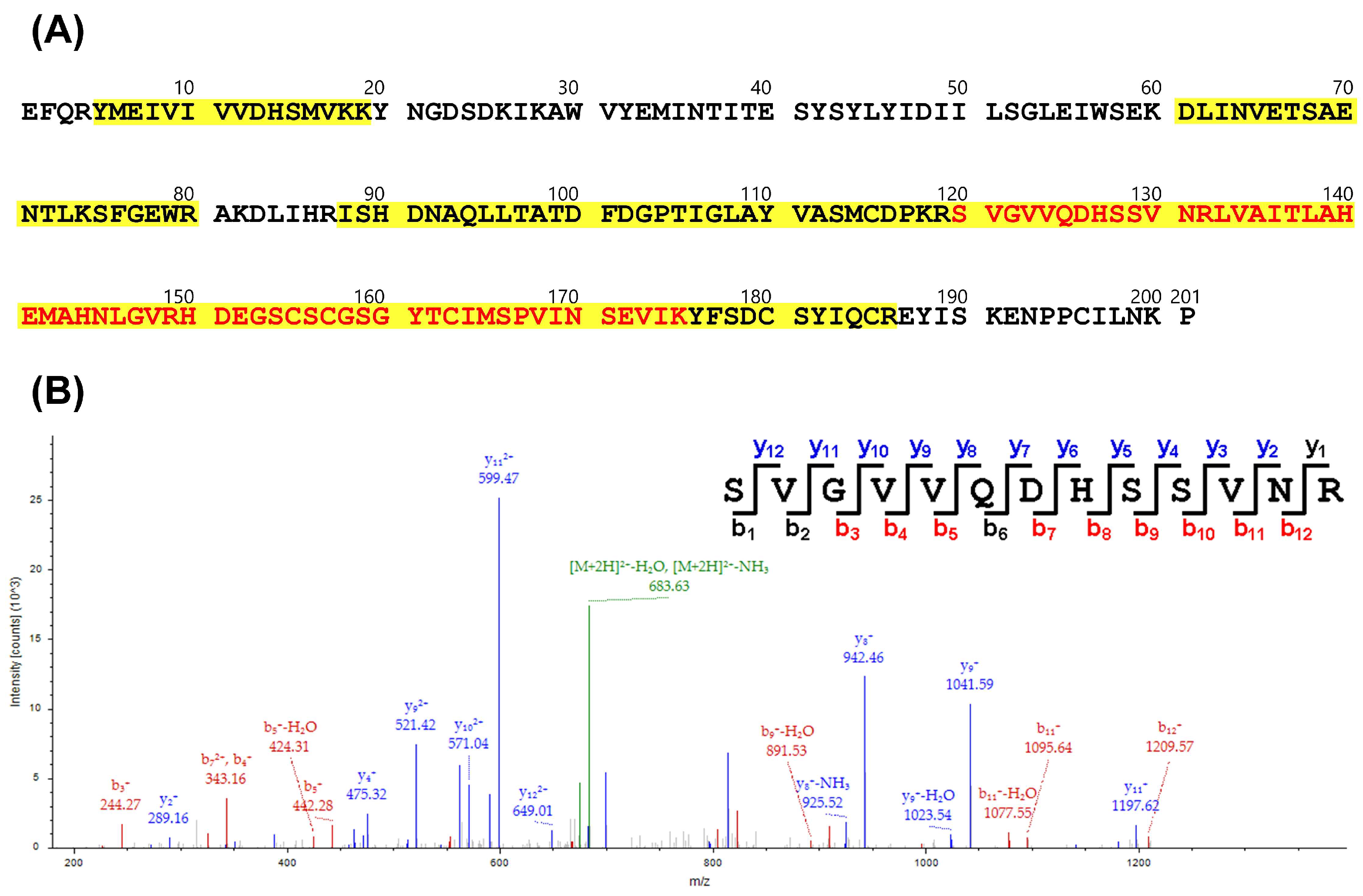
2.10. LC-MS/MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Neutralization Potency of Equine Plasma against Deinagkistrodon acutus Venom
3.2. Comparison of the Efficacy of High-Potency and Low-Potency Horse Plasma against Crude Venom of D. acutus
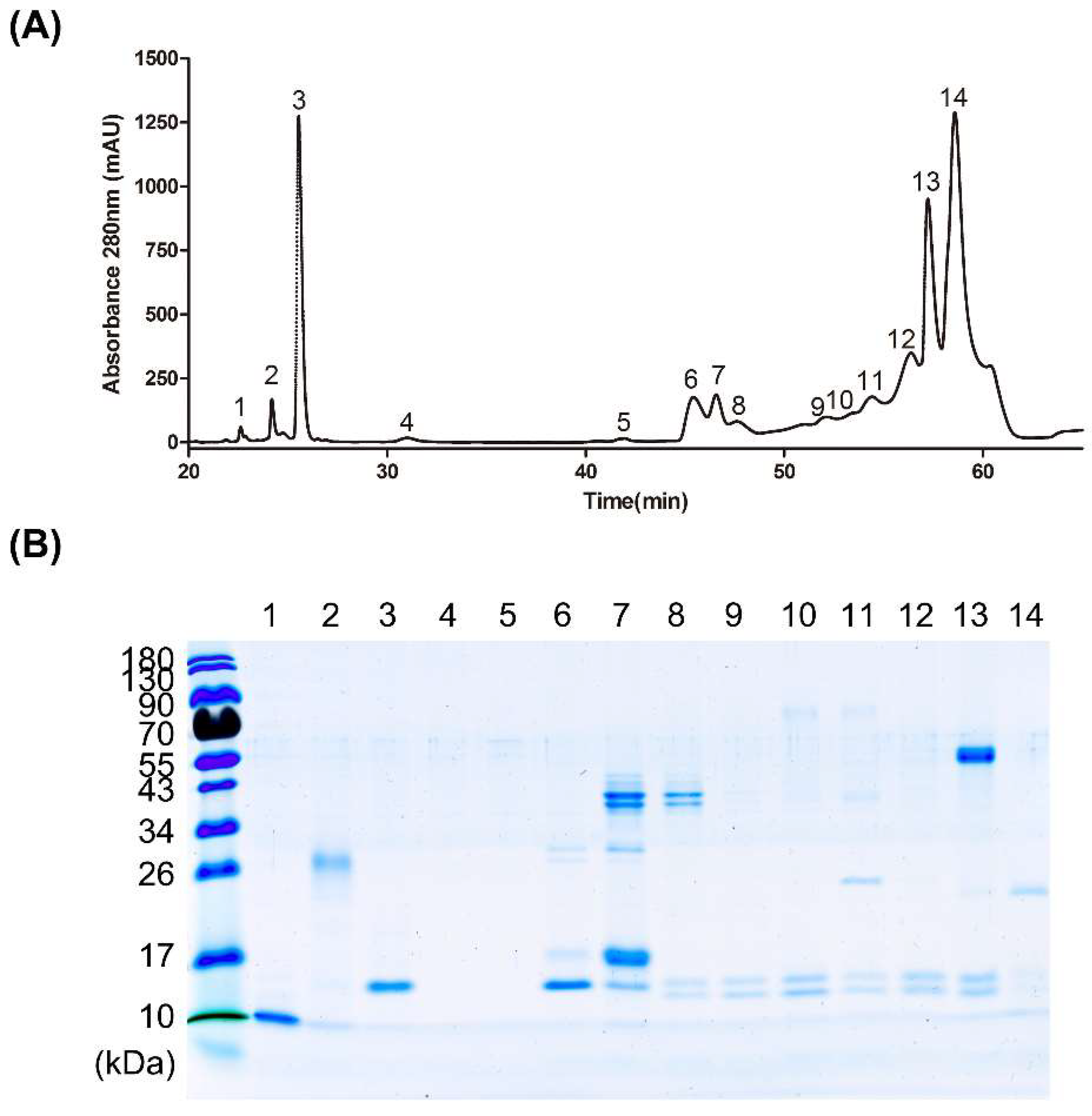
3.3. Fractionation of Venom Protein Components from D. acutus
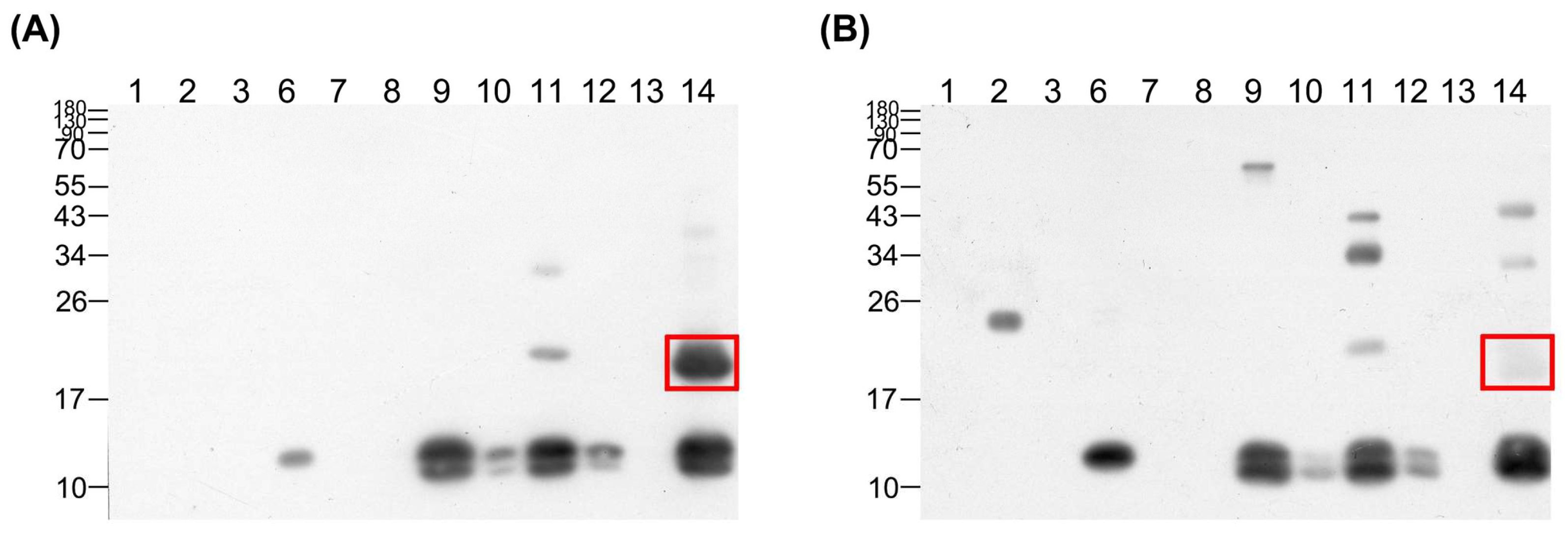
3.4. Affinity of High-Potency and Low-Potency Horse Plasma for Protein Fractions from D. acutus Venom
3.5. Differences in Antibody Titers against D. acutus Venom between High- and Low-Potency Horse Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17079. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Lai, C.S.; Lin, S.D. Management of poisonous snake bites in southern Taiwan. Kaohsiung J. Med. Sci. 2007, 23, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Huang, S.W.; Mao, Y.C.; Liu, M.W.; Lee, K.H.; Lai, P.F.; Tsai, M.J. Clinical and laboratory features distinguishing between Deinagkistrodon acutus and Daboia siamensis envenomation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 43. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, M.; Xue, C.; Liang, J.; Huang, F. Analysis of the Composition of Deinagkistrodon acutus Snake Venom Based on Proteomics, and Its Antithrombotic Activity and Toxicity Studies. Molecules 2022, 27, 2229. [Google Scholar] [CrossRef]
- Chen, P.C.; Huang, M.N.; Chang, J.F.; Liu, C.C.; Chen, C.K.; Hsieh, C.H. Snake venom proteome and immuno-profiling of the hundred-pace viper, Deinagkistrodon acutus, in Taiwan. Acta Trop. 2019, 189, 137–144. [Google Scholar] [CrossRef]
- Tan, K.Y.; Shamsuddin, N.N.; Tan, C.H. Sharp-nosed Pit Viper (Deinagkistrodon acutus) from Taiwan and China: A comparative study on venom toxicity and neutralization by two specific antivenoms across the Strait. Acta Trop. 2022, 232, 106495. [Google Scholar] [CrossRef]
- Meyer, W.P.; Habib, A.G.; Onayade, A.A.; Yakubu, A.; Smith, D.C.; Nasidi, A.; Daudu, I.J.; Warrell, D.A.; Theakston, R.D. First clinical experiences with a new ovine Fab Echis ocellatus snake bite antivenom in Nigeria: Randomized comparative trial with Institute Pasteur Serum (Ipser) Africa antivenom. Am. J. Trop. Med. Hyg. 1997, 56, 291–300. [Google Scholar] [CrossRef]
- Bochner, R. Paths to the discovery of antivenom serotherapy in France. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 20. [Google Scholar] [CrossRef]
- Dart, R.C.; McNally, J. Efficacy, safety, and use of snake antivenoms in the United States. Ann. Emerg. Med. 2001, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- De Silva, H.A.; Ryan, N.M.; de Silva, H.J. Adverse reactions to snake antivenom, and their prevention and treatment. Br. J. Clin. Pharmacol. 2016, 81, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Meenatchisundaram, S.; Parameswari, G.; Michael, A.; Ramalingam, S. Studies on pharmacological effects of Russell’s viper and Saw-scaled viper venom and its neutralization by chicken egg yolk antibodies. Int. Immunopharmacol. 2008, 8, 1067–1073. [Google Scholar] [CrossRef]
- Karlson-Stiber, C.; Persson, H. Antivenom treatment in Vipera berus envenoming—Report of 30 cases. J. Intern. Med. 1994, 235, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Leon, G.; Herrera, M.; Segura, A.; Villalta, M.; Vargas, M.; Gutierrez, J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon 2013, 76, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Worlds Health Organization. Progress in the characterization of venoms and standardization of antivenoms. WHO Offset Publ. 1981, 58, 1–44. [Google Scholar]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Brown, N.I. Consequences of neglect: Analysis of the sub-Saharan African snake antivenom market and the global context. PLoS Negl. Trop. Dis. 2012, 6, e1670. [Google Scholar] [CrossRef]
- Habib, A.G.; Lamorde, M.; Dalhat, M.M.; Habib, Z.G.; Kuznik, A. Cost-effectiveness of antivenoms for snakebite envenoming in Nigeria. PLoS Negl. Trop. Dis. 2015, 9, e3381. [Google Scholar] [CrossRef]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research strategies to improve snakebite treatment: Challenges and progress. J. Proteom. 2011, 74, 1768–1780. [Google Scholar] [CrossRef]
- Chippaux, J.P. The development and use of immunotherapy in Africa. Toxicon 1998, 36, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Ismail, A.K.; Abidin, S.A.Z.; Blanco, F.B.; Blessmann, J.; Choumlivong, K.; Comandante, J.D.; Doan, U.V.; Mohamed Ismail, Z.; Khine, Y.Y.; et al. Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Glob. Health 2022, 7, e007639. [Google Scholar] [CrossRef] [PubMed]
- Alangode, A.; Rajan, K.; Nair, B.G. Snake antivenom: Challenges and alternate approaches. Biochem. Pharmacol. 2020, 181, 114135. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K. Antivenom efficacy or effectiveness: The Australian experience. Toxicology 2010, 268, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Maria, W.S.; Cambuy, M.O.; Costa, J.O.; Velarde, D.T.; Chavez-Olortegui, C. Neutralizing potency of horse antibothropic antivenom. Correlation between in vivo and in vitro methods. Toxicon 1998, 36, 1433–1439. [Google Scholar] [CrossRef]
- Santos Barreto, G.N.; de Oliveira, S.S.; Dos Anjos, I.V.; Chalkidis, H.M.; Mourao, R.H.; Moura-da-Silva, A.M.; Sano-Martins, I.S.; Goncalves, L.R. Experimental Bothrops atrox envenomation: Efficacy of antivenom therapy and the combination of Bothrops antivenom with dexamethasone. PLoS Negl. Trop. Dis. 2017, 11, e0005458. [Google Scholar] [CrossRef]
- Muniz, E.G.; Maria, W.S.; Estevao-Costa, M.I.; Buhrnheim, P.; Chavez-Olortegui, C. Neutralizing potency of horse antibothropic Brazilian antivenom against Bothrops snake venoms from the Amazonian rain forest. Toxicon 2000, 38, 1859–1863. [Google Scholar] [CrossRef]
- Oshima-Franco, Y.; Hyslop, S.; Prado-Franceschi, J.; Cruz-Hofling, M.A.; Rodrigues-Simioni, L. Neutralizing capacity of antisera raised in horses and rabbits against Crotalus durissus terrificus (South American rattlesnake) venom and its main toxin, crotoxin. Toxicon 1999, 37, 1341–1357. [Google Scholar] [CrossRef]
- Villalta, M.; Pla, D.; Yang, S.L.; Sanz, L.; Segura, A.; Vargas, M.; Chen, P.Y.; Herrera, M.; Estrada, R.; Cheng, Y.F.; et al. Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: Keys to understand the variable immune response in horses. J. Proteom. 2012, 75, 5628–5645. [Google Scholar] [CrossRef]
- Kuwajima, Y. Immunological researches on the main Formosan poisonous snakes, especially on the venoms. 5. Therapeutic experiment of mice injected with the snake venom by means of the antivenin serum. Jpn. J. Exp. Med. 1953, 23, 457–464. [Google Scholar]
- Liau, M.Y.; Huang, R.J. Toxoids and antivenoms of venomous snakes in Taiwan. J. Toxicol. Toxin. Rev. 1997, 16, 163–175. [Google Scholar]
- Howard-Jones, N. A CIOMS ethical code for animal experimentation. WHO Chron. 1985, 39, 51–56. [Google Scholar] [PubMed]
- Liu, C.C.; Hsiao, Y.C.; Chu, L.J.; Wang, P.J.; Liu, C.H.; Hsieh, W.C.; Yu, J.S. Development of Antibody Detection ELISA Based on Immunoreactive Toxins and Toxin-Derived Peptides to Evaluate the Neutralization Potency of Equine Plasma against Naja atra in Taiwan. Toxins 2021, 13, 818. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Wu, C.J.; Hsiao, Y.C.; Yang, Y.H.; Liu, K.L.; Huang, G.J.; Hsieh, C.H.; Chen, C.K.; Liaw, G.W. Snake venom proteome of Protobothrops mucrosquamatus in Taiwan: Delaying venom-induced lethality in a rodent model by inhibition of phospholipase A2 activity with varespladib. J. Proteom. 2021, 234, 104084. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Lin, C.C.; Hsiao, Y.C.; Wang, P.J.; Yu, J.S. Proteomic characterization of six Taiwanese snake venoms: Identification of species-specific proteins and development of a SISCAPA-MRM assay for cobra venom factors. J. Proteom. 2018, 187, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, W.; Cheng, X.; Jin, G.; Shen, X.; Lou, H.; Liu, J. Molecular cloning and sequence analysis of cDNA encoding haemorrhagic toxin acutolysin A from Agkistrodon acutus. Toxicon 1999, 37, 1539–1548. [Google Scholar] [CrossRef]
- Bode, W.; Gomis-Ruth, F.X.; Stockler, W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Borkakoti, N.; Winkler, F.K.; Williams, D.H.; D’Arcy, A.; Broadhurst, M.J.; Brown, P.A.; Johnson, W.H.; Murray, E.J. Structure of the catalytic domain of human fibroblast collagenase complexed with an inhibitor. Nat. Struct. Biol. 1994, 1, 106–110. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Liu, J.; Lu, Z. Purification and characterization of hemorrhagic components from Agkistrodon acutus (hundred pace snake) venom. Toxicon 1981, 19, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Liao, S.C.; Yang, C.C. Deinagkistrodon acutus envenomation: A report of three cases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Valenta, J.; Stach, Z.; Michalek, P. Envenoming by Crotalid Snake Chinese Moccasin Agkistrodon Acutus Bite—A Case Report. Prague Med. Rep. 2015, 116, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhao, S.; Tong, F.; Liang, Y.; Le Grange, J.M.; Kuang, W.; Zhou, Y. Unexpected death in a young man associated with a unilateral swollen leg: Pathological and toxicological findings in a fatal snakebite from Deinagkistrodon acutus (Chinese moccasin). J. Forensic Sci. 2021, 66, 786–792. [Google Scholar] [CrossRef]
- Li, Q.B.; Yu, Q.S.; Huang, G.W.; Tokeshi, Y.; Nakamura, M.; Kinjoh, K.; Kosugi, T. Hemostatic disturbances observed in patients with snakebite in south China. Toxicon 2000, 38, 1355–1366. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutierrez, J.M.; Calvete, J.J.; Wuster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Karatt-Vellatt, A.; Masters, E.W.; Arias, A.S.; Pus, U.; Knudsen, C.; Oscoz, S.; Slavny, P.; Griffiths, D.T.; Luther, A.M.; et al. In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat. Commun. 2018, 9, 3928. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Samuel, S.P.; Bickler, P.E.; Lewin, M.R. Developing Small Molecule Therapeutics for the Initial and Adjunctive Treatment of Snakebite. J. Trop. Med. 2018, 2018, 4320175. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rucavado, A.; Ovadia, M. Metalloproteinase inhibitors in snakebite envenomations. Drug Discov. Today 1999, 4, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, L.O.; Xie, C.; Ainsworth, S.; Alsolaiss, J.; Crittenden, E.; Dawson, C.A.; Softley, R.; Bartlett, K.E.; Harrison, R.A.; Kool, J.; et al. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020, 11, 6094. [Google Scholar] [CrossRef] [PubMed]


| No. | Group | Administrated Venom a | Plasma | Dilution Buffer b | Survival |
|---|---|---|---|---|---|
| (mL) | (mL) | (mL) | (n = 3) | ||
| 341 | A | 0.5 | 0.5 | 0 | 3/3 |
| 341 | B | 0.5 | 0.25 | 0.25 | 3/3 |
| 341 | C | 0.5 | 0.166 | 0.334 | 3/3 |
| 341 | D | 0.5 | 0.125 | 0.375 | 3/3 |
| 341 | E | 0.5 | 0.1 | 0.4 | 3/3 |
| 341 | F | 0.5 | 0.083 | 0.417 | 1/3 |
| 410 | A | 0.5 | 0.5 | 0 | 3/3 |
| 410 | B | 0.5 | 0.25 | 0.25 | 2/3 |
| 410 | C | 0.5 | 0.166 | 0.334 | 0/3 |
| 410 | D | 0.5 | 0.125 | 0.375 | 0/3 |
| 410 | E | 0.5 | 0.1 | 0.4 | 0/3 |
| 410 | F | 0.5 | 0.083 | 0.417 | 0/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-J.; Liaw, G.-W.; Chen, C.-K.; Ouyang, C.-H.; Yang, Y.-X.; Chu, L.-C.; Hsiao, Y.-C.; Liu, C.-H.; Hsieh, W.-C.; Wang, C.-Y.; et al. Immunoprofiling of Equine Plasma against Deinagkistrodon acutus in Taiwan: Key to Understanding Differential Neutralization Potency in Immunized Horses. Trop. Med. Infect. Dis. 2023, 8, 51. https://doi.org/10.3390/tropicalmed8010051
Wu C-J, Liaw G-W, Chen C-K, Ouyang C-H, Yang Y-X, Chu L-C, Hsiao Y-C, Liu C-H, Hsieh W-C, Wang C-Y, et al. Immunoprofiling of Equine Plasma against Deinagkistrodon acutus in Taiwan: Key to Understanding Differential Neutralization Potency in Immunized Horses. Tropical Medicine and Infectious Disease. 2023; 8(1):51. https://doi.org/10.3390/tropicalmed8010051
Chicago/Turabian StyleWu, Cho-Ju, Geng-Wang Liaw, Chun-Kuei Chen, Chun-Hsiang Ouyang, Yi-Xiu Yang, Li-Chieh Chu, Yung-Chin Hsiao, Chien-Hsin Liu, Wen-Chin Hsieh, Cyong-Yi Wang, and et al. 2023. "Immunoprofiling of Equine Plasma against Deinagkistrodon acutus in Taiwan: Key to Understanding Differential Neutralization Potency in Immunized Horses" Tropical Medicine and Infectious Disease 8, no. 1: 51. https://doi.org/10.3390/tropicalmed8010051
APA StyleWu, C.-J., Liaw, G.-W., Chen, C.-K., Ouyang, C.-H., Yang, Y.-X., Chu, L.-C., Hsiao, Y.-C., Liu, C.-H., Hsieh, W.-C., Wang, C.-Y., Liou, Y.-S., Liu, C.-C., & Hsieh, C.-H. (2023). Immunoprofiling of Equine Plasma against Deinagkistrodon acutus in Taiwan: Key to Understanding Differential Neutralization Potency in Immunized Horses. Tropical Medicine and Infectious Disease, 8(1), 51. https://doi.org/10.3390/tropicalmed8010051








