Detection of Monkeypox Virus according to The Collection Site of Samples from Confirmed Cases: A Systematic Review
(This article belongs to the Section Infectious Diseases)
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Outcomes
2.6. Data Collection Process and Data Items
3. Results
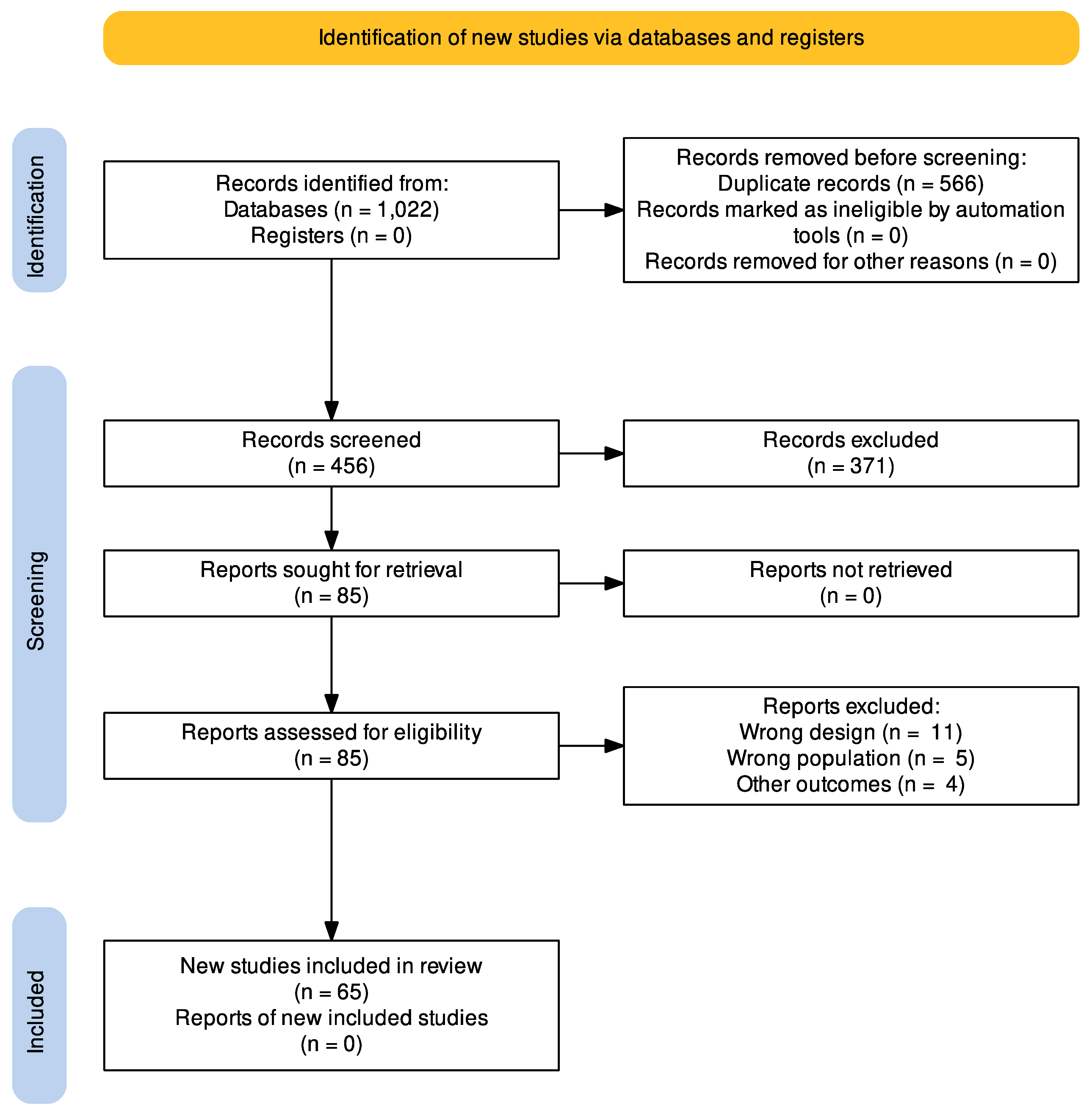
3.1. Study Selection
3.2. Study Characteristics
3.3. Demographical Characteristics and Diagnostic Method for Monkeypox
3.4. Location of Lesions, Location of Positive MPX Viral PCR Results, and the Evolution of the Disease
4. Discussion
5. Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ilic, I.; Zivanovic Macuzic, I.; Ilic, M. Global Outbreak of Human Monkeypox in 2022: Update of Epidemiology. Trop. Med. Infect. Dis. 2022, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Selvaraj, P.; Halesh, M.B.; Jeyaraman, N.; Nallakumarasamy, A.; Gupta, M.; Maffulli, N.; Gupta, A. Monkeypox: An Emerging Global Public Health Emergency. Life 2022, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- León-Figueroa, D.A.; Barboza, J.J.; Garcia-Vasquez, E.A.; Bonilla-Aldana, D.K.; Diaz-Torres, M.; Saldaña-Cumpa, H.M.; Diaz-Murillo, M.T.; Cruz, O.C.-S.; Rodriguez-Morales, A.J. Epidemiological Situation of Monkeypox Transmission by Possible Sexual Contact: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- CDC. Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 28 October 2022).
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Bonilla-Aldana, D.K.; et al. Human Monkeypox Disease (MPX). Infez. Med. 2022, 30, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeefi, M.; Rungkitwattanakul, D.; Unonu, J.; Franklin, C.-J.; Lyons, J.; Hager, K.; Daftary, M.N. The 2022 Human Monkeypox Outbreak: Clinical Review and Management Guidance. Am. J. Health Syst. Pharm. 2022, zxac300. [Google Scholar] [CrossRef]
- Mondolfi, A.P.; Guerra, S.; Muñoz, M.; Luna, N.; Hernandez, M.M.; Patino, L.H.; Reidy, J.; Banu, R.; Shrestha, P.; Liggayu, B.; et al. Evaluation and Validation of an RT-PCR Assay for Specific Detection of Monkeypox Virus (MPXV). J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic Characterization and Signs of Microevolution in the 2022 Multi-Country Outbreak of Monkeypox Virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The Never-Ending Global Emergence of Viral Zoonoses after COVID-19? The Rising Concern of Monkeypox in Europe, North America and Beyond. Travel Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Beer, E.M.; Rao, V.B. A Systematic Review of the Epidemiology of Human Monkeypox Outbreaks and Implications for Outbreak Strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- Nguyen, P.-Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Lulli, L.G.; Baldassarre, A.; Mucci, N.; Arcangeli, G. Prevention, Risk Exposure, and Knowledge of Monkeypox in Occupational Settings: A Scoping Review. Trop. Med. Infect. Dis. 2022, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Angelo, K.M.; Smith, T.; Camprubí-Ferrer, D.; Balerdi-Sarasola, L.; Díaz Menéndez, M.; Servera-Negre, G.; Barkati, S.; Duvignaud, A.; Huber, K.L.B.; Chakravarti, A.; et al. Epidemiological and Clinical Characteristics of Patients with Monkeypox in the GeoSentinel Network: A Cross-Sectional Study. Lancet Infect. Dis. 2022, in press. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox-A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance 2022, 27, 2200421. [Google Scholar] [CrossRef]
- De Baetselier, I.; Van Dijck, C.; Kenyon, C.; Coppens, J.; Michiels, J.; de Block, T.; Smet, H.; Coppens, S.; Vanroye, F.; Bugert, J.J.; et al. Retrospective Detection of Asymptomatic Monkeypox Virus Infections among Male Sexual Health Clinic Attendees in Belgium. Nat. Med. 2022, 28, 2288–2292. [Google Scholar] [CrossRef]
- Moschese, D.; Pozza, G.; Mileto, D.; Giacomelli, A.; Cutrera, M.; Cossu, M.V.; Matone, M.; Beltrami, M.; Salari, F.; Antinori, S.; et al. Isolation of Viable Monkeypox Virus from Anal and Urethral Swabs, Italy, May to July 2022. Eurosurveillance 2022, 27, 2200675. [Google Scholar] [CrossRef]
- Noe, S.; Zange, S.; Seilmaier, M.; Antwerpen, M.H.; Fenzl, T.; Schneider, J.; Spinner, C.D.; Bugert, J.J.; Wendtner, C.-M.; Wölfel, R. Clinical and Virological Features of First Human Monkeypox Cases in Germany. Infection 2022. [Google Scholar] [CrossRef]
- Ferré, V.M.; Bachelard, A.; Zaidi, M.; Armand-Lefevre, L.; Descamps, D.; Charpentier, C.; Ghosn, J. Detection of Monkeypox Virus in Anorectal Swabs from Asymptomatic Men Who Have Sex With Men in a Sexually Transmitted Infection Screening Program in Paris, France. Ann. Intern. Med. 2022, 175, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Porzucek, A.J.; Proctor, A.M.; Klinkhammer, K.E.; Tritsch, S.R.; Robertson, M.A.; Bashor, J.P.; Villani, J.; Sepulveda, J.L.; Mores, C.N. Development of an Accessible and Scalable QPCR Assay for Monkeypox Virus Detection. J. Infect. Dis. 2022, jiac414. [Google Scholar] [CrossRef] [PubMed]
- Hasso, M.; Perusini, S.; Eshaghi, A.; Tang, E.; Olsha, R.; Zhang, H.; Lau, E.; Sullivan, A.; Cronin, K.; Lee, S.; et al. Monkeypox Virus Detection in Different Clinical Specimen Types. Emerg. Infect. Dis. 2022, 28, 2513–2515. [Google Scholar] [CrossRef] [PubMed]
- Brito Caldeira, M.; Fernandes, C. Cutaneous Lesions From Monkeypox Infection. Sex. Transm. Dis. 2022, 49, 595. [Google Scholar] [CrossRef] [PubMed]
- Brundu, M.; Marinello, S.; Scaglione, V.; Ferrari, A.; Franchin, E.; Mazzitelli, M.; Cattelan, A.M. The First Case of Monkeypox Virus and Acute HIV Infection: Should We Consider Monkeypox a New Possible Sexually Transmitted Infection? J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Costello, V.; Sowash, M.; Gaur, A.; Cardis, M.; Pasieka, H.; Wortmann, G.; Ramdeen, S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1002–1005. [Google Scholar] [CrossRef]
- Davido, B.; D’Anglejan, E.; Baudoin, R.; Dahmane, L.; Chaud, A.; Cortier, M.; Vauloup-Fellous, C.; De Truchis, P.; Ghosn, J. Monkeypox Outbreak 2022: An Unusual Case of Peritonsillar Abscess in a Person Previously Vaccinated against Smallpox. J. Travel Med. 2022, 29, taac082. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef]
- Eseigbe, E.E.; Akude, C.; Osagie, I.A.; Eseigbe, P. Human Monkey Pox Virus Infection in Plateau State, North Central Nigeria: A Report of Two Cases. West Afr. J. Med. 2021, 38, 1242–1246. [Google Scholar] [CrossRef]
- Gedela, K.; Da Silva Fontoura, D.; Salam, A.; Gorman, G.; Golden, J.; O’Hara, G.; Elawaidy, A.; Tittle, V.; Girometti, N.; Whitlock, G.; et al. Infectious Proctitis Due to Human Monkeypox. Clin. Infect. Dis. 2022, ciac713. [Google Scholar] [CrossRef]
- Gomez-Garberi, M.; Sarrio-Sanz, P.; Martinez-Cayuelas, L.; Delgado-Sanchez, E.; Bernabeu-Cabezas, S.; Peris-Garcia, J.; Sanchez-Caballero, L.; Nakdali-Kassab, B.; Egea-Sancho, C.; Olarte-Barragan, E.H.; et al. Genitourinary Lesions Due to Monkeypox. Eur. Urol. 2022, 82, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L.; et al. Family Cluster of Three Cases of Monkeypox Imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Hofer, U. Case Series of Monkeypox Infections. Nat. Rev. Microbiol. 2022, 20, 445. [Google Scholar] [CrossRef] [PubMed]
- Hornuss, D.; Daehne, T.; Goetz, V.; Mueller, M.; Usadel, S.; Lorz, A.; Mockenhaupt, M.; Huzly, D.; Bierbaum, S.; Fuchs, J.; et al. Transmission Characteristics, Replication Patterns and Clinical Manifestations of Human Monkeypox Virus—An in-Depth Analysis of Four Cases from Germany. Clin. Microbiol. Infect. 2022, in press. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Casadio, L.V.B.; Polly, M.; Nastri, A.C.; Turdo, A.C.; de Araujo Eliodoro, R.H.; Sabino, E.C.; Levin, A.S.; de Proença, A.C.T.; Higashino, H.R. Monkeypox Virus Transmission to Healthcare Worker through Needlestick Injury, Brazil. Emerg. Infect. Dis. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Jarman, E.L.; Alain, M.; Conroy, N.; Omam, L.A. A Case Report of Monkeypox as a Result of Conflict in the Context of a Measles Campaign. Public Health Pract. 2022, 4, 100312. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Styczynski, A.R.; Huang, C.; Sahoo, M.K.; Srinivasan, K.; Pinsky, B.A.; Salinas, J.L. Human Monkeypox without Viral Prodrome or Sexual Exposure, California, USA, 2022. Emerg. Infect. Dis. 2022, 28, 2121–2123. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Keikha, M. Overlapping Outbreak of COVID-19 and Monkeypox in 2022: Warning for Immediate Preparedness in Iran. Int. J. Surg. 2022, 105, 106892. [Google Scholar] [CrossRef]
- Khan, S.; Razi, S.; Rao, B. It’s Here, Monkeypox: A Case Report. JAAD Case Rep. 2022, 28, 61–63. [Google Scholar] [CrossRef]
- Koh, X.Q.; Chio, M.T.W.; Tan, M.; Leo, Y.S.; Chan, R.K.W. Global Monkeypox Outbreak 2022: First Case Series in Singapore. Ann. Acad. Med. Singap. 2022, 51, 462–472. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.L.d.; Barra, L.A.C.; Borges, L.M.S.; Medeiros, L.A.; Tomishige, M.Y.S.; Santos, L.d.S.L.A.; da Silva, A.J.D.; Rodrigues, C.C.M.; de Azevedo, L.C.F.; Villas-Boas, L.S.; et al. First Case Report of Monkeypox in Brazil: Clinical Manifestations and Differential Diagnosis with Sexually Transmitted Infections. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e54. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.S.; Haddad, G.R.; Miot, H.A. Sexually-Transmitted Monkeypox: Report of Two Cases. An. Bras. Dermatol. 2022, 97, 783–785. [Google Scholar] [CrossRef]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical Characteristics of Ambulatory and Hospitalized Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clin. Microbiol. Infect. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Tutu van Furth, A.M.; van der Kuip, M.; van Els, A.L.; Fievez, L.C.; van Rijckevorsel, G.G.; van den Ouden, A.; Jonges, M.; Welkers, M.R. Paediatric Monkeypox Patient with Unknown Source of Infection, the Netherlands, June 2022. Eurosurveillance 2022, 27, 2200552. [Google Scholar] [CrossRef] [PubMed]
- Nörz, D.; Brehm, T.T.; Tang, H.T.; Grewe, I.; Hermanussen, L.; Matthews, H.; Pestel, J.; Degen, O.; Günther, T.; Grundhoff, A.; et al. Clinical Characteristics and Comparison of Longitudinal QPCR Results from Different Specimen Types in a Cohort of Ambulatory and Hospitalized Patients Infected with Monkeypox Virus. J. Clin. Virol. 2022, 155, 105254. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, Y.; Rodríguez-Morales, A.J.; Franco-Paredes, C.; Chastain, D.B.; Gharamti, A.A.; Vargas Barahona, L.; Henao-Martínez, A.F. Monkeypox-a Description of the Clinical Progression of Skin Lesions: A Case Report from Colorado, USA. Ther. Adv. Infect. Dis. 2022, 9, 20499361221117730. [Google Scholar] [CrossRef] [PubMed]
- Paparizos, V.; Nicolaidou, E.; Tryfinopoulou, K.; Papa, A.; Rigopoulos, D.; Tsiodras, S.; Stratigos, A. Monkeypox Virus Infection: First Reported Case in Greece in a Patient with a Genital Rash. J. Eur. Acad. Dermatol. Venereol. 2022. [Google Scholar] [CrossRef]
- Pembi, E.; Awang, S.; Salaudeen, S.O.; Agaba, I.A.; Omoleke, S. First Confirmed Case of Monkeypox in Adamawa State, Nigeria: A Clinico-Epidemiological Case Report. Pan Afr. Med. J. 2022, 42, 38. [Google Scholar] [CrossRef]
- Pérez-Martín, Ó.G.; Hernández-Aceituno, A.; Dorta-Espiñeira, M.M.; García-Hernández, L.; Larumbe-Zabala, E. Atypical Presentation of Sexually-Transmitted Monkeypox Lesions. Infect. Dis. 2022, 54, 940–943. [Google Scholar] [CrossRef]
- Pettke, A.; Filén, F.; Widgren, K.; Jacks, A.; Glans, H.; Andreasson, S.; Muradrasoli, S.; Helgesson, S.; Hauzenberger, E.; Karlberg, M.L.; et al. Ten-Week Follow-Up of Monkeypox Case-Patient, Sweden, 2022. Emerg. Infect. Dis. 2022, 28, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Pipitò, L.; Cascio, A. Monkeypox Virus Infection and Creatine Phosphokinase Increase: A Case from Italy. Travel Med. Infect. Dis. 2022, 50, 102412. [Google Scholar] [CrossRef] [PubMed]
- Quattri, E.; Avallone, G.; Maronese, C.A.; Cusini, M.; Carrera, C.G.; Marzano, A.V.; Ramoni, S. Unilesional Monkeypox: A Report of Two Cases from Italy. Travel Med. Infect. Dis. 2022, 49, 102424. [Google Scholar] [CrossRef] [PubMed]
- Sukhdeo, S.S.; Aldhaheri, K.; Lam, P.W.; Walmsley, S. A Case of Human Monkeypox in Canada. CMAJ 2022, 194, E1031–E1035. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Jaeranny, S.; Li, M.; Sukhdeo, S.S.; Monge, J.C.; Callejas, M.F.; Hasso, M.; Fattouh, R.; Lalonde, S.D.; Lam, J.; et al. Atypical Clinical Presentation of Monkeypox Complicated by Myopericarditis. Open Forum Infect. Dis. 2022, 9, ofac394. [Google Scholar] [CrossRef]
- Berthet, N.; Nakouné, E.; Whist, E.; Selekon, B.; Burguière, A.-M.; Manuguerra, J.-C.; Gessain, A.; Kazanji, M. Maculopapular Lesions in the Central African Republic. Lancet 2011, 378, 1354. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Heskin, J.; Belfield, A.; Milne, C.; Brown, N.; Walters, Y.; Scott, C.; Bracchi, M.; Moore, L.S.; Mughal, N.; Rampling, T.; et al. Transmission of Monkeypox Virus through Sexual Contact–A Novel Route of Infection. J. Infect. 2022, 85, 334–363. [Google Scholar] [CrossRef]
- Hammerschlag, Y.; MacLeod, G.; Papadakis, G.; Adan Sanchez, A.; Druce, J.; Taiaroa, G.; Savic, I.; Mumford, J.; Roberts, J.; Caly, L.; et al. Monkeypox Infection Presenting as Genital Rash, Australia, May 2022. Eurosurveillance 2022, 27, 2200411. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak—Nine States, May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 764. [Google Scholar] [CrossRef]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Vallée, A.; Farfour, E.; Zucman, D. Monkeypox Virus: A Novel Sexually Transmitted Disease? A Case Report from France. Travel Med. Infect. Dis. 2022, 49, 102394. [Google Scholar] [CrossRef] [PubMed]
- Oprea, C.; Ianache, I.; Piscu, S.; Tardei, G.; Nica, M.; Ceausu, E.; Popescu, C.P.; Florescu, S.A. First Report of Monkeypox in a Patient Living with HIV from Romania. Travel Med. Infect. Dis. 2022, 49, 102395. [Google Scholar] [CrossRef] [PubMed]
- Bížová, B.; Veselý, D.; Trojánek, M.; Rob, F. Coinfection of Syphilis and Monkeypox in HIV Positive Man in Prague, Czech Republic. Travel Med. Infect. Dis. 2022, 49, 102368. [Google Scholar] [CrossRef] [PubMed]
- Patrocinio-Jesus, R.; Peruzzu, F. Monkeypox Genital Lesions. N. Engl. J. Med. 2022, 387, 66. [Google Scholar] [CrossRef]
- Basgoz, N.; Brown, C.M.; Smole, S.C.; Madoff, L.C.; Biddinger, P.D.; Baugh, J.J.; Shenoy, E.S. Case 24-2022: A 31-Year-Old Man with Perianal and Penile Ulcers, Rectal Pain, and Rash. N. Engl. J. Med. 2022, 387, 547–556. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Jang, Y.R.; Lee, M.; Shin, H.; Kim, J.-W.; Choi, M.; Kim, Y.M.; Lee, M.J.; Kim, J.; Na, H.K.; Kim, J.Y. The First Case of Monkeypox in the Republic of Korea. J. Korean Med. Sci. 2022, 37, e224. [Google Scholar] [CrossRef]
- Maronese, C.A.; Beretta, A.; Avallone, G.; Boggio, F.L.; Marletta, D.A.; Murgia, G.; Cusini, M.; Gori, A.; Carrera, C.G.; Di Benedetto, A.; et al. Clinical, Dermoscopic and Histopathological Findings in Localized Human Monkeypox: A Case from Northern Italy. Br. J. Dermatol. 2022, 187, 822–823. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J.; et al. Frequent Detection of Monkeypox Virus DNA in Saliva, Semen, and Other Clinical Samples from 12 Patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.I.; Montalbán, E.G.; Bueno, S.J.; Martínez, F.M.; Juliá, A.N.; Díaz, J.S.; Marín, N.G.; Deorador, E.C.; Forte, A.N.; García, M.A.; et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Yinka-Ogunleye, A. Sexual History of Human Monkeypox Patients Seen at a Tertiary Hospital in Bayelsa, Nigeria. Int. J. STD AIDS 2022, 33, 928–932. [Google Scholar] [CrossRef]
- Orviz, E.; Negredo, A.; Ayerdi, O.; Vázquez, A.; Muñoz-Gomez, A.; Monzón, S.; Clavo, P.; Zaballos, A.; Vera, M.; Sánchez, P.; et al. Monkeypox Outbreak in Madrid (Spain): Clinical and Virological Aspects. J. Infect. 2022, 85, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- Pfäfflin, F.; Wendisch, D.; Scherer, R.; Jürgens, L.; Godzick-Njomgang, G.; Tranter, E.; Tober-Lau, P.; Stegemann, M.S.; Corman, V.M.; Kurth, F.; et al. Monkeypox In-Patients with Severe Anal Pain. Infection 2022. [Google Scholar] [CrossRef]
- Philpott, D. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1018–1022. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Candela, C.; Mileto, D.; Canetti, D.; Bruzzesi, E.; Rizzo, A.; Castagna, A.; Nozza, S. Monkeypox Infection among Men Who Have Sex with Men: PCR Testing on Seminal Fluids. J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef]
- Rodríguez, B.S.; Herrador, B.R.G.; Franco, A.D.; Fariñas, M.P.S.-S.; Valero, J.D.A.; Llorente, A.H.A.; de Agreda, J.P.A.P.; Malonda, R.C.; Castrillejo, D.; López, M.D.C.; et al. Epidemiologic Features and Control Measures during Monkeypox Outbreak, Spain, June 2022. Emerg. Infect. Dis. 2022, 28, 1847–1851. [Google Scholar] [CrossRef]
- Vusirikala, A.; Charles, H.; Balasegaram, S.; Macdonald, N.; Kumar, D.; Barker-Burnside, C.; Cumiskey, K.; Dickinson, M.; Watson, M.; Olufon, O.; et al. Epidemiolog y of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg. Infect. Dis. 2022, 28, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Ramoni, S.; Maronese, C.A.; Morini, N.; Avallone, G.; Quattri, E.; Carrera, C.G.; Boggio, F.L.; Marzano, A.V. Syphilis and Monkeypox Co-Infection: Coincidence, Synergy or Asymptomatic Carriage? Travel Med. Infect. Dis. 2022, 50, 102447. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. First Two Cases of Monkeypox Virus Infection in Travellers Returned from UAE to India, July 2022. J. Infect. 2022, 85, e145–e148. [Google Scholar] [CrossRef]
- Turco, M.; Mancuso, F.R.; Pisano, L. A Monkeypox Virus Infection Mimicking Primary Syphilis. Br. J. Dermatol. 2022, 187, e194–e195. [Google Scholar] [CrossRef]
- Pisano, L.; Turco, M.; Mancuso, F.R.; Lastrucci, I.; Pimpinelli, N. Atypical Oral Presentation of Monkeypox Virus: A Report of Two Cases from Florence, Italy. Travel Med. Infect. Dis. 2022, 50, 102457. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Colavita, F.; Antinori, A.; Nicastri, E.; Focosi, D.; Girardi, E.; Vaia, F.; Maggi, F. Monkeypox Virus in Human Body Sites and Fluids: Evidence for Transmission. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Sitaula, C.; Shahi, T.B. Monkeypox Virus Detection Using Pre-Trained Deep Learning-Based Approaches. J. Med. Syst. 2022, 46, 78. [Google Scholar] [CrossRef]
- Laboratory Testing for the Monkeypox Virus: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-MPX-laboratory-2022.1 (accessed on 2 November 2022).
- Gul, I.; Liu, C.; Yuan, X.; Du, Z.; Zhai, S.; Lei, Z.; Chen, Q.; Raheem, M.A.; He, Q.; Hu, Q.; et al. Current and Perspective Sensing Methods for Monkeypox Virus. Bioengineering 2022, 9, 571. [Google Scholar] [CrossRef]
- Laboratory Guidelines for the Detection and Diagnosis of Monkeypox Virus Infection-PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection (accessed on 2 November 2022).
- Català, A.; Clavo-Escribano, P.; Riera-Monroig, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles-Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox Outbreak in Spain: Clinical and Epidemiological Findings in a Prospective Cross-Sectional Study of 185 Cases. Br. J. Dermatol. 2022, 187, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Abdelaal, A.; Brakat, A.M.; Lashin, B.I.; Abouelkheir, M.; Abdelazeem, B.; Rodriguez-Morales, A.J.; Sah, R. Monkeypox Viral Detection In Semen Specimens of Confirmed Cases: A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 95, e28250. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Paran, N.; Yahalom-Ronen, Y.; Shifman, O.; Lazar, S.; Ben-Ami, R.; Yakubovsky, M.; Levy, I.; Wieder-Feinsod, A.; Amit, S.; Katzir, M.; et al. Monkeypox DNA Levels Correlate with Virus Infectivity in Clinical Samples, Israel, 2022. Eurosurveillance 2022, 27, 2200636. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Outbreak of Monkeypox-External Situation Report 8, Published 19 October 2022-World|ReliefWeb. Available online: https://reliefweb.int/report/world/multi-country-outbreak-monkeypox-external-situation-report-8-published-19-october-2022 (accessed on 2 November 2022).
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral Loads in Clinical Samples of Men with Monkeypox Virus Infection: A French Case Series. Lancet Infect. Dis. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcon-Braga, E.A.; Hernandez-Bustamante, E.A.; Mosquera-Rojas, M.D.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Clinical Features, Hospitalisation and Deaths Associated with Monkeypox: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 36. [Google Scholar] [CrossRef]
| Base | Search Strategy |
|---|---|
| PubMed | #1 (“Monkeypox” OR “Monkey Pox”) #2 (“Specimen Handling” OR “Handling, Specimen” OR “Handlings, Specimen” OR “Specimen Handlings” OR “Specimen Collection” OR “Collection, Specimen” OR “Collections, Specimen” OR “Specimen Collections” OR “blood” OR “saliva” OR “skin” OR “semen” OR “genitals” OR “feces”) #3 = #1 AND #2 |
| Scopus | #1 TITLE-ABS-KEY (“Monkeypox” OR “Monkey Pox”) #2 TITLE-ABS-KEY (“Specimen Handling” OR “Specimen Handlings” OR “Specimen Collection” OR “Specimen Collections” OR “blood” OR “saliva” OR “skin” OR “semen” OR “genitals” OR “feces”) #3 = #1 AND #2 |
| Web of Science | #1 ALL = (“Monkeypox” OR “Monkey Pox”) #2 ALL = (“Specimen Handling” OR “Handling, Specimen” OR “Handlings, Specimen” OR “Specimen Handlings” OR “Specimen Collection” OR “Collection, Specimen” OR “Collections, Specimen” OR “Specimen Collections” OR “blood” OR “saliva” OR “skin” OR “semen” OR “genitals” OR “feces”) #3 = #1 AND #2 |
| Embase | #1 ‘monkeypox’/exp OR ‘monkeypox’ OR ‘monkeypox virus’/exp OR ‘monkeypox virus’ #2 ‘specimen’ OR ‘blood’ OR ‘saliva’ OR ‘skin’ OR ‘semen’ OR ‘genitals’ OR ‘feces’ #3 = #1 AND #2 |
| Authors | Year | Design | Country | Number of Cases (N) | Age (Years) | Sex (M/F) | Risk Factor | STIs | Site of Positive MPX Viral PCR | Localization of Skin Lesions | Diagnostic Method for Monkeypox |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brito Caldeira, M., et al. [25] | 2022 | Case report | Portugal | 1 | 33 | M | MSM | Gonorrhea and chlamydia | Skin lesions | Face and buttocks | RT-PCR |
| Brundu, M., et al. [26] | 2022 | Case report | Italy | 1 | 35 | M | MSM | HIV | Skin and anal lesions, seminal fluid, oropharynx, blood, and urine. | Abdomen, chest, back, and perianal. | RT-PCR |
| Costello. V., et al. [27] | 2022 | Case report | United States | 1 | 28 | M | None | None | Skin lesions. | Abdomen, face, neck, and hands. | RT-PCR |
| Davido, B., et al. [28] | 2022 | Case report | France | 1 | 48 | M | MSM | HIV | Throat | Peritonsillar abscess. | RT-PCR |
| Erez, N., et al. [29] | 2019 | Case report | Israel | 1 | 38 | M | None | None | Skin lesions. | Face, penis, trunk, and extremities. | RT-PCR |
| Eseigbe, E.E., et al. [30] | 2021 | Case report | Nigeria | 2 | 20 | M | None | None | Skin lesions and blood. | Skin | RT-PCR |
| Gedela, K., et al. [31] | 2022 | Case report | United Kingdom | 2 | 40 | M | MSM | Herpes simplex (n = 2), HIV (n = 1) | Perianal, rectum, nose, and throat ulcers. | Skin | RT-PCR |
| 30 | |||||||||||
| Gomez-Garberi, M., et al. [32] | 2022 | Observational study | Spain | 14 | Median: 40 (20–56) | M | MSM (n = 10) and Heterosexuals (n = 2) | HIV (n = 8), Chlamydia (n = 2), Herpes type 2 (n = 1), Syphilis (n = 1), Mycoplasma genitalium (n = 1), and Gonococcus (n = 1) | Skin lesions (vesicles, pustules, and crusts) | Skin lesion, genital area, penis, and scrotum. | RT-PCR |
| Hobson, G., et al. [33] | 2022 | Case report | United Kingdom | 3 | NR | M | None | None | Skin lesions | Skin | RT-PCR |
| Hofer, U., [34] | 2022 | Case series | United Kingdom | 7 | NR | NR | None | None | Nose and throat | Skin | RT-PCR |
| Hornuss, D., et al. [35] | 2022 | Case series | Germany | 4 | Range (29–53) | M | MSM | Chlamydia (n = 1), Gonorrhoea (n = 1), and Mycoplasma (n = 1) | Typical lesions (vesicle, encrustation, erosion, ulceration), forearms/palms, pharyngeal/anal mucosa, blood, urine, and seminal fluid. | Mons pubis (n = 1), Perioral (n = 1), Perianal (n = 2), Nose (n = 1), and Genital (n = 2) | RT-PCR |
| Carvalho, L.B., et al. [36] | 2022 | Case report | Brazil | 1 | 20 | F | None | None | Blood, skin lesions and oropharyngeal lesions. | Hands, left thigh, and face. | RT-PCR |
| Jarman, E.L., et al. [37] | 2022 | Case report | Cameroon | 1 | 14 | M | None | None | Suprapubic injury and hand | Face, head, chest, arms, and legs. | RT-PCR |
| Karan, A., et al. [38] | 2022 | Case report | United States | 1 | 20 | M | MSM | Syphilis | Plasma and skin lesions. | Skin lesions, hands, lip, and back. | RT-PCR |
| Karbalaei, M., et al. [39] | 2022 | Case report | Iran | 1 | 34 | F | None | None | Skin lesions | Skin lesions and hands. | RT-PCR |
| Khan, S., et al. [40] | 2022 | Case report | United States | 1 | 31 | M | MSM | Herpes simplex, varicella-zoster, and syphilis. | Skin lesions | Hands, feet, and trunk. | RT-PCR |
| Koh, X.Q., et al. [41] | 2022 | Case series | Singapore | 15 | Median: 38.4 (25–54) | M | MSM | NR | Skin lesions | Skin lesions (n = 11), inguinal and anogenital regions (n = 5), and abdomen (n = 1). | RT-PCR |
| Lapa, D., et al. [42] | 2022 | Case report | Italy | 1 | 39 | M | MSM | HIV | Plasma, semen, rash, or skin lesion. | Anus, head, thorax, legs, arms, hand, and penis. | RT-PCR |
| Lima, E.L., et al. [43] | 2022 | Case report | Brazil | 1 | 41 | M | MSM | None | Skin lesions | Face, periumbilical region, back, upper extremities, trunk, and genitals. | RT-PCR |
| Lopes, P.S., et al. [44] | 2022 | Case report | Brazil | 2 | 28 | M | MSM | Syphilis (n = 2) | Skin lesions | Lip (n = 2) and penis (n = 1) | RT-PCR |
| Mailhe, M., et al. [45] | 2022 | Observational study | France | 264 | Median: 35 (30-41) | M (n = 262) F (n = 1) Trans (n = 1) | MSM (n = 245) | HIV (n = 73) and history of STI (n = 209) | Skin (n = 252), oropharynx (n = 150), and blood (n = 8). | Genital area (n = 135), limbs (n = 121), torso (n = 105), perianal area (n = 100), face (n = 88), and palmoplantar area (n = 36). | RT-PCR |
| Tutu van Furth, A.M., et al. [46] | 2022 | Case report | Netherlands | 1 | 10 | M | None | None | Blood, throat, anal region, and skin vesicles. | Face, ear, jaw, forearms, shoulder, thighs, and back. | RT-PCR |
| Nörz, D., et al. [47] | 2022 | Case reports | Germany | 16 | Range (20–40) | M | MSM | HIV (n = 2) | Skin lesions (n = 16), blood (n = 4) and oropharynx (n = 3) | Anal/perianal (n = 3), genital/perigenital (n = 8), oral (n = 2), face (n = 1), oral (n = 1), and arm (n = 2). | RT-PCR |
| Ortiz-Martínez, Y., et al. [48] | 2022 | Case report | United States | 1 | 36 | M | MSM | None | Skin lesions | Penis, neck, thigh, and nipple | RT-PCR |
| Paparizos, V., et al. [49] | 2022 | Case report | Greece | 1 | 59 | M | MSM | HIV | Skin lesions | Pubic area and penis. | RT-PCR |
| Pembi, E., et al. [50] | 2022 | Case report | Nigeria | 1 | 30 | M | None | Syphilis | Skin lesions | Forehead, groin, genitals, chest, back, feet, and hands. | RT-PCR |
| Pérez-Martín, Ó.G., et al. [51] | 2022 | Case report | Spain | 1 | 40 | M | MSM | None | Skin lesions | Perineum, penis, and testicles. | RT-PCR |
| Pettke, A., et al. [52] | 2022 | Case report | Sweden | 1 | NR | M | MSM | None | Genital lesions, blood, urine, saliva, nasopharynx, and semen. | Genital skin lesions. | RT-PCR |
| Pipitò, L., et al. [53] | 2022 | Case report | Italy | 1 | 45 | M | MSM | HIV | Skin lesions | Face, neck, genitalia, extremities, and trunk. | RT-PCR |
| Quattri, E., et al. [54] | 2022 | Case report | Italy | 2 | 35 and 39 | M | MSM | HIV (n = 2), Syphilis (n = 2), Gonorrhea (n = 1) | Skin lesions | Lesión cutánea (n = 2), prepucio (n = 1), pene (n = 1), and tronco (n = 1). | RT-PCR |
| Sukhdeo, S.S., et al. [55] | 2022 | Case report | Canada | 1 | 33 | M | MSM | None | Pustules of each arm and serum. | Face, extremities, torso, forearm, and wrist. | RT-PCR |
| Tan, D.H.S., et al. [56] | 2022 | Case report | Canada | 1 | 40 | M | MSM | HIV | Skin lesions, saliva, and semen. | Cutaneous, genital, labial, chest, and arm lesions. | RT-PCR |
| Berthet, N., et al. [57] | 2011 | Case report | Central African Republic | 2 | 14 and 15 | M | None | Syphilis (n = 1) | Skin lesions | Skin, face, torso, and extremities. | RT-PCR |
| Thornhill, J.P., et al. [58] | 2022 | Case report | Multicountry (n = 16) | 528 | Median: 38 (18–68) | M (n = 527) Trans (n = 1) | Homosexual (n = 509) Bisexual (n = 10) | HIV (n = 218) Gonorrhea (n = 32/377), Chlamydia (n = 20/377), Syphilis (n = 33/377), Herpes simplex (n = 3/377), and Lymphogranuloma venereum (n = 2/377) | Skin or anogenital lesion (n = 512), Nose or throat swab (n = 138), Blood (n = 35), Urine (n = 14), and Semen (n = 29) | Anogenital area (n = 383), face (n = 134), trunk or limbs (n = 292), palms or soles (n = 51), and mu-cosal lesions present (n = 217). | RT-PCR |
| Antinori, A., et al. [18] | 2022 | Case reports | Italy | 4 | Median: 30 | M | MSM | Hepatitis C (n = 1), syphilis (n = 3), hepatitis B (n = 1), Hepatitis A (n = 1), HIV (n = 2). | Serum (n = 1), Plasma (n = 1), Genital or rectal lesions (n = 4), Nasopharyngeal swab (n = 3), Skin lesions (n = 3), Seminal fluid (n = 3), Scab (n = 2), Faeces (n = 2), and Saliva (n = 1) | Genital (n = 3), thorax (n = 2), Anal (n = 2), arms (n = 2) | RT-PCR |
| Heskin, J., et al. [59] | 2022 | Case reports | United Kingdom | 2 | NR | M | MSM | None | Serum (n = 2), genital lesions (n = 2), and urine (n = 2). | Genital (n = 2), pubic and tongue (n = 2), oral and buccal mucous membranes (n = 2) | RT-PCR |
| Hammerschlag, Y., et al. [60] | 2022 | Case report | Australia | 1 | 30 | M | MSM | Syphilis | skin lesions and nasal throat | Penis, trunk, face, extremities, hand, calf, nasal throat. | RT-PCR |
| Minhaj, F.S., et al. [61] | 2022 | Case reports | United States | 17 | Median 40 (28–61) | M | GBMSM (n = 16) | NR | Skin lesions | Arm (n = 9), Trunk (n = 9), Leg (n = 8), Face (n = 7), Hand (n = 6), Perianal (n = 6), Oral (n = 5), Neck (n = 5), Genital (penis or vagina) (n = 4), Feet (n = 4). | RT-PCR |
| Perez Duque, M., et al. [62] | 2022 | Case reports | Portugal | 27 | Median: 33 (22–51) | M | MSM (18/19), MSW (1/19) | HIV (n = 14) | Lesions on the palms of the hands, genital area, and/or oral mucosa. | Anus (n = 14) and genitalia (n = 12) | RT-PCR |
| Vallée, A., et al. [63] | 2022 | Case report | France | 1 | NR | M | MSM | HIV | Pharyngeal area | Genitalia | RT-PCR |
| Oprea, C., et al. [64] | 2022 | Case report | Romania | 1 | 26 | M | MSM | HIV | Skin, nasopharyngeal, urine, and blood lesions. | Anogenital, buttocks, neck, trunk, upper and lower limbs, and sole of one foot. | RT-PCR |
| Bížová, B., et al. [65] | 2022 | Case report | Czech Republic | 1 | 34 | M | MSM | Syphilis and HIV | Perianal | The perianal and left side of the body. | RT-PCR |
| Patrocinio-Jesus, R., et al. [66] | 2022 | Case report | Portugal | 1 | 31 | M | MSM | HIV | Genitals and hands | Genitals and hands | RT-PCR |
| Basgoz, S.N., et al. [67] | 2022 | Case report | United States | 1 | 31 | M | MSM | Syphilis, herpes simplex | Skin lesions, throat, and serum | Perianal, penis, arms, and legs. | RT-PCR |
| Mileto, D., et al. [68] | 2022 | Case report | Italy | 1 | 33 | M | MSM | HIV | Oropharynx, anus, perianal ulcerated lesion, a foot vesicle, and plasma. | Perianal, face, both elbows, trunk, buttock, and right foot. | RT-PCR |
| Girometti, N., et al. [69] | 2022 | Cohort study | United Kingdom | 54 | Median: 41 (34–45) | M | MSM | HIV (n = 13) syphilis (n = 14), herpes simplex (n = 24) and gonorrhea (n = 13) | Blood, urine, and skin lesions. | Skin (n = 54), genitalia (n = 33), perianal (n = 24), upper and lower extremities (n = 27), facial (n = 11), oropharyngeal (n = 4) and torso (n = 14). | RT-PCR |
| Noe, S., et al. [21] | 2022 | Case report | Germany | 2 | 26 | M | MSM | HIV (n = 1) | Blood, semen, throat, and skin lesions. | Tonsils, trunk, limbs, and head. | RT-PCR |
| 32 | |||||||||||
| Jang, Y.R., et al. [70] | 2022 | Case report | Korea | 1 | 34 | M | MSM | None | Penile, oropharyngeal, and nasopharyngeal. | Penis, oropharynx, nasopharynx, face, abdomen, and trunk. | RT-PCR |
| Maronese, C.A., et al. [71] | 2022 | Case report | Italy | 1 | 44 | M | MSM | Hepatitis C, HIV, syphilis | Pharyngeal and skin lesions. | Penis, scrotum, and extremities. | RT-PCR |
| Peiró-Mestres, A., et al. [72] | 2022 | Case report | Spain | 12 | Range (30–50) | M | MSM | HIV (n = 4), Syphilis (n = 2) Chlamydia (n = 1) y gonorrhea (n = 1). | Saliva (n = 12), rectal (n = 11), nasopharyngeal (n = 10), semen (n = 7), urine (n = 9), and feces (n = 8). | Arm (n = 1), trunk (n = 3), genital area (n = 5), anal area (n = 6), chest (n = 2), legs (n = 1), pectoral (n = 1), fingers (n = 1), and hand (n = 1). | RT-PCR |
| Iñigo Martínez, J., et al. [73] | 2022 | Case report | Spain | 508 | Median: 35 (18–67) | M (n = 503) F (n = 5) | MSM (n = 397) | HIV (n = 225) | Skin lesions, urine, pharyngeal exudates, and mucosal exudates. | Anogenital and/or perineal area (n = 359), legs and/or arms (n = 222), face (n = 177), chest and/or abdomen (n = 159), back (n = 132), palms and/or plants (n = 124). | RT-PCR |
| Tarín-Vicente, E.J., et al. [74] | 2022 | Cohort study | Spain | 181 | Median: 37 (31–42) | M (n = 175) F (n = 6) | MSM (n = 166) MSW (n = 15) | HIV (n = 72), Chlamydia (n = 10), gonorrhea (n = 6), herpes simplex virus (n = 2), and syphilis (n = 13). | Skin (n = 178/180), throat (n = 82/117), and anal (n = 43/55) lesions. | Genital (n = 100), Perianal (n = 66), Oral ulcer (n = 45), Perioral (n = 51), Hands and feet (n = 108), Trunk and extremities (n = 104) | RT-PCR |
| Ogoina, D., et al. [75] | 2022 | Cross-sectional study | Nigeria | 16 | Median: 28 (22–43) | M (n = 12) F (n = 4) | MSW | HIV (n = 3) | Skin lesions | Genital (n = 13) | RT-PCR |
| Orviz, E., et al. [76] | 2022 | Observational study | Spain | 48 | Median: 35 (29–44) | M | MSM (n = 42) | HIV (n = 19) | Skin lesions | Vesicular-umbilicated skin lesions location (n = 45), Genitals (n = 26), Upper extremities (n = 20), Perianal (n = 17), Trunk (n = 16), Facial (n = 12), Periorally (n = 9), Lower extremities (n = 10), and Palms and soles (n = 2) | RT-PCR |
| Patel, A., et al. [77] | 2022 | Case report | United Kingdom | 197 | Median: 38 (32–42) | M | MSM | HIV (n = 70), Chlamydia (n = 11), gonor-rhea (n = 34), herpes simplex virus (n = 11), and syphilis (n = 6). | Skin lesions | Face (n = 71), Trunk (n = 70), Arms/legs (n = 74), Hands/feet (n = 56), Genitals (n = 111), Anus or perianal area (n = 82), and Oropharyngeal (n = 27) | RT-PCR |
| Pfäfflin, F., et al. [78] | 2022 | Case report | Germany | 6 | Range (21–50) | M | MSM | Syphilis (n = 1), gonorrhea (n = 3), myco-plasma homi-nis (n = 1) | Skin blister fluid | Limbs (n = 3), arm (n = 2), trunk (n = 2), genital (n = 1), head (n = 1), neck (n = 1) | RT-PCR |
| Philpott, D., et al. [79] | 2022 | Case report | United States | 1195 | Median: 35 (30–41) | M (n = 1178) F (n = 5) | MSM | HIV (n = 490) | Skin rash | Genitals (n = 333), Arms (n = 284), Face (n = 276), Legs(n = 265), Perianal (n = 225), Mouth, lips, or oral mucosa (n = 179), Palms of hands (n = 157), Trunk (n = 156), Neck (n = 130), Head (n = 97), and Soles of feet (n = 77) | RT-PCR |
| Raccagni, A.R., et al. [80] | 2022 | Case report | Italy | 36 | Median: 41.5 (31.25–35.5) | M | MSM | HIV (n = 15) | Hyssop (n = 36), Seminal fluids (n = 22), Urines (n = 8), and Serum/Plasma (n = 24) | Genital (n = 13), Rectal (n = 18), cutaneous (n = 20) | RT-PCR |
| Rodríguez, B.S., et al. [81] | 2022 | Case report | Spain | 1256 | Median: 37 | M (n = 1242) F (n = 14) | MSM | NR | Skin lesions | Report of some cases (n = 530): Anogenital (n = 355), other than anogenital or oro/peribuccal (n = 293) | RT-PCR |
| Vusirikala, A., et al. [82] | 2022 | Case report | United Kingdom | 45 | Median: 37 | M | GBMSM (n = 44) | HIV (n = 11) | Skin lesions | Skin lesions | RT-PCR |
| Moschese, D., et al. [20] | 2022 | Case report | Italy | 33 | Median: 38 (34–43) | M | MSM | HIV (n = 17) | Anus (n = 13/18) and urethra (n = 11/15). | Cutaneous (n = 33), perianal (n = 7) and glans (n = 3) lesions. | RT-PCR |
| Ramoni, S., et al. [83] | 2022 | Case report | Italy | 2 | 24 | M | MSM | Syphilis | Skin lesions | Pubic area | RT-PCR |
| 38 | M | MSM | Syphilis | Skin and pharyngeal lesions | Penis, perianal region, and forehead. | ||||||
| Yadav, P.D., et al. [84] | 2022 | Case report | India | 2 | 35 | M | None | None | Skin lesions, oropharyngeal, nasopharyngeal, blood, serum, and urine. | Oral cavity, lips, genitalia, and navel. | RT-PCR |
| 31 | M | None | None | Skin lesions, oropharyngeal, nasopharyngeal, and urine. | Genitals, hands, face, back, neck, and forearm. | ||||||
| Turco, M., et al. [85] | 2022 | Case report | Italy | 1 | 46 | M | MSM | None | Skin lesions and the oropharynx. | Face, hands, and penis. | RT-PCR |
| Pisano, L., et al. [86] | 2022 | Case report | Italy | 2 | 45 | M | MSM | HIV, syphilis, gonorrhoea. | Oral lesions and the oropharynx. | Oral mucosa and trunk | RT-PCR |
| 69 | M | MSM | HIV, syphilis, hepatitis C. | Oral lesions, oropharynx, and nipple. | Oral mucosa and nipple |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Figueroa, D.A.; Barboza, J.J.; Saldaña-Cumpa, H.M.; Moreno-Ramos, E.; Bonilla-Aldana, D.K.; Valladares-Garrido, M.J.; Sah, R.; Rodriguez-Morales, A.J. Detection of Monkeypox Virus according to The Collection Site of Samples from Confirmed Cases: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 4. https://doi.org/10.3390/tropicalmed8010004
León-Figueroa DA, Barboza JJ, Saldaña-Cumpa HM, Moreno-Ramos E, Bonilla-Aldana DK, Valladares-Garrido MJ, Sah R, Rodriguez-Morales AJ. Detection of Monkeypox Virus according to The Collection Site of Samples from Confirmed Cases: A Systematic Review. Tropical Medicine and Infectious Disease. 2023; 8(1):4. https://doi.org/10.3390/tropicalmed8010004
Chicago/Turabian StyleLeón-Figueroa, Darwin A., Joshuan J. Barboza, Hortencia M. Saldaña-Cumpa, Emilly Moreno-Ramos, D. Katterine Bonilla-Aldana, Mario J. Valladares-Garrido, Ranjit Sah, and Alfonso J. Rodriguez-Morales. 2023. "Detection of Monkeypox Virus according to The Collection Site of Samples from Confirmed Cases: A Systematic Review" Tropical Medicine and Infectious Disease 8, no. 1: 4. https://doi.org/10.3390/tropicalmed8010004
APA StyleLeón-Figueroa, D. A., Barboza, J. J., Saldaña-Cumpa, H. M., Moreno-Ramos, E., Bonilla-Aldana, D. K., Valladares-Garrido, M. J., Sah, R., & Rodriguez-Morales, A. J. (2023). Detection of Monkeypox Virus according to The Collection Site of Samples from Confirmed Cases: A Systematic Review. Tropical Medicine and Infectious Disease, 8(1), 4. https://doi.org/10.3390/tropicalmed8010004










