Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Agents
2.3. Fosfomycin Effect on Planktonic MDR UPEC
2.4. Biofilm-Formation Assay for MDR UPEC
2.5. Fosfomycin Effect on Biofilm-Associated MDR UPEC
2.6. Statistical Analysis
3. Results
3.1. Susceptibility of Planktonic MDR UPEC to Fosfomycin
3.2. Biofilm Producer Capacity of MDR UPEC
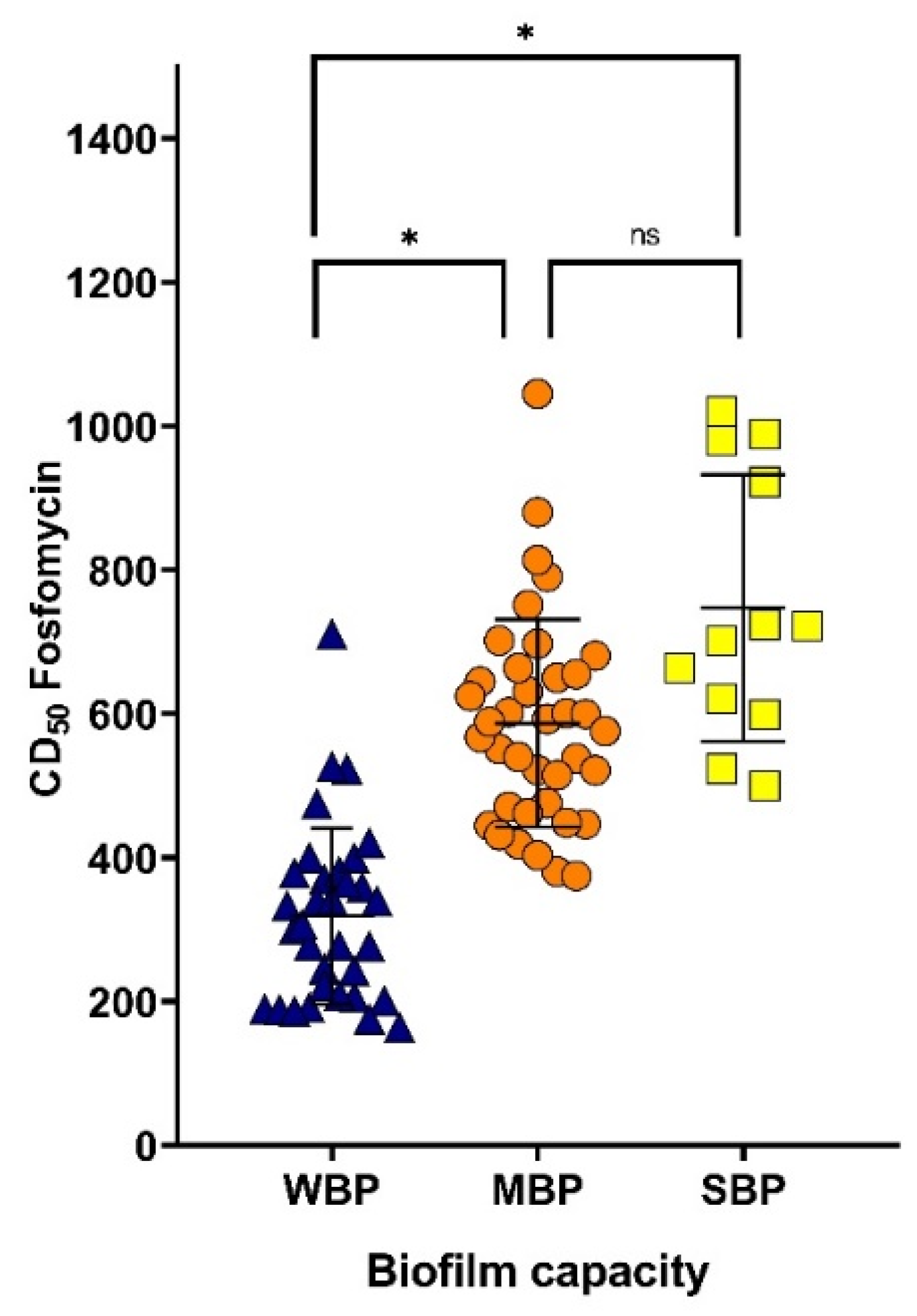
3.3. Effect of Fosfomycin on Biofilm-Associated MDR UPEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance, and development of effective vaccines against uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combat antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52, 397–428. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Vestby, L.K.; Torstein, G.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Marti, R.; Schmid, M.; Kulli, S.; Schneeberger, K.; Naskova, J.; Knøchel, S.; Hummerjohann, J. Biofilm formation potential of heat-resistant Escherichia coli dairy isolates and the complete genome of multidrug-resistant, heat-resistant strain FAM21845. Appl. Environ. Microbiol. 2017, 83, e00628-17. [Google Scholar] [CrossRef]
- Zamani, H.; Salehzadeh, A. Biofilm formation in uropathogenic Escherichia coli: Association with adhesion factor genes. Turk. J. Med. Sci. 2018, 48, 162–167. [Google Scholar] [CrossRef]
- Candel, F.J.; Cantón, R. Current approach to Fosfomycin: From bench to bedside. Enferm. Infecc. Microbiol. Clin. 2019, 37, 1–3. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuín, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Sastry, S.; Doi, Y. Fosfomycin: Resurgence of an old companion. J. Infect. Chemother. 2016, 22, 273–280. [Google Scholar] [CrossRef]
- Candel, F.J.; David, M.M.; López, J.B. Nuevas perspectivas para la reevaluación de la fosfomicina: Aplicabilidad en la práctica clínica actual. Rev. Esp. Quimioter. 2019, 32, 1–7. [Google Scholar]
- Iarikov, D.; Wassel, R.; Farley, J.; Nambiar, S. Adverse Events Associated with Fosfomycin Use: Review of the Literature and Analyses of the FDA Adverse Event Reporting System Database. Infect. Dis. Ther. 2015, 4, 433–458. [Google Scholar] [CrossRef] [PubMed]
- Amladi, A.U.; Abirami, B.; Devi, S.M.; Sudarsanam, T.D.; Kandasamy, S.; Kekre, N.; Veeraraghavan, B.; Sahni, R.D. Susceptibility profile, resistance mechanisms & efficacy ratios of Fosfomycin, Nitrofurantoin & Colistin for carbapenem-resistant Enterobacteriaceae causing urinary tract infections. Indian J. Med. Res. 2019, 149, 185–191. [Google Scholar] [CrossRef]
- Mezzatesta, M.L.; La Rosa, G.; Maugeri, G.; Zingali, T.; Caio, C.; Novelli, A.; Stefani, S. In vitro activity of Fosfomycin Trometamol and other oral antibiotics against multidrug-resistant uropathogens. Int. J. Antimicrob. Agents 2017, 49, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Urbán, E.; Gajdács, M. Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Sugathan, S.; Mandal, J. An in vitro experimental study of the effect of Fosfomycin in combination with Amikacin, Ciprofloxacin or Meropenem on biofilm formation by multidrug-resistant urinary isolates of Escherichia coli. J. Med. Microbiol. 2019, 68, 1699–1706. [Google Scholar] [CrossRef]
- Gopichand, P.; Agarwal, G.; Natarajan, M.; Mandal, J.; Deepanjali, S.; Parameswaran, S.; Dorairajan, L.N. In vitro effect of Fosfomycin on multi-drug resistant Gram-negative bacteria causing urinary tract infections. Infect. Drug. Resist. 2019, 12, 2005–2013. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jiménez-Guillermo, J.G.; Molina-Salinas, G.M. High prevalence of antimicrobial resistance among Gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing 2017, 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari-Ayezloy, E.; Hosseini-Jazani, N.; Yousefi, S.; Habibi, N. Eradication of Methicillin resistant S. aureus biofilm by the combined use of Fosfomycin and β-chloro-L-alanine. Iranian. J. Microbiol. 2017, 9, 1–10. [Google Scholar]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. Uropathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Manseck, A.S.; Otto, W.; Schnabel, M.; Denzinger, S.; Burger, M.; Spachmann, P.J. Geriatric patients and symptomatic urinary tract infections: Analysis of bacterial range and resistance rates at a 3rd level of care hospital in Germany. Urol. Int. 2021, 106, 298–303. [Google Scholar] [CrossRef]
- Kettani Halabi, M.; Lahlou, F.; Diawara, I.; El Adouzi, Y.; Marnaoui, R.; Benmessaoud, R.; Smyej, I. Antibiotic resistance pattern of extended spectrum beta lactamase producing Escherichia coli isolated from patients with urinary tract infection in Morocco. Front. Cell Infect. Microbiol. 2021, 11, 720701. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rani, H.; Singla, N.; Kaistha, N.; Chander, J. Determination of extended-spectrum β-lactamases and AmpC production in uropathogenic isolates of Escherichia coli and susceptibility to Fosfomycin. J. Lab. Physicians. 2013, 5, 90–93. [Google Scholar] [CrossRef]
- Bi, W.; Li, B.; Song, J.; Hong, Y.; Zhang, X.; Liu, H.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial susceptibility and mechanisms of Fosfomycin resistance in extended-spectrum β-lactamase-producing Escherichia coli strains from urinary tract infections in Wenzhou, China. Int. J. Antimicrob. Agents 2017, 50, 29–34. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.; Enciso-Martínez, Y.; Martínez-de la Peña, C.F.; Rocha-Gracia, R.; Lozano-Zaraín, P.; Navarro-Ocaña, A.; Martínez-Laguna, Y.; de la Rosa-López, R. Virulence and resistance determinants of uropathogenic Escherichia coli strains isolated from pregnant and non-pregnant women from two states in Mexico. Infect. Drug Resist. 2020, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. Antimicrobial susceptibility profiles of bacteria causing urinary tract infections in Mexico: Single-centre experience with 10 years of results. J. Glob. Antimicrob. Resist. 2018, 14, 90–94. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Cantón, R. Nuevos aspectos microbiológicos de la fosfomicina. Rev. Esp. Quimioter. 2019, 32, 8–18. [Google Scholar]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Kengkla, K.; Kongpakwattana, K.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Antimicrob. Chemother. 2018, 73, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug resistant and extensively drug resistant bacteria: A study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [Green Version]
- Blaskovich, M.A.T.; Pitt, M.E.; Elliott, A.G.; Cooper, M.A. Can octapeptin antibiotics combat extensively drug-resistant (XDR) bacteria? Expert. Rev. Anti. Infec. Ther. 2018, 16, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Tenke, P.; Köves, B.; Nagy, K.; Hultgren, S.J.; Mendling, W.; Wullt, B.; Bjerklund Johansen, T.E. Update on biofilm infections in the urinary tract. World J. Urol. 2012, 30, 51–57. [Google Scholar] [CrossRef]
- Naziri, Z.; Kilegolan, J.A.; Moezzi, M.S.; Derakhshandeh, A. Biofilm formation by uropathogenic Escherichia coli: A complicating factor for treatment and recurrence of urinary tract infections. J. Hosp. Infect. 2021, 117, 9–16. [Google Scholar] [CrossRef]
- Rafaque, Z.; Abid, N.; Liaqat, N.; Afridi, P.; Siddique, S.; Masood, S.; Kanwal, S.; Dasti, J.I. In-vitro investigation of antibiotics efficacy against uropathogenic Escherichia coli biofilms and antibiotic induced biofilm formation at sub-minimum inhibitory concentration of Ciprofloxacin. Infect. Drug. Resist. 2020, 13, 2801–2810. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control. 2016, 5, 5. [Google Scholar] [CrossRef]
- Tewawong, N.; Kowaboot, S.; Pimainog, Y.; Watanagul, N.; Thongmee, T.; Poovorawan, Y. Distribution of phylogenetic groups, adhesin genes, biofilm formation, and antimicrobial resistance of uropathogenic Escherichia coli isolated from hospitalized patients in Thailand. PeerJ 2020, 8, e10453. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, S.; Chaudhary, U. Biofilm production in uropathogenic Escherichia coli. Indian J. Pathol. Microbiol. 2009, 52, 294. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodríguez-Cerrato, V.; Ponte, M.C.; Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship between biofilm formation and antimicrobial resistance in Gram-negative bacteria. Microb. Drug. Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Walkty, A.J.; Karlowsky, J.A. Fosfomycin: A first-line oral therapy for acute uncomplicated cystitis. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 2082693. [Google Scholar] [CrossRef] [PubMed]
- González, M.J.; Da Cunda, P.; Notejane, M.; Zunino, P.; Scavone, P.; Robino, L. Fosfomycin tromethamine activity on biofilm and intracellular bacterial communities produced by uropathogenic Escherichia coli isolated from patients with urinary tract infection. Pathog. Dis. 2019, 77, ftz022. [Google Scholar] [CrossRef]
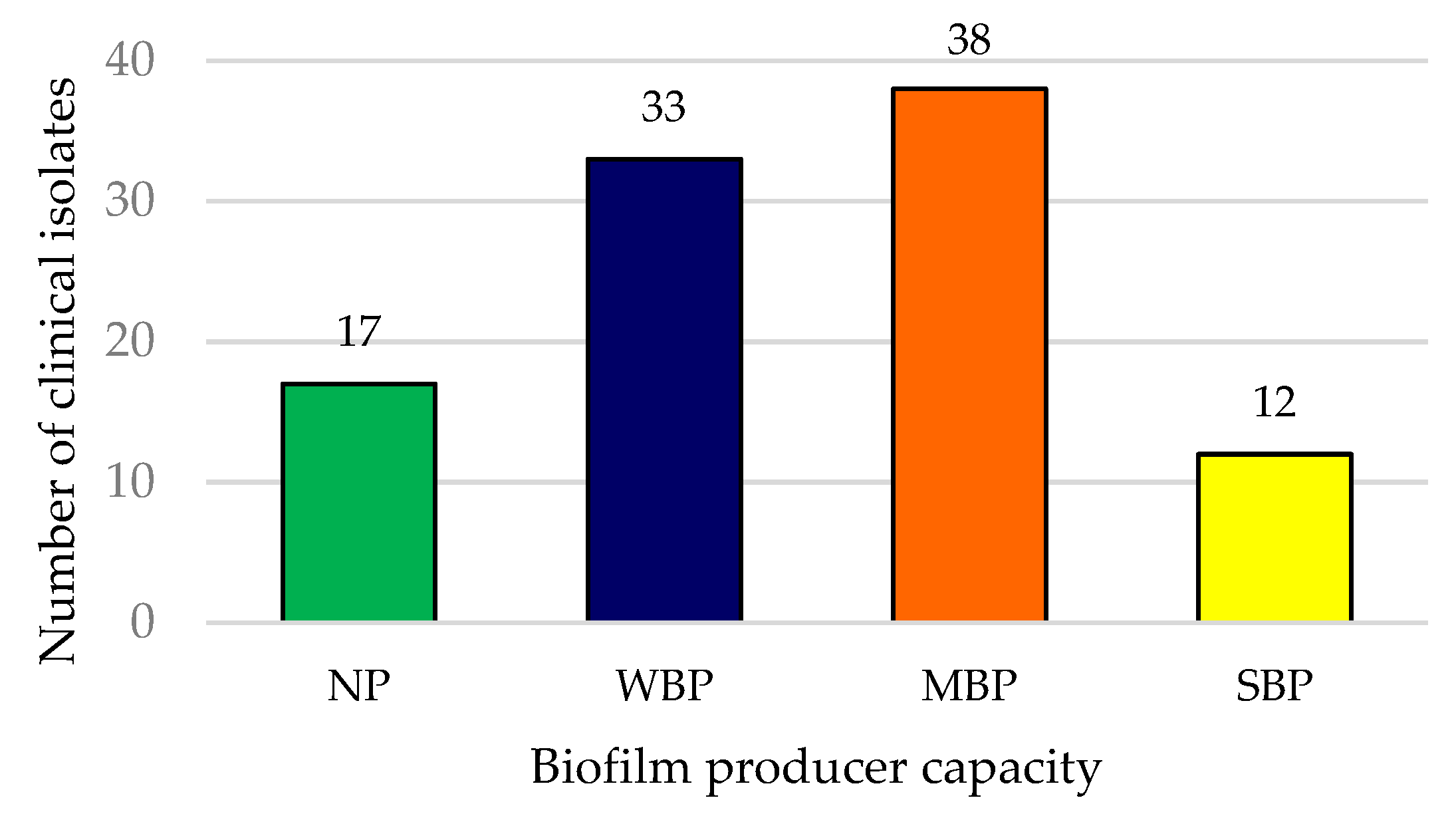
| Antimicrobial Category | Antibiotic |
|---|---|
| Penicillins | AMP, PIP |
| Penicillins + beta-lactamase inhibitors | SAM |
| Antipseudomonal penicillins + beta-lactamase inhibitors | TZP |
| 1st- and 2nd-generation cephalosporins | CFX |
| 3rd- and 4th-generation cephalosporins | FEP, CTX, CRO, CAZ |
| Cephamycins | CTT |
| Aminoglycosides | AMK, GEN, TOB |
| Carbapenems | IMP, MEM, ETP |
| Fluoroquinolones | LVX, CIP |
| Folate pathway inhibitors | SXT |
| Monobactams | ATM |
| Glycylcyclines | TGG |
| Tetracyclines | TET |
| Phenicols | CLO |
| Polymyxins | CST |
| Criteria | Biofilm-Formation Capacity |
|---|---|
| ODs ≤ODc | Not a biofilm producer |
| ODc ≤ ODs ≤ 2 × ODc | Weak biofilm producer |
| 2 × ODc ≤ ODs ≤ 4 × ODc | Moderate biofilm producer |
| 4 × ODc < ODs | Strong biofilm producer |
| ID Isolate | Resistance Drugs | Susceptibility Drugs |
|---|---|---|
| 120 | AMP, PIP, SAM, TZP, FEP, CTX, CFX, CRO, CAZ, CTT, AMK, GEN, TOB, IMP, ERT, LVX, CIP, SXT, ATM, TET, TGG | CLO, FOF, CST |
| 76 | AMP, SAM, PIP, FEP, CTX, CFX, CRO, CAZ, GEN, TOB, LVX, CIP, SXT, ATM, TET, CLO, FOF, CST | TZP, CTT, AMK, IMP, MEM, ETP, TGG |
| 77 | AMP, SAM, PIP, TZP, FEP, CTX, CFX, CRO, CAZ, TOB, IMP, LVX, CIP, SXT, ATM, TET, CLO, FOF | CTT, GEN, IMP, MEM, ETP, TGG, CST |
| 126 | AMP, SAM, PIP, TZP, FEP, CTX, CFX, CRO, CAZ, AMK, TOB, LVX, CIP, SXT, ATM, TET, CLO | CTT, AMK, GEN, IMP, MEM, ETP, TGG, CST, FOF, CST |
| Antimicrobials | Biofilm Producers | Non-Producers | p-Value | |
|---|---|---|---|---|
| Amikacin | R | 18 | 2 | 0.51 |
| S | 66 | 14 | ||
| Piperacillin/tazobactam | R | 37 | 6 | 0.78 |
| S | 47 | 10 | ||
| Gentamicin | R | 40 | 7 | >0.99 |
| S | 44 | 9 | ||
| Tobramycin | R | 55 | 11 | >0.99 |
| S | 29 | 5 | ||
| Trimethoprim/sulfamethoxazole | R | 54 | 12 | 0.57 |
| S | 30 | 4 | ||
| Tetracycline | R | 66 | 12 | 0.75 |
| S | 18 | 4 |
| ID Isolate | Biofilm-Formation Capacity | Anti-Biofilm Activity DC50 (μg/mL) | ID isolate | Biofilm-Formation Capacity | Anti-Biofilm Activity DC50 (μg/mL) |
|---|---|---|---|---|---|
| 61 | WBP | 164.4 ± 1.9 | 140 | MBP | 514.7 ± 3.0 |
| 117 | WBP | 175.6 ± 2.2 | 93 | MBP | 521.8 ± 6.9 |
| 116 | WBP | 187.1 ± 1.8 | 7 | MBP | 522.0 ± 3.1 |
| 95 | WBP | 188.6 ± 4.7 | 1 | WBP | 523.1 ± 1.8 |
| 43 | WBP | 189.6 ± 0.7 | 70 | SBP | 523.9 ± 5.9 |
| 48 | WBP | 192.0 ± 2.5 | 127 | WBP | 526.8 ± 5.7 |
| 38 | WBP | 200.8 ± 1.0 | 67 | MBP | 538.4 ± 3.1 |
| 27 | WBP | 206.0 ± 5.1 | 56 | MBP | 540.0 ± 4.8 |
| 119 | WBP | 209.0 ± 1.4 | 21 | MBP | 551.0 ± 0.9 |
| 65 | WBP | 222.4 ± 4.1 | 40 | MBP | 567.7 ± 2.7 |
| 52 | WBP | 243.1 ± 4.6 | 105 | MBP | 576.2 ± 2.3 |
| 16 | WBP | 245.1 ± 1.9 | 63 | MBP | 589.1 ± 5.7 |
| 54 | WBP | 276.9 ± 1.4 | 49 | MBP | 593.2 ± 1.2 |
| 34 | WBP | 278.0 ± 3.0 | 138 | SBP | 599.5 ± 4.8 |
| 91 | WBP | 278.0 ± 5.9 | 87 | MBP | 599.8 ± 1.4 |
| 15 | WBP | 301.1 ± 3.7 | 55 | MBP | 601.2 ± 1.8 |
| 122 | WBP | 307.5 ± 3.3 | 20 | MBP | 602.2 ± 2.1 |
| 39 | WBP | 333.2 ± 1.4 | 112 | SBP | 620.9 ± 8.5 |
| 68 | WBP | 339.8 ± 1.2 | 81 | MBP | 624.6 ± 2.9 |
| 53 | WBP | 340.0 ± 1.6 | 109 | MBP | 631.0 ± 2.5 |
| 88 | WBP | 341.8 ± 1.2 | 74 | MBP | 645.1 ± 2.1 |
| 2 | WBP | 359.0 ± 2.1 | 62 | MBP | 650.6 ± 1.3 |
| 33 | WBP | 364.7 ± 1.0 | 124 | MBP | 655.7 ± 4.7 |
| 11 | WBP | 369.6 ± 1.7 | 71 | SBP | 663.9 ± 2.6 |
| 118 | MBP | 375.2 ± 5.7 | 129 | MBP | 664.0 ± 1.6 |
| 92 | WBP | 378.2 ± 5.2 | 36 | MBP | 680.9 ± 2.6 |
| 41 | MBP | 380.8 ± 3.2 | 60 | MBP | 698.0 ± 2.3 |
| 18 | WBP | 380.9 ± 2.8 | 136 | SBP | 701.5 ± 2.7 |
| 5 | WBP | 400.0 ± 6.1 | 22 | MBP | 702.1 ± 1.4 |
| 19 | WBP | 400.1 ± 2.4 | 29 | WBP | 710.9 ± 2.6 |
| 66 | MBP | 403.1 ± 1.9 | 64 | SBP | 722.0 ± 1.1 |
| 10 | MBP | 419.0 ± 1.2 | 3 | SBP | 723.6 ± 2.0 |
| 26 | WBP | 420.7 ± 0.9 | 80 | MBP | 751.4 ± 1.8 |
| 47 | MBP | 431.6 ± 1.4 | 120 | MBP | 790.8 ± 4.1 |
| 28 | MBP | 444.8 ± 1.3 | 142 | MBP | 814.2 ± 5.1 |
| 50 | MBP | 447.0 ± 1.7 | 128 | MBP | 880.4 ± 1.7 |
| 114 | MBP | 449.1 ± 1.6 | 108 | SBP | 922.6 ± 6.3 |
| 139 | MBP | 461.5 ± 1.2 | 77 | SBP | 980.1 ± 1.1 |
| 97 | MBP | 470.9 ± 3.1 | 126 | SBP | 989.2 ± 2.6 |
| 131 | WBP | 474.8 ± 1.3 | 121 | SBP | 1021.2 ± 1.3 |
| 107 | MBP | 476.3 ± 5.7 | 76 | MBP | 1045.0 ± 3.4 |
| 101 | SBP | 500.2 ± 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzib-Baak, H.E.; Uc-Cachón, A.H.; Dzul-Beh, A.d.J.; Rosado-Manzano, R.F.; Gracida-Osorno, C.; Molina-Salinas, G.M. Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates. Trop. Med. Infect. Dis. 2022, 7, 235. https://doi.org/10.3390/tropicalmed7090235
Dzib-Baak HE, Uc-Cachón AH, Dzul-Beh AdJ, Rosado-Manzano RF, Gracida-Osorno C, Molina-Salinas GM. Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates. Tropical Medicine and Infectious Disease. 2022; 7(9):235. https://doi.org/10.3390/tropicalmed7090235
Chicago/Turabian StyleDzib-Baak, Haziel Eleazar, Andrés Humberto Uc-Cachón, Angel de Jesús Dzul-Beh, Rey Fernando Rosado-Manzano, Carlos Gracida-Osorno, and Gloria María Molina-Salinas. 2022. "Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates" Tropical Medicine and Infectious Disease 7, no. 9: 235. https://doi.org/10.3390/tropicalmed7090235
APA StyleDzib-Baak, H. E., Uc-Cachón, A. H., Dzul-Beh, A. d. J., Rosado-Manzano, R. F., Gracida-Osorno, C., & Molina-Salinas, G. M. (2022). Efficacy of Fosfomycin against Planktonic and Biofilm-Associated MDR Uropathogenic Escherichia coli Clinical Isolates. Tropical Medicine and Infectious Disease, 7(9), 235. https://doi.org/10.3390/tropicalmed7090235







