Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area and Health Facilities
2.2. Data Collection and Structuring
| Data Type | Variable | Source | Description |
|---|---|---|---|
| Topographic | Elevation, slope and Terrain Shape Index (TSI) | RNRA L&M | Using the DEM, an elevation map was created and further used to generated Slope and Terrain Shape Index [26] using ArcGIS. The outputs have a high spatial resolution with a cell size of 10 × 10 m. |
| Climatic | Rain, temperature humidity, evapo-transpiration | NMS | Data are measured nationwide at 183 meteorological and agro-meteorological weather stations and were interpolated and resampled to a cell size of 800 m. |
| Soil characteristics | pH, Clay and sand percentage | MINAGRI | The soil geo-database of Rwanda created in 2000 by MINAGRI in collaboration with Ghent University, Belgium. Resolution: 500 m (1/50,000) |
| Water bodies & Wetlands | Wetland area, wetland proportion, wetland use | REMA & RNRA | National inventory of wetlands by REMA in 2008 updated in collaboration with RNRA in 2012. Ortho-photograph resolution: 0.25 m. |
| Rice crop area | MINAGRI | The GIS database of the Rural Sector Support Program of MINAGRI considering new wetland management or rehabilitation for intensive cropping. Resolution: 25 m. | |
| Socio-economic conditions | Demography: Population, pop. density, number of households | NISR | The fourth Rwandan Population and Housing Census, August 2012 |
| Residential area | |||
| Sanitation | |||
| Water source | |||
| Education level |
2.3. Comparison of Spatial Patterns of STH Incidence and Prevalence at District and HFSA Level
2.4. Converting Confirmed Cases of STH Infection to Incidence Rates
2.5. Spatial Autocorrelation Analysis and Test for Spatial Clustering
2.6. Assessment of Relationship between STHs Incidence and Environmental Covariates
3. Results
3.1. Spatial Patterns of STH Incidence at District Level
3.2. Comparing District Level Prevalence and Incidence Data
3.3. Spatial Patterns and Spatial Clusters of STH Transmission at the HFSA Level
3.4. STH Incidence at District and HFSA Level and Their Association with Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, Y.S.; Zhou, X.N.; Utzinger, J.; Vounatsou, P. Bayesian geostatistical modelling of soil-transmitted helminth survey data in the People’s Republic of China. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef][Green Version]
- TRAC+. National Prevalence Survey on Soil-Transmitted Helminths and Schistosomiasis in School-Aged Children; Access Project; TRAC PLUS-Centre for Infectious Disease Control: Kigali, Rwanda, 2008. [Google Scholar]
- WHO. Rwanda-Country Profile. Preventive Chemotherapy and Transmission Control; WHO: Department of Control of Neglected Tropical Diseases: Geneva, Switzerland, 2010. [Google Scholar]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar]
- Ruxin, J.; Negin, J. Removing the neglect from neglected tropical diseases: The Rwandan experience 2008–2010. Glob. Public Health Int. J. Res. Policy Pract. 2012, 7, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Rujeni, N.; Morona, D.; Ruberanziza, E.; Mazigo, H.D. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: An update on their epidemiology and control. Infect. Dis. Poverty 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ruberanziza, E.; Owada, K.; Clark, N.J.; Umulisa, I.; Ortu, G.; Lancaster, W.; Munyaneza, T.; Mbituyumuremyi, A.; Bayisenge, U.; Fenwick, A.; et al. Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations. Trop. Med. Infect. Dis. 2019, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Owada, K.; Ruberanziza, E.; Ortu, G.; Umulisa, I.; Bayisenge, U.; Mbonigaba, J.B.; Mucaca, J.B.; Lancaster, W.; Fenwick, A.; et al. Parasite associations predict infection risk: Incorporating co-infections in predictive models for neglected tropical diseases. Parasites Vectors 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Abera, A.; Nibret, E. Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pac. J. Trop. Med. 2014, 7, 525–530. [Google Scholar] [CrossRef]
- Pullan, R.L.; Kabatereine, N.B.; Quinnell, R.J.; Brooker, S. Spatial and Genetic Epidemiology of Hookworm in a Rural Community in Uganda. PLoS Negl. Trop. Dis. 2010, 4, e713. [Google Scholar] [CrossRef]
- Kaliappan, S.P.; George, S.; Francis, M.R.; Kattula, D.; Sarkar, R.; Minz, S.; Mohan, V.R.; George, K.; Roy, S.; Ajjampur, S.S.R.; et al. Prevalence and clustering of soil-transmitted helminth infections in a tribal area in southern India. Trop. Med. Int. Health 2013, 18, 1452–1462. [Google Scholar] [CrossRef]
- Clements, A.C.A.; Deville, M.-A.; Ndayishimiye, O.; Brooker, S.; Fenwick, A. Spatial co-distribution of neglected tropical diseases in the East African Great Lakes region: Revisiting the justification for integrated control. Trop. Med. Int. Health 2010, 15, 198–207. [Google Scholar] [CrossRef]
- Halpenny, C.M.; Paller, C.; Koski, K.G.; Valdés, V.E.; Scott, M.E. Regional, Household and Individual Factors that Influence Soil Transmitted Helminth Reinfection Dynamics in Preschool Children from Rural Indigenous Panamá. PLoS Negl. Trop. Dis. 2013, 7, e2070. [Google Scholar] [CrossRef]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, Sanitation, Hygiene, and Soil-Transmitted Helminth Infection: A Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef]
- Larsen, M.N.; Roepstorff, A. Seasonal variation in development and survival of Ascaris suum and Trichuris suis eggs on pastures. Parasitology 1999, 119, 209–220. [Google Scholar] [CrossRef]
- Nyandwi, E.; Veldkamp, A.; Amer, S.; Karema, C.; Umulisa, I. Schistosomiasis mansoni incidence data in Rwanda can improve prevalence assessments, by providing high-resolution hotspot and risk factors identification. BMC Public Health 2017, 17, 845. [Google Scholar] [CrossRef]
- NISR. Fourth Rwanda Population and Housing Census. In Thematic Report: Population Projections; NISR: Kigali, Rwanda, 2014. [Google Scholar]
- Rwanda. Ministerial Instruction/Min/2012 Defining the Functioning Norms of Private Health Establishment in Rwanda, Kigali; Rwanda: Kigali, Rwanda, 2012. [Google Scholar]
- National Institute of Statistics of Rwanda. The Third Integrated Household Living Conditions Survey; National Institute of Statistics of Rwanda: Kigali, Rwanda, 2011. [Google Scholar]
- Swedesurvey. Project of Rwanda National Land Use and Development Master Plan, Report for Production of Ortho Photo in Rwanda; Swedesurvey: Stockholm, Sweden, 2010. [Google Scholar]
- McNab, W.H. Terrain Shape Index: Quantifying Effect of Minor Landforms on Tree Height. For. Sci. 1989, 35, 91–104. [Google Scholar]
- Verdoodt, A.; van Ranst, E. Environmental assessment tools for multi-scale land resources information systems: A case study of Rwanda. Agric. Ecosyst. Environ. 2006, 114, 170–184. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- REMA. Etablissement d’un Inventaire National Rapide des Marais et Élaboration de 5 Avant-Projets D’arrêtés Ministériels Relatifs Aux Marais; Rwanda Environment Management Authority Kigali: Kigali, Rwanda, 2008; p. 58. [Google Scholar]
- Nyandwi, E.; Veldkamp, T.A.; Amer, S. Regional climate sensitivity of wetland environments in Rwanda: The need for a location-specific approach. Reg. Environ. Change 2015, 16, 1635–1647. [Google Scholar] [CrossRef]
- Hurlimann, E.; Schur, N.; Boutsika, K.; Stensgaard, A.; Laserna de Himpsl, M.; Ziegelbauer, K.; Laizer, N.; Camenzind, L.; Di Pasquale, A.; Ekpo, U.; et al. Toward an open-access global database for mapping, control, and surveillance of neglected tropical diseases. PLoS Negl. Trop. Dis. 2011, 5, e1404. [Google Scholar] [CrossRef]
- Rothman, K.J. Epidemiology: An Introduction, 2nd ed.; Oxford University Press, Inc.: Oxford, UK, 2012; p. 268. [Google Scholar]
- Goovaerts, P. Accounting for rate instability and spatial patterns in the boundary analysis of cancer mortality maps. Environ. Ecol. Stat. 2008, 15, 421–446. [Google Scholar] [CrossRef]
- CDC. BioMedware SpaceStat-Empirical Bayesian Smoothing. Available online: https://www.biomedware.com/files/documentation/spacestat/Statistics/Smoothing/Empirical_Bayesian_Smoothing.htm (accessed on 5 November 2014).
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Getis, A. A History of the Concept of Spatial Autocorrelation: A Geographer’s Perspective. Geogr. Anal. 2007, 40, 297–309. [Google Scholar] [CrossRef]
- Hessami, M.; Gachon, P.; Ouarda, T.B.M.J.; St-Hilaire, A. Automated regression-based statistical downscaling tool. Environ. Model. Softw. 2008, 23, 813–834. [Google Scholar] [CrossRef]
- Ortega Hinojosa, A.M.; Davies, M.M.; Jarjour, S.; Burnett, R.T.; Mann, J.K.; Hughes, E.; Balmes, J.R.; Turner, M.C.; Jerrett, M. Developing small-area predictions for smoking and obesity prevalence in the United States for use in Environmental Public Health Tracking. Environ. Res. 2014, 134, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.L. Nonparametrics: Statistical Methods Based on Ranks, 1st ed.; Prentice Hall: Hoboken, NJ, USA, 1998; p. 463. [Google Scholar]
- Van Oost, K.; Govers, G.; Cerdan, O.; Thauré, D.; Van Rompaey, A.; Steegen, A.; Nachtergaele, J.; Takken, I.; Poesen, J. Spatially distributed data for erosion model calibration and validation: The Ganspoel and Kinderveld datasets. CATENA 2005, 61, 105–121. [Google Scholar] [CrossRef]
- Kabo, G.J.; Paulechka, Y.U.; Zaitsau, D.H.; Firaha, A.S. Prediction of the enthalpies of vaporization for room-temperature ionic liquids: Correlations and a substitution-based additive scheme. Thermochim. Acta 2015, 609, 7–19. [Google Scholar] [CrossRef]
- Wang, Z.J.; Chapiro, J.; Schernthaner, R.; Duran, R.; Chen, R.X.; Geschwind, J.F.; Lin, M.D. Multimodality 3D Tumor Segmentation in HCC Patients Treated with TACE. Acad. Radiol. 2015, 22, 840–845. [Google Scholar] [CrossRef]
- Lemeshow, S.; Moeschberger, M.L. Review of Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models by Vittinghoff, Glidden, Shiboski, and McCulloch. Strata 2005, 5, 274–278. [Google Scholar] [CrossRef]
- Legesse, M.; Erko, B.; Medhin, G. Efficacy of alebendazole and mebendazole in the treatment of Ascaris and Trichuris infections. Ethiop. Med. J. 2002, 40, 335–343. [Google Scholar] [PubMed]
- Montresor, A.; Crompton, D.W.T.; Hall, A.; Bundy, D.A.P.; Savioli, L. Guidelines for Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Malesu, M.M.; Oduor, A.R.; Chrogony, K.; Nyolei, D.; Gachene, C.K.K.; Biamah, E.K.; O’Neil, M.; Ilyama, M.; Mogoi, J.; Rwanda Irrigation Master Plan. The Government of Rwanda, Ministry of Agriculture and Animal Resources, Ebony Company Limited and World Agroforestry Centre (ICRAF). Nairobi, Kenya. Available online: http://www.worldagroforestry.org/downloads/Publications/PDFS/B16738.pdf (accessed on 30 January 2017).
- Abera, B.; Alem, G.; Melaku, M. Epidemiology of soil-transmitted helminths, Schistosoma mansoni, and haematocrit values among schoolchildren in Ethiopia. J. Infect. Dev. Ctries. 2012, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- MINECOFIN. Demographic and Health Survey [DHS] 2014/2015 Key Findings; MINECOFIN: Kigali, Rwanda, 2015. [Google Scholar]
- Brooker, S.; Clements, A.C.; Bundy, D.A. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv. Parasitol. 2006, 62, 221–261. [Google Scholar] [CrossRef] [PubMed]
- Mascarini-Serra, L. Prevention of Soil-transmitted Helminth Infection. J. Glob. Infect. Dis. 2011, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, F.; Segundo, H.; Gabrielli, S.; Totino, V.; Gonzales, P.R.; Salazar, E.; Bozo, R.; Bartoloni, A.; Cancrini, G. Dramatic Decrease in Prevalence of Soil-Transmitted Helminths and New Insights Into Intestinal Protozoa in Children Living in the Chaco Region, Bolivia. Am. J. Trop. Med. Hyg. 2015, 92, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, M. Integrating Population, Health, and Environment in Rwanda; Population Reference Bureau: Washington, DC, USA, 2009. [Google Scholar]
- Phillips, D.R. Health and Health Care in the Third World; Longman Scientific & Technical: Harlow, UK, 1990; p. 334. [Google Scholar]
- NISR; MOH; ICF International. Rwanda Demographic and Health Survey 2010; National Institute of Statistics of Rwanda (NISR): Kigali, Rwanda; Ministry of Health (MOH): Singapore; ICF International: Calverton, MD, USA, 2012. [Google Scholar]
- USAID/Rwanda. Rwanda HMIS Assessment Report; 9 May 2006; USAID: Kigali, Rwanda, 2006. [Google Scholar]

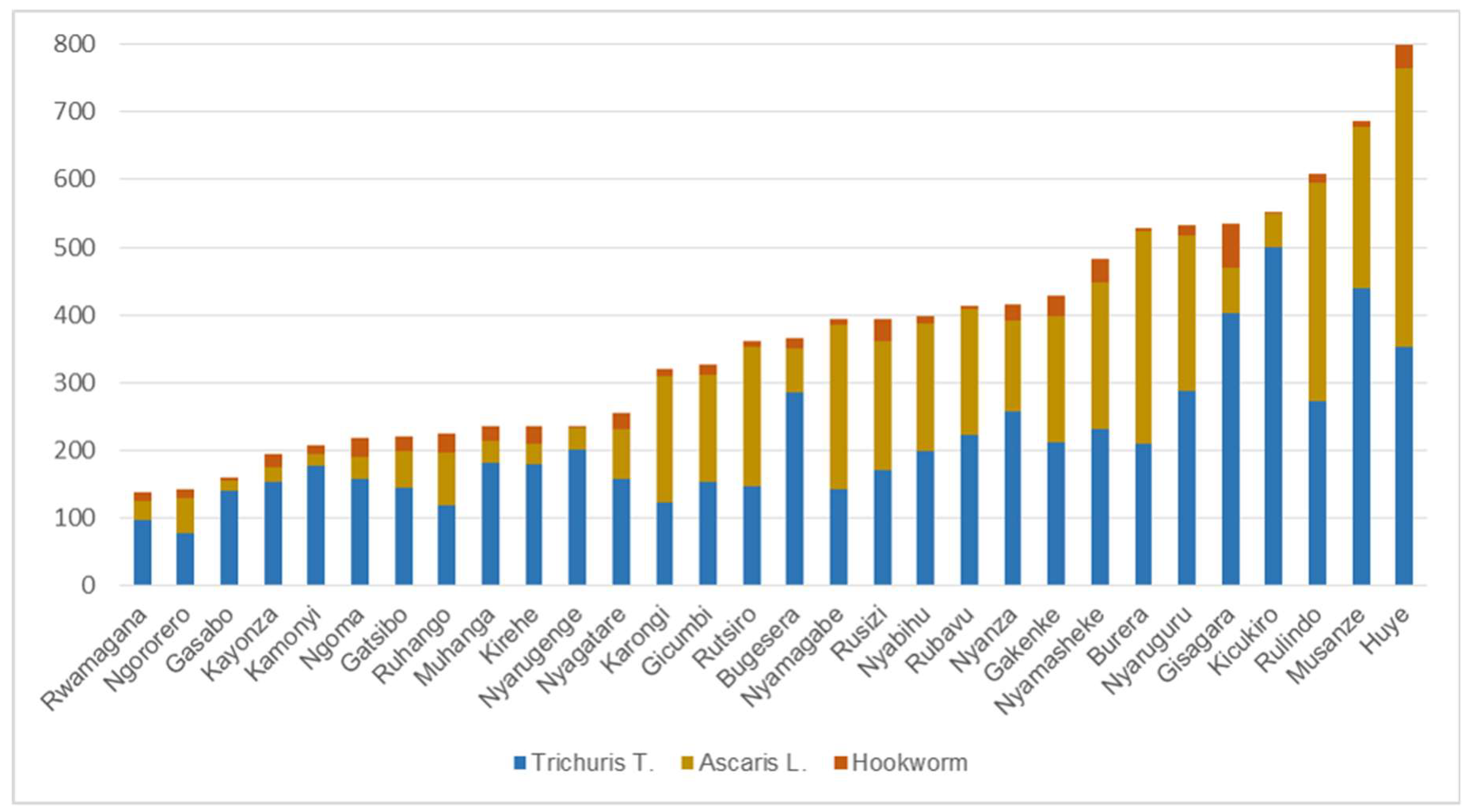
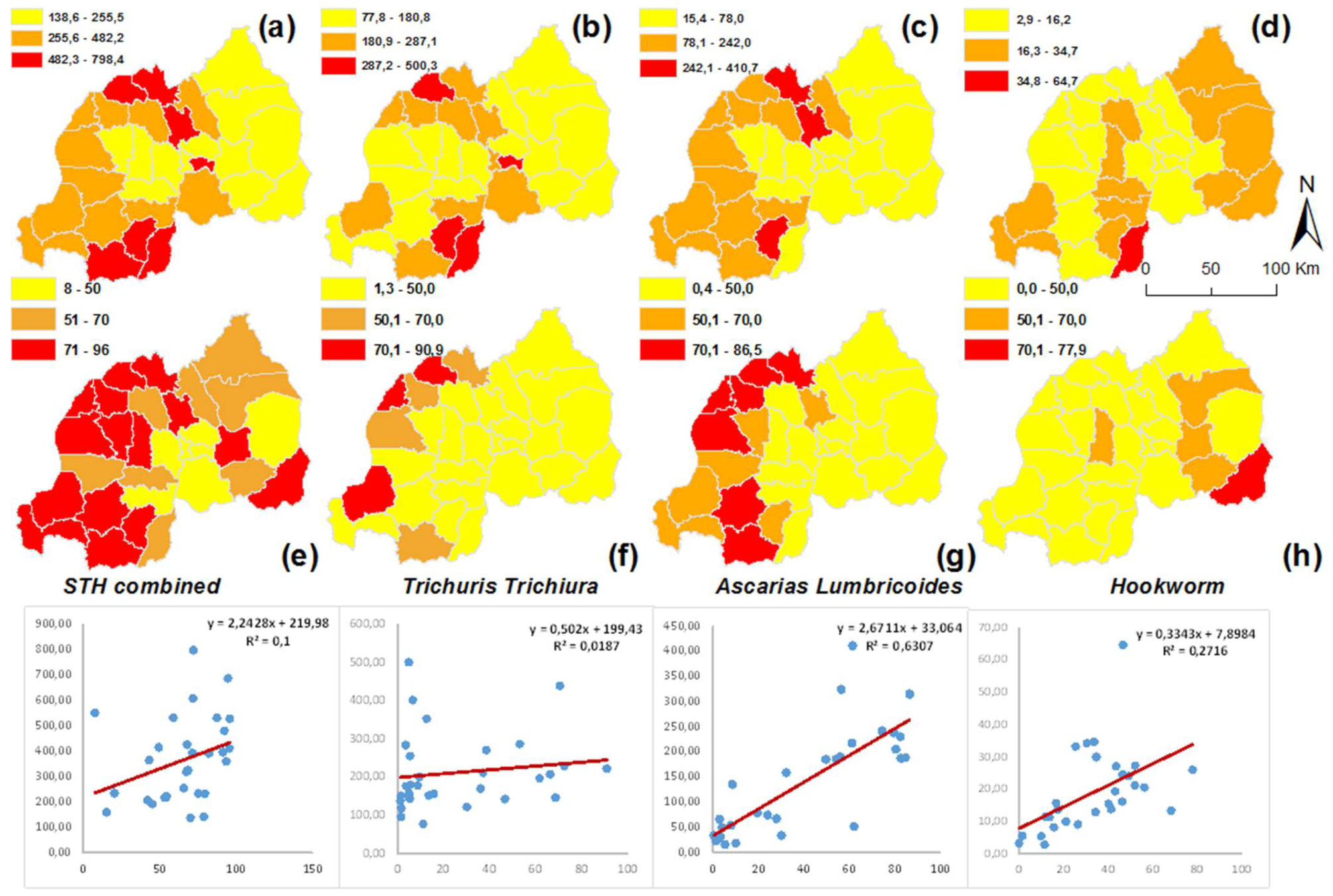
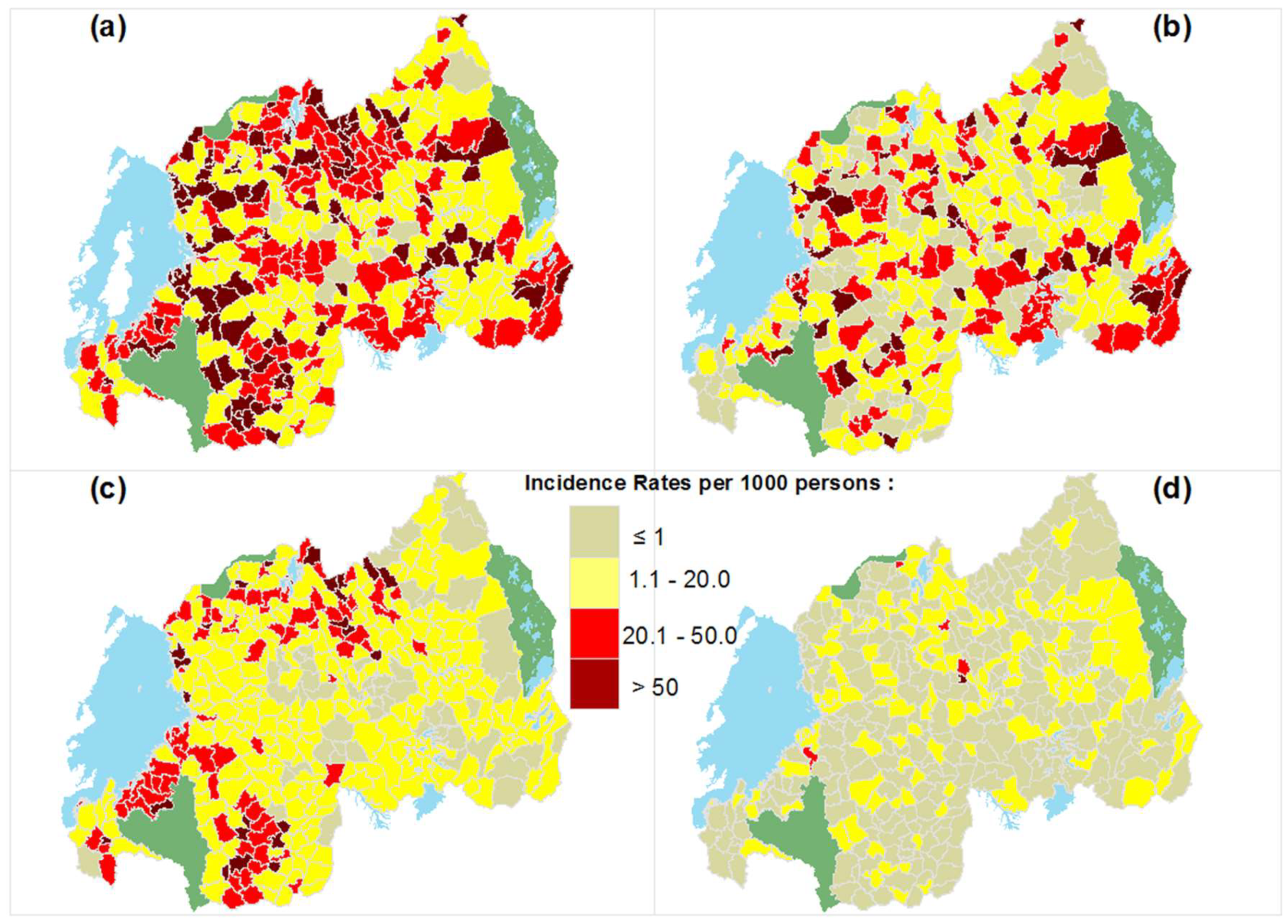

| Year | Moran’s I | p-Value | z-Score |
|---|---|---|---|
| Combined STH | 0.113 | 0.000006 | 4.51 |
| Trichuris trichiura | 0.052 | 0.037 | 2.08 |
| Ascaris lumbricoides | 0.375 | 0.000000 | 14.433 |
| Hookworm | 0.0056 | 0.744 | 0.326 |
| Factors | Combined | Trichuris Trichiura | Ascaris Lumbricoides | Hookworm | ||||
|---|---|---|---|---|---|---|---|---|
| District | HFSA | District | HFSA | District | HFSA | district | hfsa | |
| pH | - | - | 0.43 ** | - | - | - | - | - |
| Sand prop (%) | −0.38 ** | −0.41 ** | - | - | - | −0.43 ** | - | - |
| Elevation (m) | −0.53 ** | - | - | - | - | - | - | −0.29 ** |
| Wetland area (ha) | - | - | - | - | - | - | - | - |
| Wetland proportion (%) | 0.39 ** | 0.22 ** | - | 0.46 ** | - | - | - | - |
| Wetland rice cultivation (ha) | - | - | - | 0.26 * | - | - | - | - |
| Rainfall | 0.43 ** | 0.29 ** | - | - | - | 0.31 ** | - | - |
| Number of households | - | - | - | - | 0.37 * | - | - | - |
| Unimproved sanitation | - | - | 0.37 * | - | - | - | - | - |
| Rural | - | 0.125 * | - | - | - | 0.12 * | - | - |
| Urban | - | - | - | −0.27 * | - | - | - | - |
| Spatial Level | Factor | Significant Variables | R2 | Std. Error of the Estimate |
|---|---|---|---|---|
| Ascaris lumbricoides | ||||
| HFSA | Climatic | Rain | 0.27 | 337.802 |
| Physical | Sand percentage | |||
| Socio-economic | Percentage rural population | |||
| District | Demographic | Number of households | 0.13 | 2952.698 |
| Hookworm | ||||
| HFSA | Physical | Elevation | 0.08 | 52.635 |
| District | ||||
| Trichuris trichiura | ||||
| HFSA | Ecological | Wetland cultivated area | 0.22 | 90.302 |
| Wetland proportion | ||||
| Demographic | Urban proportion | |||
| District | Physical | pH | 0.30 | 395.764 |
| Demographic | Unimproved sanitation | |||
| Combined STH | ||||
| HFSA | Climatic | Rain | 0.24 | 362.725 |
| Physical | Sand percentage | |||
| Ecological | Wetland prop | |||
| Demographic | Rural proportion | |||
| District | Physical | Elevation, Sand percentage | 0.823 | 1553.642 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyandwi, E.; Veldkamp, T.; Amer, S.; Ruberanziza, E.; Rujeni, N.; Umulisa, I. Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda. Trop. Med. Infect. Dis. 2022, 7, 202. https://doi.org/10.3390/tropicalmed7080202
Nyandwi E, Veldkamp T, Amer S, Ruberanziza E, Rujeni N, Umulisa I. Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda. Tropical Medicine and Infectious Disease. 2022; 7(8):202. https://doi.org/10.3390/tropicalmed7080202
Chicago/Turabian StyleNyandwi, Elias, Tom Veldkamp, Sherif Amer, Eugene Ruberanziza, Nadine Rujeni, and Ireneé Umulisa. 2022. "Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda" Tropical Medicine and Infectious Disease 7, no. 8: 202. https://doi.org/10.3390/tropicalmed7080202
APA StyleNyandwi, E., Veldkamp, T., Amer, S., Ruberanziza, E., Rujeni, N., & Umulisa, I. (2022). Using Routinely Collected Health Records to Identify the Fine-Resolution Spatial Patterns of Soil-Transmitted Helminth Infections in Rwanda. Tropical Medicine and Infectious Disease, 7(8), 202. https://doi.org/10.3390/tropicalmed7080202






